Abstract
Dendritic cells (DCs) are the key cell type in the regulation of an adaptive immune response. Under inflammatory conditions monocytes can give rise to immunostimulatory DCs, depending on microenvironmental stimuli. Here we show that oxidized phospholipids (Ox-Pls), which are generated during inflammatory reactions, dysregulate the differentiation of DCs. DCs generated in the presence of Ox-Pls up-regulated the typical DC marker DC-SIGN but did not express CD1a, CD1b, and CD1c. These DCs generated in the presence of Ox-Pls had a substantially diminished T cell–stimulating capacity after stimulation with Toll-like receptor ligands. Toll-like receptor ligand–induced production of interleukin-12 also was strongly diminished, whereas induction of CD83 was not altered. In addition, we found that Ox-Pls strongly inhibit inflammatory stimuli-induced phosphorylation of histone H3, a key step of interleukin-12 production, yet leaving activation of nuclear factor-κB unaltered. Taken together, Ox-Pls present during differentiation yielded DCs with a reduced capacity to become immunostimulatory mature DCs. Furthermore, the presence of Ox-Pls blocked histone modifications required for full activation of DCs. Therefore, inflammation-derived Ox-Pls control DC functions in part by epigenetic mechanisms.
Introduction
Dendritic cells (DCs) are the most potent antigen-presenting cells, and they are considered key players for the induction of adaptive immune responses.1 Monocytes are a large precursor pool for DCs as well as macrophages under inflammatory conditions.2-4 They are recruited to the sites of inflammation and differentiate in either direction depending on the nature of the local environment.5-7 Factors released during inflammation, such as interleukin-6 (IL-6), IL-15, but also lipid mