Abstract
Host responses controlling blood-stage malaria include both innate and acquired immune effector mechanisms. During Plasmodium chabaudi infection in mice, a population of CD11bhighLy6C+ monocytes are generated in bone marrow, most of which depend on the chemokine receptor CCR2 for migration from bone marrow to the spleen. In the absence of this receptor mice harbor higher parasitemias. Most importantly, splenic CD11bhighLy6C+ cells from P chabaudi–infected wild-type mice significantly reduce acute-stage parasitemia in CCR2−/− mice. The CD11bhighLy6C+ cells in this malaria infection display effector functions such as production of inducible nitric oxide synthase and reactive oxygen intermediates, and phagocytose P chabaudi parasites in vitro, and in a proportion of the cells, in vivo in the spleen, suggesting possible mechanisms of parasite killing. In contrast to monocyte-derived dendritic cells, CD11bhighLy6C+ cells isolated from malaria-infected mice express low levels of major histocompatibility complex II and have limited ability to present the P chabaudi antigen, merozoite surface protein-1, to specific T-cell receptor transgenic CD4 T cells and fail to activate these T cells. We propose that these monocytes, which are rapidly produced in the bone marrow as part of the early defense mechanism against invading pathogens, are important for controlling blood-stage malaria parasites.
Introduction
Malaria infections induce strong innate immune responses from the host, which play several roles during infection. They are necessary to initiate protective acquired immunity mediated mainly through CD4 T cells and antibody.1 However, if too strong, they may contribute to the pathology associated with severe malaria.2,3 Innate responses can also have direct antiparasite effects, but the extent to which they contribute to early control of blood-stage parasitemia is not fully understood. Activation of macrophages or monocytes can result in release of parasiticidal mediators and in receptor-mediated phagocytosis.4,5 Natural killer (NK), NKT, and γδ T cells may also be involved in parasite control at this early stage3 through direct recognition and killing of parasites within red blood cells (RBCs) or merozoite stages of the parasite or by cytokine release and subsequent macrophage activation.
Because the erythrocyte stages of Plasmodium circulate in the blood and do not enter tissues, the most likely location for such innate mechanisms to take place is within the spleen, and this organ is crucial for development of immunity to malaria.6 In humans, mechanical retention of infected RBCs (iRBCs) in the spleen has been suggested to contribute to the clearance of Plasmodium falciparum.7 Although there are differences between the splenic structure of mice and humans, splenomegaly8 and disruption of the microarchitecture are characteristic features of both human malaria infections and mouse models of infection.9 We have previously observed in Plasmodium chabaudi infections of mice that cellularity of the spleen increases during the acute stage of infection10 and 10% or more of splenocytes express low levels of CD11c on their surface.11 This increase in myeloid cells in response to infection may be important either in directly controlling blood-stage parasitemia or in activating CD4 T cells and B cells or both to bring about a protective acquired immune response.
Whether these cells have migrated into the spleen or have been generated as a result of extramedullary hematopoiesis in situ from myeloid precursors12-14 in response to the malaria infection is not known. Leukocyte trafficking into sites of infection is a highly regulated process. During systemic bacterial infections inflammatory monocytes migrate from the bone marrow to the spleen where they produce tumor necrosis factor-α (TNF-α) and inducible nitric oxide synthase (iNOS), phagocytose bacteria, and activate and stimulate marginal zone B cells to produce immunoglobulin M.15,16 Egress of these cells from the bone marrow depends on the presence of the chemokine receptor CCR2.17,18 Consequently, mice deficient in CCR2 expression fail to recruit a population of inflammatory monocytes to the sites of infection and are susceptible to many infections.19-22 Here, we have characterized CD11bhighLy6C+ cells that appear in the spleen of mice after an acute infection of P chabaudi. They resemble inflammatory monocytes and require the chemokine receptor CCR2 for their egress from the bone marrow. Most importantly, we provide strong evidence that these migrating monocytes are important for the control of this acute blood-stage malaria infection. Phagocytosis of P chabaudi–iRBCs in vivo and parasiticidal mediators such as reactive oxygen intermediates are possible candidates for the protective effect of these monocytes in malaria infections.
Methods
Mice and parasites
C57Bl/6, BALB/c, BALB/c Rag2−/− mice, and B5 MSP1-specific T-cell receptor (TCR) transgenic (Tg) mice23 interleukin-10−/− (IL-10−/−), IL-6−/−, and IL-12p40−/− mice on C57Bl/6 background (6-12 weeks old) were bred in the specific pathogen–free unit at the National Institute for Medical Research. CCR2−/− mice on C57Bl/6 or BALB/c backgrounds17 were used with kind permission from W. Kuziel (PDL Biopharma). Mice were housed in reverse light conditions and were infected intraperitoneally with 105 iRBCs containing P chabaudi chabaudi (AS) parasites. Body weights and parasitemias were monitored as described.24 All mouse experiments were approved by the British Home Office.
Transfection of P chabaudi–expressing GFP
P chabaudi schizonts were episomally transfected with the pL0016 plasmid containing the genes for green fluorescent protein (GFP) and the drug resistance marker DHFR,25 a kind gift of Dr Blandine Franke-Fayarde (University of Leiden). The transfected parasites expressed GFP under drug selection. P chabaudi trophozoites were purified from the blood of infected BALB/c RAG2−/− mice on a 74% Percoll cushion prepared as previously described.26 Parasites were matured to schizonts in vitro in RPMI containing 14% fetal calf serum (FCS) for up to 6 hours. Schizonts (108) were transfected with 10 μg of pL0016 plasmid with Nucleofector technology (Lonza), using the U33 program and the T-cell line Nucleofector kit (VCA-1002).27 Transfected parasites were immediately injected intravenously into a BALB/c RAG2−/− mouse. One day after transfection, 0.07 mg/mL Pyrimethamine (Sigma-Aldrich) was administered in acidified drinking water for 12 to 15 days to select for transfected parasites. GFPbright-iRBCs were purified by cell sorting on a MoFlo (Dako-Cytomation) and injected into BALB/c RAG2−/− mice. The GFPbright enriched parasites were used for infection as described in “Mice and parasites.” For flow cytometric analysis of RBCs containing P chabaudi–expressing GFPs (PcAS-GFPs), mice were exsanguinated, the blood was collected and stained with 2 μg/mL Hoechst 33342 dye (BD Biosciences). Samples were acquired on a BD LSR II Flow Cytometer (BD Biosciences) and analyzed with FlowJo software (TreeStar).
Cell lines and peptides
The B5 T-cell hybridoma recognizing a peptide1151-1171 of merozoite surface protein 1 (MSP1) and I-Ed of P chabaudi MSP1 has been described.28 The hybridoma was cultured in Iscove medium (IMDM; Sigma-Aldrich) with 10% FCS, 1mM l-glutamine, 10mM HEPES, 5 × 10−5M 2ME, 100 μg/mL penicillin, 100 U/mL streptomycin, and 1mM sodium pyruvate (complete IMDM). The IL-2–dependent T-cell line, CTLL-2, was cultured with complete IMDM supplemented with 10 U of human recombinant IL-2/mL as described.29
Antibodies and reagents
Antibodies used were anti-CD8αbiotin, CD19biotin, Ter119biotin, CD11cAPC–Alexa Fluor 750, CD11bPE-Cy7 (eBioscience, Insight Biotechnology), Ly6CAPC (Serotec), H-2A, CD3PerCP, CD86PE, CD40PE, Ly6GPE, CD68FITC, Dx5PE, F4/80FITC, CD8αPE, streptavidin-PerCP, (BD Biosciences), streptavidin–Alexa 610 (Molecular Probes), and iNOS-PE (Insight Biotechnology). Rabbit immunoglobulin G isotype control PE (Southern Biotec). An Alexa 488–labeled antibody that recognized plasmacytoid dendritic cells (DCs), 120G8,30 was a kind gift of Giorgio Trinchieri (National Cancer Institute–Frederick, MD) and Anne O'Garra (National Institute for Medical Research). Isotype control Ab (BD Biosciences) were included in each staining, 7-Aminoactinomycin D (7AAD; Sigma-Aldrich), DHR 123 (Molecular Probes).
Quantitative reverse transcription–polymerase chain reaction
Sorted cells from C57Bl/6 and BALB/c mice and IL-12p40, IL-10, and I-L6−/− mice were placed in Trizol (Invitrogen), and RNA extraction was performed as described.31 cDNA was synthesized and analyzed for expression of cytokines (primers are listed in supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article) by real-time polymerase chain reaction assay on an ABI Prism 7000 Sequence Detection System (Applied Biosystems). Specific gene mRNA expression was quantified with SYBR Green (Fisher-Scientific) and normalized to ubiquitin levels, using the 2−ΔΔCt method as previously described.32
Cell sorting and enrichment
CD11bhighLy6C+ monocytes were purified from spleens of P chabaudi–infected mice by cell sorting. Single-cell suspensions were treated for 30 minutes at 37°C with 0.4 mg/mL Liberase CI (Roche) in serum-free IMDM. After washing and centrifugation at 500g for 10 minutes, cell pellets were resuspended for 5 minutes in 8.3 g/L NH4Cl in 0.01M Tris-HCl pH 7.5 to lyse RBCs. Spleen cells were washed, incubated with FcR block, followed by anti-CD11b magnetic beads and enrichment on magnetic columns (Miltenyi Biotec Inc). Enriched cells were stained with biotinylated CD19, CD8, Ter119, CD3 antibodies and streptavidin Alexa 610, CD11bPE-Cy7, Ly6CAPC, CD11cAlexa 750 as well as 7AAD. Viable CD11bhighLy6C+ monocytes were sorted on a MoFlo cytometer (Dako-Cytomation) to greater than 95% purity. Gates were set on lineage-negative cells (CD19, CD8, CD3, Ter119 negative), and CD11bhighLy6C+ monocytes with intermediate levels of CD11c were sorted (shown in supplemental Figure 5Ci). Viable CD11chigh DCs were enriched with CD11c magnetic beads (Miltenyi Biotec Inc) and stained with 7AAD, Ter119, CD3, CD19 antibodies as described. Viable CD11chigh, CD19−, CD3−, Ter119− (DCs) were sorted from the CD11c-enriched population to greater than 95% purity. TCR transgenic CD4+ T cells were isolated from spleens of uninfected B5 mice with the use of anti-CD4 beads (Miltenyi Biotec Inc).
Flow cytometric analysis
Spleen cells were isolated as described in “Cell sorting and enrichment,” incubated with Fc receptor block (BD Biosciences), and stained with antibodies listed above as described.11 Intracellular iNOS-PE staining was performed after surface staining and fixation in 2% paraformaldehyde. Reactive oxygen intermediate (ROI) staining on monocytes was performed by incubating 2 × 106 surface-labeled splenocytes with 10μM DHR 123 for 30 minutes at room temperature before fixing in 2% paraformaldehyde for 20 minutes. Samples were acquired on a BD LSR II Flow Cytometer and analyzed with FlowJo software.
CD4 T-cell hybridoma and B5 CD4 Tg cell proliferation and cytokine production
For T-cell proliferation assays, B5 CD4 Tg T cells (105) were cultured with sorted CD11bhighLy6C+ and CD11chigh cells isolated from BALB/c mice 8 days after infection as described above in 200 μL of complete IMDM at 37°C, 7% CO2 in 96-well round-bottom plates for 4 days and pulsed with 3H thymidine (Amersham) for the final 12 hours.28
For detection of cytokines, 105 Tg T cells and 5 × 104 sorted CD11chigh or CD11bhighLy6C+ cells were incubated for 6 days in 200 μL of complete IMDM at 37°C, 7% CO2 in 96-well round-bottom plates. After incubation, cells were washed and resuspended in 200 μL of complete IMDM containing 2 μg/mL anti-CD28 Abs, and 105 cells/well were transferred to a flat-bottomed plate pretreated with 10 μg/mL anti-CD3. Interferon-γ (IFNγ), IL-4 and IL-10 were determined in supernatants after 48-hour culture by enzyme-linked immunoabsorbent assays as described.33 Recombinant IFNγ, IL-4, and IL-10 standards were purchased from Invitrogen. To measure the IL-2 response of MSP1-specific T-cell hybridoma, 2 × 104 B5 cells were cultured with sorted CD11chigh and CD11bhighLy6C+ cells isolated from BALB/c mice 8 days after infection as described above in 200 μL of complete IMDM at 37°C, 7% CO2 in 96-well round-bottom plates for 24 hours, and supernatants were cultured with the IL-2–dependent cell line CTLL2 for 24 hours before being pulsed with 3H-thymidine (Amersham Biosciences) as described.28
Phagocytosis of P chabaudi–iRBCs
In vitro phagocytosis assay.
Blood was collected from mice infected with WT or PcAS-GFP (day 7) when parasites were predominantly late trophozoites. iRBCs were enriched by centrifugation on 74% isotonic Percoll (Amersham Biosciences) as described.26 Trophozoite-iRBCs, from which contaminating leukocytes had been removed with Plasmodipur filters (Euro-Diagnostica), were stained with Giemsa and found to be greater than 95% pure. WT iRBCs (5 × 107/mL) were incubated with 1μM CFSE (Molecular Probes) for 5 minutes at room temperature and then washed 3 times in complete IMDM.
CD11b+ spleen cells were enriched from infected mice by using Miltenyi magnetic beads and prepared as described. iRBCs at 100:1 ratio (iRBC/splenocytes) were cultured in 500 μL of complete IMDM for 2 hours at 37°C, 7% CO2, in a 48-well tissue-culture plate (Corning Life Sciences). After incubation, nonadherent cells were removed, and adherent cells were dislodged with cold 2mM EDTA in PBS. iRBCs that were not phagocytosed were lysed in water for 10 seconds, cells were washed with HBSS 5% FCS and labeled with biotinylated CD19, CD3, Ter119 antibodies and streptavidin PerCP as well as CD11bPE-Cy7, Ly6CAPC, and CD11cAlexa Fluor 750. Samples were acquired on a BD LSR II Flow Cytometer and analyzed with FlowJo software.
In vivo phagocytosis.
PcAS-GFP parasites were injected into BALB/c and C57BL/6 mice maintained on pyrimethamine-containing drinking water. Parasitemias were monitored daily, and the mice were killed on day 8, at peak parasitemia. Spleen cells were prepared as described and stained with antibodies against CD19, CD3, CD8, Ter119, Ly6C, CD11c, and CD11b. Samples were acquired on a BD LSR II Flow Cytometer and analyzed with FlowJo software. Alternatively, PcAs-GFP parasites were purified on Percoll gradients and injected intravenously (109 parasites) into C57Bl/6 mice infected 13 days previously with 105 WT P chabaudi. The recipient mice were treated with 20 mg/kg chloroquine (Sigma-Aldrich) 2 hours before injection. Splenocytes were harvested after 15 minutes and prepared, stained, and analyzed as in “Flow cytometric analysis.”
Adoptive transfer experiments
Single spleen-cell suspensions were prepared from C57Bl/6 mice 7 days after intraperitoneal infection with 105P chabaudi as described. CD8+ and CD4+ T cells were depleted as described.33 The remaining cells were incubated with FcR block, and CD11bhigh cells were enriched (> 90%) by using CD11b magnetic beads (Miltenyi Biotec Inc). Enriched cells were stained with CD19, CD3, CD8, Ter119, as well as Ly6C, CD11c, and CD11b, and samples were acquired and analyzed as described. Enriched CD11b+ cells (15 × 106) were injected intravenously into CCR2−/− C57Bl/6 mice or WT C57BL/6 mice infected 7 days previously with 105P chabaudi. Parasitemias were determined as described in “Mice and parasites.”
Statistical analysis
Statistical significance was evaluated by using the 2-tailed Student t test and the Mann-Whitney test.
Results
Increased numbers of CD11bhighLy6C+ monocytes in the spleen during the first 20 days of acute blood-stage infection of C57Bl/6 mice with P chabaudi
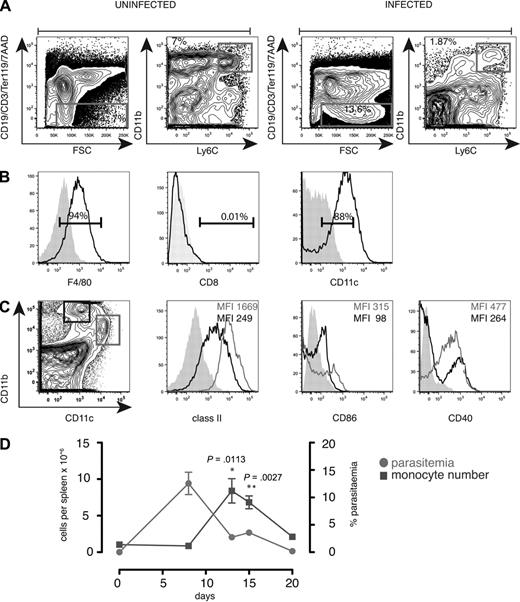
We reported previously that during and after primary infection with P chabaudi, 10% to 15% of splenocytes comprised a population of CD8− cells expressing low levels of CD11c.11 These may be cells generated in situ or recruited into the spleen, either as a component of the innate effector response to control parasite growth or to provide an expanded population of antigen-presenting cells (APCs) for the activation of T cells. On further analysis, we found that the spleen contained cells expressing high levels of CD11b and were positive for the monocyte marker Ly6C (Figure 1A). This cell population also expressed the F4/80 antigen (Figure 1B) and CD68 (not shown) as well as low levels of CD11c, class II, CD40 and CD86 (Figure 1B-C), and were of monocytic appearance (not shown). Only a small proportion expressed CD8α (Figure 1B), the granulocyte marker Ly6G, the NK marker, DX5, or the plasmacytoid DC marker recognized by the Ab 120G8 (data not shown). The monocyte-like population increased up to day 13 to 15 of infection as the parasitemia was controlled and was still elevated at day 20 of infection (Figure 1D).
Increased numbers of CD11bhighLy6C+ monocytes in the spleen during the first 20 days of acute P chabaudi infection. (A) Spleen cells from uninfected and day 8–infected C57Bl/6 mice were stained with antibodies against CD11c, CD8, Ly6C, CD11b, CD19, CD3, Ter119, as well as 7AAD. (Left) Gating strategy was a FACS plot of CD19, CD3, Ter119, 7AAD, and forward scatter (FSC) on spleen cells. Gate was set on viable lineage-negative cells as well as side scatter (SSC) and FSC. (Right) FACS plot showing CD11b and Ly6C profiles of gated cells. (B) Histogram of gated CD11bhighLy6C+ cells from day 8–infected cells: (left) F4/80, (middle) CD8, and (right) CD11c. Shaded histogram shows unstained control. Numbers show the percentage of positive cells within the region. (C left) Dot plot of CD11b and CD11c on day 8–infected spleen cells gated on FSC and SSC. Histograms of class II, CD86, and CD40 on gated CD11chigh cells (shown in left panel) and CD11bhighLy6C+ cells gated as described in panel A. Each experiment was performed with at least 3 mice per time point and repeated 3 times. (D) Total numbers of CD11bhighLy6C+CD11c low monocytes (left y-axis) in the spleen of C57Bl/6 mice during the first 20 days of a primary intraperitoneal infection with P chabaudi and percentage of parasitemia (right y-axis). Error bars represent SEM of the values from 3 individual mice. Each experiment was performed with at least 3 mice per time point and repeated 3 times. Significant differences were determined using the Student t test.
Increased numbers of CD11bhighLy6C+ monocytes in the spleen during the first 20 days of acute P chabaudi infection. (A) Spleen cells from uninfected and day 8–infected C57Bl/6 mice were stained with antibodies against CD11c, CD8, Ly6C, CD11b, CD19, CD3, Ter119, as well as 7AAD. (Left) Gating strategy was a FACS plot of CD19, CD3, Ter119, 7AAD, and forward scatter (FSC) on spleen cells. Gate was set on viable lineage-negative cells as well as side scatter (SSC) and FSC. (Right) FACS plot showing CD11b and Ly6C profiles of gated cells. (B) Histogram of gated CD11bhighLy6C+ cells from day 8–infected cells: (left) F4/80, (middle) CD8, and (right) CD11c. Shaded histogram shows unstained control. Numbers show the percentage of positive cells within the region. (C left) Dot plot of CD11b and CD11c on day 8–infected spleen cells gated on FSC and SSC. Histograms of class II, CD86, and CD40 on gated CD11chigh cells (shown in left panel) and CD11bhighLy6C+ cells gated as described in panel A. Each experiment was performed with at least 3 mice per time point and repeated 3 times. (D) Total numbers of CD11bhighLy6C+CD11c low monocytes (left y-axis) in the spleen of C57Bl/6 mice during the first 20 days of a primary intraperitoneal infection with P chabaudi and percentage of parasitemia (right y-axis). Error bars represent SEM of the values from 3 individual mice. Each experiment was performed with at least 3 mice per time point and repeated 3 times. Significant differences were determined using the Student t test.
Sustained acute-stage parasitemia in P chabaudi–infected C57Bl/6 CCR2−/− mice compared with WT mice
Many bone marrow–derived cells, including monocytes, produced during a variety of infections depend on the chemokine receptor CCR2 for their migration out of the bone marrow.19 This was also the case for the CD11bhighLy6C+ monocytes observed in this P chabaudi infection. C57Bl/6 CCR2−/− mice infected with P chabaudi had significantly more CD11b+ monocytes present in the bone marrow (Figure 2B) and significantly fewer CD11bhighLy6C+ cells in the spleen (Figure 2B) than their infected WT counterparts on day 8 after infection. Deletion of CCR2 has been shown to affect T-cell migration17 ; however, no significant difference in the numbers of CD3+ T cells or CD19+ B cells was evident at day 8 of infection (supplemental Figure 1). These data suggest that a population of monocytes leaves the bone marrow in a CCR2-dependent manner and traffics to the spleen in response to infection and that this may represent part of a host response for controlling P chabaudi infection. Reduced numbers of monocytes in the spleens of CCR2−/− mice were transient and returned to levels of those in WT mice by day 11 (Figure 2B). However, they were still retained in the bone marrow at this time, suggesting these cells may also be produced elsewhere during infection.
Sustained acute-stage parasitemia and delayed weight gain in P chabaudi–infected C57Bl/6 CCR2−/− mice compared with WT mice. (A) Contour plots showing CD11b and Ly6C expression in bone marrow (left) and spleen (right) of CCR2-deficient and WT C57Bl/6 mice infected for 8 days with P chabaudi. Number indicates percentage of CD11bhighLy6Chigh bone marrow monocytes within the region shown. The same gating strategy as described in Figure 1 was used to obtain the CD11b versus Ly6C contour plots of the spleen cells. Number indicates percentage of CD11bhighLy6C+ monocytes in the gated populations. (B) Total numbers of CD11bhighLy6Chigh monocytes in the bone marrow (left) and the CD11bhighLy6C+ monocytes in the spleen (right) of CCR2−/− (□) and WT C57Bl/6 (■) mice after 8 and 11 days of infection. The values shown represent the means and SEMs of 4 mice. P values are shown (Student t test). (C) Parasitemia (left) and body weight (right) in P chabaudi–infected CCR2−/− (open symbols) and WT (closed symbols) C57Bl/6 mice. Mice were infected intraperitoneally with 105 iRBCs, and the course of infection and weight was followed for 22 days. Weight is expressed as the percentage of the starting weight at day 0. Significant differences: *P = .001-.01, **P = .01-.05 (Mann-Whitney test) between WT and CCR2−/− mice. The values shown represent the means and SEMs of 5 to 10 mice in each group.
Sustained acute-stage parasitemia and delayed weight gain in P chabaudi–infected C57Bl/6 CCR2−/− mice compared with WT mice. (A) Contour plots showing CD11b and Ly6C expression in bone marrow (left) and spleen (right) of CCR2-deficient and WT C57Bl/6 mice infected for 8 days with P chabaudi. Number indicates percentage of CD11bhighLy6Chigh bone marrow monocytes within the region shown. The same gating strategy as described in Figure 1 was used to obtain the CD11b versus Ly6C contour plots of the spleen cells. Number indicates percentage of CD11bhighLy6C+ monocytes in the gated populations. (B) Total numbers of CD11bhighLy6Chigh monocytes in the bone marrow (left) and the CD11bhighLy6C+ monocytes in the spleen (right) of CCR2−/− (□) and WT C57Bl/6 (■) mice after 8 and 11 days of infection. The values shown represent the means and SEMs of 4 mice. P values are shown (Student t test). (C) Parasitemia (left) and body weight (right) in P chabaudi–infected CCR2−/− (open symbols) and WT (closed symbols) C57Bl/6 mice. Mice were infected intraperitoneally with 105 iRBCs, and the course of infection and weight was followed for 22 days. Weight is expressed as the percentage of the starting weight at day 0. Significant differences: *P = .001-.01, **P = .01-.05 (Mann-Whitney test) between WT and CCR2−/− mice. The values shown represent the means and SEMs of 5 to 10 mice in each group.
Lack of CCR2 rendered C57Bl/6 mice more susceptible to a primary P chabaudi infection; CCR2−/− mice displayed significantly higher parasitemias with delayed reduction of the acute infection (days 13-17, P = .031-.007, Mann-Whitney test; Figure 2C) and had lost significantly more weight on day 16 than did WT mice (Figure 2C).
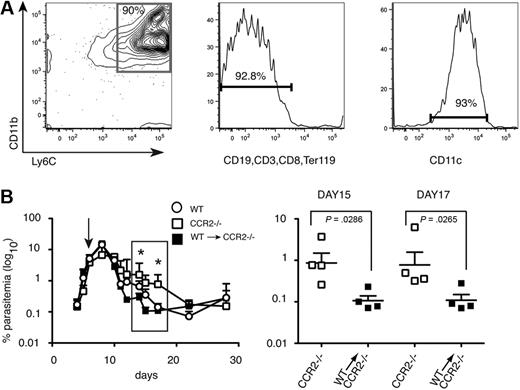
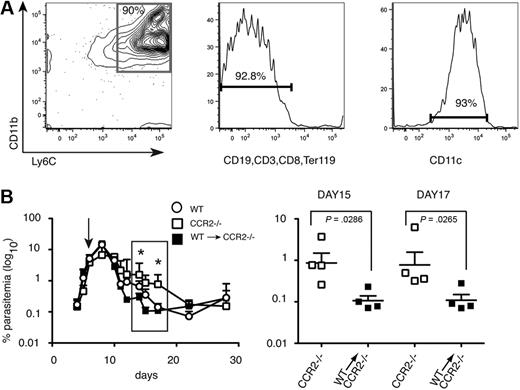
Transfer of CD11bhighLy6C+ spleen cells from P chabaudi–infected WT C57Bl/6 mice into CCR2−/− mice accelerates clearance of blood-stage parasitemia
Because CCR2 controls migration of other cells in addition to monocytes,17 it was possible that delay in parasite clearance in the CCR2−/− mice was not due to reduced numbers of CD11bhighLy6C+ monocytes in the spleen. To determine whether these cells could restore the ability of C57Bl/6 CCR2−/− mice to reduce acute parasitemias to the levels of WT mice, P chabaudi–infected CCR2−/− C57Bl/6 mice were injected with purified CD11bhighLy6C+ cells from day 7 infected C57Bl/6 WT mice (Figure 3A). Parasitemias were significantly reduced in the reconstituted mice between days 12 and 20 compared with unreconstituted CCR2−/− mice and were equivalent or lower than those observed in infected C57Bl/6 control mice (Figure 3B). Because CCR2 is required for egress from the bone marrow, and not for entry of inflammatory monocytes into the spleen, a control transfer of CCR2−/− inflammatory monocytes into CCR2−/− mice would not be informative. However, transfer of similarly purified WT monocytes into WT C57Bl/6 recipients (supplemental Figure 2) had no effect on parasitemia, suggesting that adoptive transfer of cells per se does not affect parasitemia. These data suggest that these CD11bhighLy6C+ CD11clow monocytes have an important role in controlling acute-stage parasitemia.
Intravenous transfer of CD11bhighLy6C+ spleen cells from P chabaudi–infected WT mice into CCR2-deficient mice reduces blood-stage parasitemia. (A) Characterization of cells used for adoptive transfer. Dual parameter contour plots and histogram showing purity and FACS profile of enriched CD11bhighLy6C+ spleen cells by magnetic-activated cell sorting (MACS) from WT C57BL/6 mice infected for 7 days with 105P chabaudi and used in the transfer experiments. (Left) Contour plot showing CD11b and Ly6C expression. The number indicates the percentage of cells within the gated population. (Middle) Histogram showing expression of lineage markers. Number indicates the percentage of lineage-negative cells. (Right) Histogram of CD11c staining of the cells used for transfer. The number indicates the percentage of cells expressing low levels of CD11c. (B left) Course of a P chabaudi infection in CCR2−/− mice (□) and CCR2−/− given 15 × 106 enriched CD11bhighLy6C+ cells on day 7 of their infection (■). As a comparison, the infection in C57Bl/6 mice is also shown (○). The values shown are the geometric means and SEMs of 4 mice. (Right) enlargement showing significant differences in parasitemias at days 15 and 17 of infection between CCR2−/− mice (□) and CCR2−/− given CCR2+/+CD11bhighLy6C+ cells (■). Each symbol represents an individual mouse, and horizontal lines are the geometric means of 4 mice. P values were derived from the Mann-Whitney test; significant differences: *P = .001-.01, **P = .01-.05. The experiment was repeated twice.
Intravenous transfer of CD11bhighLy6C+ spleen cells from P chabaudi–infected WT mice into CCR2-deficient mice reduces blood-stage parasitemia. (A) Characterization of cells used for adoptive transfer. Dual parameter contour plots and histogram showing purity and FACS profile of enriched CD11bhighLy6C+ spleen cells by magnetic-activated cell sorting (MACS) from WT C57BL/6 mice infected for 7 days with 105P chabaudi and used in the transfer experiments. (Left) Contour plot showing CD11b and Ly6C expression. The number indicates the percentage of cells within the gated population. (Middle) Histogram showing expression of lineage markers. Number indicates the percentage of lineage-negative cells. (Right) Histogram of CD11c staining of the cells used for transfer. The number indicates the percentage of cells expressing low levels of CD11c. (B left) Course of a P chabaudi infection in CCR2−/− mice (□) and CCR2−/− given 15 × 106 enriched CD11bhighLy6C+ cells on day 7 of their infection (■). As a comparison, the infection in C57Bl/6 mice is also shown (○). The values shown are the geometric means and SEMs of 4 mice. (Right) enlargement showing significant differences in parasitemias at days 15 and 17 of infection between CCR2−/− mice (□) and CCR2−/− given CCR2+/+CD11bhighLy6C+ cells (■). Each symbol represents an individual mouse, and horizontal lines are the geometric means of 4 mice. P values were derived from the Mann-Whitney test; significant differences: *P = .001-.01, **P = .01-.05. The experiment was repeated twice.
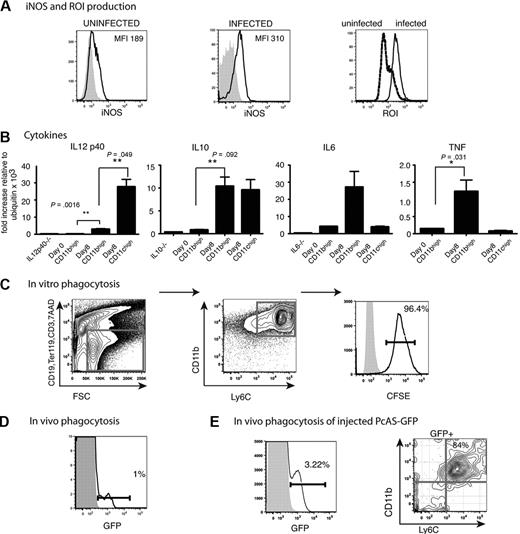
CD11bhighLy6C+ monocytes from infected mice produce inflammatory cytokines as well as iNOS and ROI and can phagocytose P chabaudi–infected erythrocytes
It was possible that these monocytes mediated their antiparasitic effects directly either by phagocytosing iRBCs in the spleen or in intracellular bacterial infections such as Listeria monocytogenes, by the production of ROIs and NOS.16 Therefore, it was important to determine whether the CD11bhighLy6C+ cells in the P chabaudi infection also produced these molecules. This was the case; most splenic CD11bhighLy6C+cells in the P chabaudi–infected mice up-regulated iNOS, ROI, and TNFα by day 8 of infection. In addition, IL-6 and IL-10 but not IL-12p40 mRNAs were also increased in CD11bhighLy6C+ cells. CD11chigh cells, in contrast, up-regulated IL-12p40 and IL-10 but not IL-6 and TNFα (Figure 4A-B).
Functional capacities of CD11bhighLy6C+ cells from the spleens of mice infected with P chabaudi. (A) FACS analysis of CD11bhighLy6C+ spleen cells from C57Bl/6 mice. Expression of iNOS and ROI in CD11bhighLy6C+ monocytes from the spleens of uninfected and day 8–infected C57Bl/6 mice. Cells were stained with antibodies against CD11c, Ly6C, CD11b, CD19, CD3, Ter119 and gated as described in Figure 1A. Live cells were gated on forward and side scatter and CD19, CD3, Ter119-positive cells were excluded. The fluorescence profiles of gated CD11bhighLy6C+ cells from uninfected (left) and day 8–infected mice (middle) labeled with Ab to iNOS (black line) and the isotype control Ab (shaded histogram) are indicated. (Right) Fluorescence is shown from DHR 123 taken up by gated CD11bhighLy6C+ cells from uninfected (stippled lines) and infected (solid lines) mice as indicator of ROI activity. (B) Expression of cytokine mRNA in CD11bhighLy6C+ spleen cells from uninfected and day 8–infected C57Bl/6 mice. CD11bhighLy6C+ monocytes and CD11chigh DCs were sorted to greater than 95% purity, and quantitative polymerase chain reaction was performed as described in “Quantitative reverse transcription–polymerase chain reaction.” (Primer sequences are shown in supplemental Table 1). The data are shown as fold increase relative to ubiquitin. These values shown are the means and SEMs of 3 independent experiments, each using total RNA from a pool of 3 mice. P values were derived from the Student t test. (C) Ability of CD11bhighLy6C+ splenic cells to phagocytose CFSE-labeled P chabaudi–iRBCs in vitro. Dual parameter contour plots and histogram of C57Bl/6 spleen cells isolated at day 8 of a P chabaudi infection. Cells were enriched with the use of CD11b MACS beads as described and stained with antibodies against CD11c, Ly6C, CD11b, CD19, CD3, CD8, and Ter119 after incubation with 100:1 iRBCs as described. (Left) Cells were gated for live cells on forward and side scatter, and CD19, CD3, Ter119, CD8 lineage-positive and 7AAD+ cells were excluded. (Middle) Contour plot of CD11b and Ly6C profiles of lineage-negative cells. (Right) Histogram of CFSE on gated CD11bhighLy6C+ cells incubated with CFSE-labeled iRBCs (solid lines) and without iRBCs (shaded histogram). iRBCs were prepared as described in “Methods.” (D) Ability of CD11bhighLy6C+ spleen cells to phagocytose GFP-expressing P chabaudi parasites in vivo during an infection. Histogram shows GFP expression in CD11bhighLy6C+ cells from splenocytes of C57Bl/6 mice 8 days after infection with 105 GFP–P chabaudi (solid lines) or WT P chabaudi (shaded histogram). Cells were stained with antibodies against CD11c, Ly6C, CD11b, CD19, CD3, Ter119 and gated as described in Figure 1A. Live cells were gated on forward and side scatter. and CD19, CD3, Ter119-positive cells were excluded. Each experiment was performed with at least 3 mice per group and repeated 3 times. Numbers show the percentage of GFP+ cells within the regions. (E) The proportion of CD11b+Ly6C+ splenocytes that contain GFP 15 minutes after intravenous injection of 109 GFP P chabaudi parasites into day 13–infected mice. Splenocytes were stained with antibodies against CD11c, Ly6C, CD11b, CD19, CD3, Ter119, and 7AAD as described in Figure 1. (Left) GFP expression on FSC and SSC gated cells from GFP–P chabaudi–injected (solid lines) and uninjected (shaded histograms) mice. (Right) CD11b and Ly6C expression on GFP+ cells. Similar gating strategies were used as described above. Number shows the percentage of cells within the region. Experiment was performed with 3 mice per group.
Functional capacities of CD11bhighLy6C+ cells from the spleens of mice infected with P chabaudi. (A) FACS analysis of CD11bhighLy6C+ spleen cells from C57Bl/6 mice. Expression of iNOS and ROI in CD11bhighLy6C+ monocytes from the spleens of uninfected and day 8–infected C57Bl/6 mice. Cells were stained with antibodies against CD11c, Ly6C, CD11b, CD19, CD3, Ter119 and gated as described in Figure 1A. Live cells were gated on forward and side scatter and CD19, CD3, Ter119-positive cells were excluded. The fluorescence profiles of gated CD11bhighLy6C+ cells from uninfected (left) and day 8–infected mice (middle) labeled with Ab to iNOS (black line) and the isotype control Ab (shaded histogram) are indicated. (Right) Fluorescence is shown from DHR 123 taken up by gated CD11bhighLy6C+ cells from uninfected (stippled lines) and infected (solid lines) mice as indicator of ROI activity. (B) Expression of cytokine mRNA in CD11bhighLy6C+ spleen cells from uninfected and day 8–infected C57Bl/6 mice. CD11bhighLy6C+ monocytes and CD11chigh DCs were sorted to greater than 95% purity, and quantitative polymerase chain reaction was performed as described in “Quantitative reverse transcription–polymerase chain reaction.” (Primer sequences are shown in supplemental Table 1). The data are shown as fold increase relative to ubiquitin. These values shown are the means and SEMs of 3 independent experiments, each using total RNA from a pool of 3 mice. P values were derived from the Student t test. (C) Ability of CD11bhighLy6C+ splenic cells to phagocytose CFSE-labeled P chabaudi–iRBCs in vitro. Dual parameter contour plots and histogram of C57Bl/6 spleen cells isolated at day 8 of a P chabaudi infection. Cells were enriched with the use of CD11b MACS beads as described and stained with antibodies against CD11c, Ly6C, CD11b, CD19, CD3, CD8, and Ter119 after incubation with 100:1 iRBCs as described. (Left) Cells were gated for live cells on forward and side scatter, and CD19, CD3, Ter119, CD8 lineage-positive and 7AAD+ cells were excluded. (Middle) Contour plot of CD11b and Ly6C profiles of lineage-negative cells. (Right) Histogram of CFSE on gated CD11bhighLy6C+ cells incubated with CFSE-labeled iRBCs (solid lines) and without iRBCs (shaded histogram). iRBCs were prepared as described in “Methods.” (D) Ability of CD11bhighLy6C+ spleen cells to phagocytose GFP-expressing P chabaudi parasites in vivo during an infection. Histogram shows GFP expression in CD11bhighLy6C+ cells from splenocytes of C57Bl/6 mice 8 days after infection with 105 GFP–P chabaudi (solid lines) or WT P chabaudi (shaded histogram). Cells were stained with antibodies against CD11c, Ly6C, CD11b, CD19, CD3, Ter119 and gated as described in Figure 1A. Live cells were gated on forward and side scatter. and CD19, CD3, Ter119-positive cells were excluded. Each experiment was performed with at least 3 mice per group and repeated 3 times. Numbers show the percentage of GFP+ cells within the regions. (E) The proportion of CD11b+Ly6C+ splenocytes that contain GFP 15 minutes after intravenous injection of 109 GFP P chabaudi parasites into day 13–infected mice. Splenocytes were stained with antibodies against CD11c, Ly6C, CD11b, CD19, CD3, Ter119, and 7AAD as described in Figure 1. (Left) GFP expression on FSC and SSC gated cells from GFP–P chabaudi–injected (solid lines) and uninjected (shaded histograms) mice. (Right) CD11b and Ly6C expression on GFP+ cells. Similar gating strategies were used as described above. Number shows the percentage of cells within the region. Experiment was performed with 3 mice per group.
To investigate whether CD11bhighLy6C+ cells could phagocytose P chabaudi–iRBCs, we used CFSE-labeled iRBCs, and P chabaudi parasites were episomally transfected with a PcAS-GFP. The proportions of GFP-expressing parasites corresponded well with measurement of parasitemia by microscopy of Giemsa-stained blood films and by fluorescence-activated cell sorting (FACS; supplemental Figure 3A-B). CD11bhighLy6C+ cells enriched from spleens of day 8 infected mice were highly effective at phagocytosing P chabaudi–iRBCs in vitro; greater than 90% of the cells phagocytosed CFSE-iRBCs (Figure 4C).
Although the CD11bhigh subset of splenic cells were able to phagocytose P chabaudi in vitro, we wanted to establish whether this was taking place in vivo during infection. At maximum parasitemia (day 8; supplemental Figure 3B), GFP was detected in a small proportion of splenic CD11bhighLy6C+ cells (1% in Figure 4D), indicating that a proportion of these cells were also able to phagocytose iRBCs in vivo. The lower mean fluorescence intensity of GFP within the phagocytic cells compared with that within RBCs in blood (supplemental Figure 3A) is probably due to the quenching effect of the acidic lysosomes on GFP as described35 and indicates that the percentage of cells that have ingested GFP parasite material may be an underestimate in vivo. In an attempt to increase the sensitivity of the assay and to reduce GFP quenching in vivo, we injected a large dose of PcAS-GFP (109 iRBCs) into already infected mice in the presence of chloroquine to neutralize phagolysosome pH and analyzed the splenocytes after 15 minutes. In this case, most splenocytes containing GFP expressed CD11b and Ly6C (Figure 4E) and low levels of CD11c (data not shown) showing that inflammatory monocytes were among the phagocytosing cells in the spleen.
Splenic CD11bhighLy6C+ cells display lower levels of class II and costimulatory molecules and are poor stimulators of CD4 T cells
Another possibility for the positive effect of inflammatory monocytes on control of parasitemia is that they develop into monocyte-derived DCs36,37 and act as antigen-presenting cells for malaria-specific T cells, thus inducing a protective CD4 T-cell response. Previously, we demonstrated that CD8−CD11c+ DCs were the major antigen-presenting cells for activating naive transgenic MSP1-specific CD4 T cells during acute P chabaudi infection.11 Because those cells were sorted for high expression of CD11c, the contribution of CD11bhighLy6C+CD11clow cells to this response was not known.
To investigate this, we used PcMSP-1–specific CD4 T cells from a CD4 T-cell transgenic mouse23 and the CD4 T-cell hybridoma28 from which the transgenic TCR was derived. The TCR is restricted to I-Ed (BALB/c), and C57BL/6 (H-2b) monocytes could not be used as APCs. The BALB/c CD11bhighLy6C+ monocyte response to P chabaudi was essentially similar to that of C57BLl/6 mice in terms of cell-surface phenotype and effects of deletion of the CCR2 gene (supplemental Figures 4 and 5); therefore, we reasoned that the antigen-presenting capacities would also be similar. CD11bhighLy6C+ cells from BALB/c mice expressed lower levels of IL-12p40 mRNA (supplemental Figure 5) as well as lower levels of major histocompatibility complex (MHC) class II, CD40, and CD86 than CD11chigh DCs (Figure 5A), suggesting that they may not be effective APCs. Indeed, sorted CD11bhighLy6C+ cells isolated from infected mice at day 8 were unable to present MSP1 peptides processed in vivo; they induced little proliferation, no IL-4, and only low levels of IFNγ and IL-10 from the transgenic P chabaudi MSP1-specific CD4 T cells compared with stimulation by CD11chigh DCs (Figure 5B; supplemental Figure 5). This could be because no peptide had been processed and presented in vivo on the CD11bhighcells or because there was insufficient MHC class II or costimulatory molecules. Exogenously added peptide restored the Tg T-cell response to some extent, suggesting that, although MHC class II/costimulation may not be optimal, MSP1 peptides had not been processed and presented in vivo. We used the MSP1 CD4 T-cell hybridoma, B5, which carries the same TCR as the Tg T cells and which requires only sufficient peptide and MHC class II on the APCs for induction of an IL-2 response,28 to investigate whether the amount of MHC II/peptide was sufficient. There was little response of the hybridoma cultured in the presence of the CD11bhighLy6C+ cells from infected mice. Addition of saturating amounts of exogenous peptide to CD11bhigh cells enhanced the response of the T-cell hybridoma minimally, compared with the level achieved after presentation by CD11chigh cells, suggesting that MHC class II levels on CD11bhigh cells were limiting to some extent, (Figure 5C) and that peptide is not processed and presented by these cells in vivo.
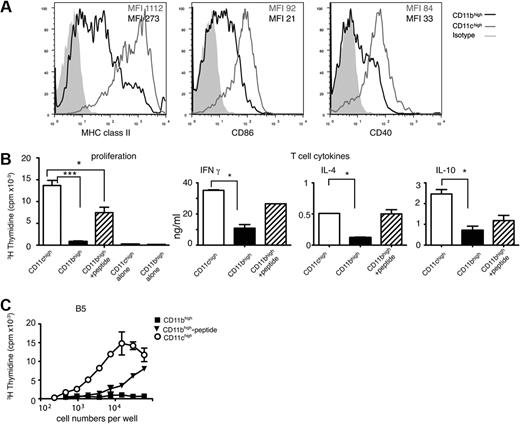
Splenic CD11bhighLy6C+cells are poor stimulators of CD4 T cells and express less MHC class II and costimulatory molecules than CD11chigh cells during a P chabaudi infection. (A) Representative examples showing surface expression of MHC class II, CD86, and CD40 on CD11chigh (gray line) and CD11bhighLy6C+ (black line) on spleen cells obtained from BALB/c mice infected for 8 days with P chabaudi. CD11bhighLy6C+ cells were gated as shown in Figure 1. The gray-shaded area represents the staining pattern of the cells incubated with an isotype-control antibody. Each experiment was performed with at least 3 mice per time point and repeated 3 times. (B) Proliferation and T-cell–cytokine production by malaria-specific TCR Tg T cells. (Left) Proliferation of MSP1-specific transgenic CD4 T cells (B5) after coculture with 5 × 104 purified CD11bhighLy6C+ (■) and CD11chigh (□) subpopulations of splenocytes obtained from mice infected for 8 days with P chabaudi. CD11bhighLy6C+ cells were cultured in the presence ( ) and absence of 1μM of specific peptide. T-cell proliferation was determined by the incorporation of 3H-thymidine after 4 days. The values shown represent the means and SEMs of the mean of triplicate cultures of a representative experiment of 3 performed. Proliferation of APCs alone is shown. (Middle and right) IFNγ, IL-4, and IL-10 produced in the supernatants of B5 transgenic CD4 T cells cultured with CD11chigh (□) and CD11bhighLy6C+ in the absence (■) and presence of 1μM peptide (
) and absence of 1μM of specific peptide. T-cell proliferation was determined by the incorporation of 3H-thymidine after 4 days. The values shown represent the means and SEMs of the mean of triplicate cultures of a representative experiment of 3 performed. Proliferation of APCs alone is shown. (Middle and right) IFNγ, IL-4, and IL-10 produced in the supernatants of B5 transgenic CD4 T cells cultured with CD11chigh (□) and CD11bhighLy6C+ in the absence (■) and presence of 1μM peptide ( ) cells purified from spleens of P chabaudi–infected mice 8 days after infection. After 6 days of coculture, T cells were transferred into plates coated with anti-CD3 Ab and cultured for a further 48 hours. Cytokines were measured in the culture supernatant by enzyme-linked immunoabsorbent assay. The bars and error bars represent the means and SEMs of triplicate cultures of a representative experiment of 3 performed. (C) IL-2 production by MSP1-specific CD4 T-cell hybridoma, B5, after coculture with purified CD11bhighLy6C+ (■) and CD11chigh (○) subpopulations of splenocytes obtained from mice infected for 8 days with P chabaudi. CD11bhighLy6C+ cells were also cultured in the presence (▾) of 1μM specific peptide. The amount of IL-2 in the culture supernatant was determined after 24 hours using the CTLL2 proliferation assay. Each symbol is the mean and SEM of triplicate samples of 1 representative experiment of 3 performed. SEM less than 10% of the mean is not shown.
) cells purified from spleens of P chabaudi–infected mice 8 days after infection. After 6 days of coculture, T cells were transferred into plates coated with anti-CD3 Ab and cultured for a further 48 hours. Cytokines were measured in the culture supernatant by enzyme-linked immunoabsorbent assay. The bars and error bars represent the means and SEMs of triplicate cultures of a representative experiment of 3 performed. (C) IL-2 production by MSP1-specific CD4 T-cell hybridoma, B5, after coculture with purified CD11bhighLy6C+ (■) and CD11chigh (○) subpopulations of splenocytes obtained from mice infected for 8 days with P chabaudi. CD11bhighLy6C+ cells were also cultured in the presence (▾) of 1μM specific peptide. The amount of IL-2 in the culture supernatant was determined after 24 hours using the CTLL2 proliferation assay. Each symbol is the mean and SEM of triplicate samples of 1 representative experiment of 3 performed. SEM less than 10% of the mean is not shown.
Splenic CD11bhighLy6C+cells are poor stimulators of CD4 T cells and express less MHC class II and costimulatory molecules than CD11chigh cells during a P chabaudi infection. (A) Representative examples showing surface expression of MHC class II, CD86, and CD40 on CD11chigh (gray line) and CD11bhighLy6C+ (black line) on spleen cells obtained from BALB/c mice infected for 8 days with P chabaudi. CD11bhighLy6C+ cells were gated as shown in Figure 1. The gray-shaded area represents the staining pattern of the cells incubated with an isotype-control antibody. Each experiment was performed with at least 3 mice per time point and repeated 3 times. (B) Proliferation and T-cell–cytokine production by malaria-specific TCR Tg T cells. (Left) Proliferation of MSP1-specific transgenic CD4 T cells (B5) after coculture with 5 × 104 purified CD11bhighLy6C+ (■) and CD11chigh (□) subpopulations of splenocytes obtained from mice infected for 8 days with P chabaudi. CD11bhighLy6C+ cells were cultured in the presence ( ) and absence of 1μM of specific peptide. T-cell proliferation was determined by the incorporation of 3H-thymidine after 4 days. The values shown represent the means and SEMs of the mean of triplicate cultures of a representative experiment of 3 performed. Proliferation of APCs alone is shown. (Middle and right) IFNγ, IL-4, and IL-10 produced in the supernatants of B5 transgenic CD4 T cells cultured with CD11chigh (□) and CD11bhighLy6C+ in the absence (■) and presence of 1μM peptide (
) and absence of 1μM of specific peptide. T-cell proliferation was determined by the incorporation of 3H-thymidine after 4 days. The values shown represent the means and SEMs of the mean of triplicate cultures of a representative experiment of 3 performed. Proliferation of APCs alone is shown. (Middle and right) IFNγ, IL-4, and IL-10 produced in the supernatants of B5 transgenic CD4 T cells cultured with CD11chigh (□) and CD11bhighLy6C+ in the absence (■) and presence of 1μM peptide ( ) cells purified from spleens of P chabaudi–infected mice 8 days after infection. After 6 days of coculture, T cells were transferred into plates coated with anti-CD3 Ab and cultured for a further 48 hours. Cytokines were measured in the culture supernatant by enzyme-linked immunoabsorbent assay. The bars and error bars represent the means and SEMs of triplicate cultures of a representative experiment of 3 performed. (C) IL-2 production by MSP1-specific CD4 T-cell hybridoma, B5, after coculture with purified CD11bhighLy6C+ (■) and CD11chigh (○) subpopulations of splenocytes obtained from mice infected for 8 days with P chabaudi. CD11bhighLy6C+ cells were also cultured in the presence (▾) of 1μM specific peptide. The amount of IL-2 in the culture supernatant was determined after 24 hours using the CTLL2 proliferation assay. Each symbol is the mean and SEM of triplicate samples of 1 representative experiment of 3 performed. SEM less than 10% of the mean is not shown.
) cells purified from spleens of P chabaudi–infected mice 8 days after infection. After 6 days of coculture, T cells were transferred into plates coated with anti-CD3 Ab and cultured for a further 48 hours. Cytokines were measured in the culture supernatant by enzyme-linked immunoabsorbent assay. The bars and error bars represent the means and SEMs of triplicate cultures of a representative experiment of 3 performed. (C) IL-2 production by MSP1-specific CD4 T-cell hybridoma, B5, after coculture with purified CD11bhighLy6C+ (■) and CD11chigh (○) subpopulations of splenocytes obtained from mice infected for 8 days with P chabaudi. CD11bhighLy6C+ cells were also cultured in the presence (▾) of 1μM specific peptide. The amount of IL-2 in the culture supernatant was determined after 24 hours using the CTLL2 proliferation assay. Each symbol is the mean and SEM of triplicate samples of 1 representative experiment of 3 performed. SEM less than 10% of the mean is not shown.
Discussion
Myeloid cells are important for an effective immune response to most pathogens. They are multifunctional cells, which play a role in all facets of immune responses, producing proinflammatory cytokines, inducing and regulating T and B cells destroying pathogens. Tissue-resident myeloid cells can perform all these functions, but during infection monocytes are additionally recruited from bone marrow to the site of inflammation. These inflammatory monocytes from different infections have shared characteristics but also some specific to the infection in question. In most cases they produce inflammatory cytokines such as TNFα, as well as producing ROI and iNOS that are important for killing bacteria in the phagolysosome before they can escape to replicate in the cytoplasm. In infections such as influenza inflammatory monocytes can differentiate into DCs, which regulate the T-cell response, and in leishmaniasis, toxoplasmosis, and tuberculosis they also induce inflammatory T helper type 1 (Th1) cells crucial to pathogen clearance.18,20,21,34
Here, we investigated inflammatory monocytes in the host response to P chabaudi. We show that CD11bhighLy6C+ monocytes increased in numbers in bone marrow and were depleted in spleens early in infection of CCR2−/− mice, suggesting that they are generated in bone marrow and require CCR2 for egress. CCR2−/− mice exhibited prolonged acute parasitemias, which could be reduced to the levels seen in WT mice by adoptive transfer of CD11bhighLy6C+ cells derived from CCR2+/+-infected mice, suggesting an important role for these cells in controlling malaria infections. This requirement for CCR2 is similar to that of inflammatory monocytes, monocyte-derived inflammatory DCs or TNF-α, iNOS-producing (TIP) DCs described in other infections such Listeria monocytogenes, Mycobacterium tuberculosis, Leishmania major, Cryptococcus neoformans, and Toxoplasma gondii.18,20,21,34 The CD11bhighLy6C+ cells found during P chabaudi infection also resembled TIP-DCs and inflammatory DCs in that they expressed low levels of CD11c and produced iNOS and ROI. However, there were important differences. Although CCR2−/− mice rapidly succumb to Listeria infection, most mice were able eventually to clear P chabaudi. The much slower replication rate of P chabaudi compared with Listeria possibly allowed time for activation of the specific acquired immune response before a fulminant parasitemia. CD4 T-cell responses are thought to play a role in control of primary infection.38 In addition, as supported by our observations here, and in contrast to other infections, generation of inflammatory monocytes may take place outside of the bone marrow. In this regard, the spleen is a site of hematopoiesis in Plasmodium infections39 ; thus, CD11bhighLy6C+ monocytes and other myeloid cells may be generated directly in the spleen independently of a requirement for immigration and CCR2.
In contrast to CD11bhighLy6C+ monocyte-derived inflammatory DCs and TIP-DCs, which express high levels of MHC class II and induce potent inflammatory Th1 responses crucial for pathogen removal,36 CD11bhighLy6C+ monocytes from day 8 of a P chabaudi infection expressed only low levels of MHC class II, did not present MSP1 peptides, or did not stimulate T cells to proliferate or produce cytokines, including IFNγ. It is, of course, possible that on entry into the spleen, or after encounter with parasites, CD11bhighLy6C+ cells undergo further differentiation into CD11chighCD11b+ DCs as described,18,37,40 which then could present and activate CD4 T cells. Although there is experimental evidence that bone marrow–derived inflammatory monocytes do not replenish the splenic DCs,13 or the tissue resident macrophage pool,41 other data suggest that this is possible.42 Inflammatory monocytes, which develop into classic DCs able to induce Th2 responses have been reported.43 Alternatively, it has been proposed that monocytes develop into other phagocytic macrophages, which do not express CD11c and only low levels of MHC class II, some of which no longer express CD11b.44 Our findings that the CD11bhighLy6C+ cells are phagocytic, express only low levels of MHC class II, and do not activate CD4 T cells are more in line with this view. Identification of pathways of monocyte differentiation from their generation in the bone marrow to their destination in lymphoid organs and inflamed tissues would elucidate whether these cells indeed become the main populations of phagocytic cells or DCs found in spleens of malaria-infected mice. Influx of monocytes correlated with a sharp drop in parasitemia, and, more importantly, transferring monocytes back into CCR2−/− mice increased parasite clearance, suggesting rapid removal of parasites by these cells. Because peak parasitemias were similar in CCR2−/− and WT mice and the delayed clearance was only observed after day 10, we suggest that CD11bhighLy6C+ cells are part of a second wave of mechanisms controlling postpeak parasitemia, rather than part of the first line of defense.
There are several possible mechanisms whereby the CD11bhighLy6C+ monocytes could contribute to the removal and killing of blood-stage malaria parasites: phagocytosis, production of potentially parasiticidal mediators such as ROI and NO, and stimulation of marginal zone B cells to produce antibodies,15,45 which could clear parasites. We think it unlikely that antibody-mediated mechanisms are responsible for the parasite removal because malaria-specific antibodies of any isotype are at extremely low levels at this stage of acute infection.29
Plasmodium-iRBCs are readily phagocytosed in vitro,46,47 and it has been proposed that this is an important mechanism of parasite clearance.3 Unlike Listeria, blood stages of Plasmodium develop only within RBCs. Phagocytosis of merozoites and iRBCs is therefore an efficient way of killing parasites. Our data suggest that CD11bhighLy6C+ cells may phagocytose P chabaudi in acute infection. They were highly phagocytic in vitro, and, most importantly, they phagocytosed parasites in vivo in the acute infection. Using P chabaudi–expressing GFP, we detected phagocytosed parasite material in a proportion of CD11bhighLy6C+ cells. However, we believe this is a significant underestimate of the real uptake of parasites and iRBCs. GPF fluorescence is quenched in the phagolysosome,35 and the CD11bhighLy6C+ cells have very active phagolysosomes as evidenced by the presence of ROI. Furthermore, efficient uptake and rapid destruction of parasites by the monocytes will make it difficult to detect large numbers of GFP-expressing parasites. In this regard, phagocytosis of apoptotic cells in lymphoid tissues can only be detected by sensitive transferase-mediated dUTP nick end labeling assays.48 ROI and iNOS produced by the CD11bhighLy6C+ cells may contribute to parasite killing brought about by these cells in vivo. Although there is little, if any, evidence of a role for NO in killing Plasmodium,49 there is strong evidence that ROI contributes to parasite control in rodent malaria infections.50
Monocytes derived from bone marrow hematopoiesis are important components of host responses to many types of pathogens,18 including Plasmodium. To recruit sufficient cells rapidly to sites of pathogen replication, some change from steady-state hematopoiesis may be required to deviate increased numbers of progenitor cells into the myeloid lineage. However, increased myelopoiesis may deplete other hematopoietic pathways, particularly erythropoiesis, which is required to replace RBCs destroyed by the parasite. Understanding how hematopoietic processes are regulated in malaria in mouse models as well as in humans could provide us with important information, not only about innate responses to the parasite but also about the interplay between host responses to the parasite and severe malarial anaemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Philip Spence for careful reading of the manuscript, Vicky Harrison for skilled technical assistance, and Dr William Kuziel for permission to use the CCR2−/− mice.
This work was supported by the Medical Research Council, United Kingdom, and is part of the BioMalPar European Network of Excellence supported by a European grant (LSHP-CT-2004-503578) from the Priority 1 “Life Sciences, Genomics, and Biotechnology for Health” in the 6th Framework Program. B.M. is the recipient of an MRC PhD studentship.
Authorship
Contribution: A.-M.S. designed and performed experiments and analyzed results; A.P.F.d.R., C.V., B.M., W.J., L.R., M.M. performed experiments; J.T. and S.K. generated vital reagents used in this work; A.J.P. analyzed data; J.L. conceptualized and designed experiments and interpreted results; A.-M.S. and J.L. wrote the paper; and all authors read and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean Langhorne, Division of Parasitology, National Institute for Medical Research, The Ridgeway, London NW7 1AA, United Kingdom; e-mail: jlangho@nimr.mrc.ac.uk.







 ) and absence of 1μM of specific peptide. T-cell proliferation was determined by the incorporation of 3H-thymidine after 4 days. The values shown represent the means and SEMs of the mean of triplicate cultures of a representative experiment of 3 performed. Proliferation of APCs alone is shown. (Middle and right) IFNγ, IL-4, and IL-10 produced in the supernatants of B5 transgenic CD4 T cells cultured with CD11chigh (□) and CD11bhighLy6C+ in the absence (■) and presence of 1μM peptide (
) and absence of 1μM of specific peptide. T-cell proliferation was determined by the incorporation of 3H-thymidine after 4 days. The values shown represent the means and SEMs of the mean of triplicate cultures of a representative experiment of 3 performed. Proliferation of APCs alone is shown. (Middle and right) IFNγ, IL-4, and IL-10 produced in the supernatants of B5 transgenic CD4 T cells cultured with CD11chigh (□) and CD11bhighLy6C+ in the absence (■) and presence of 1μM peptide (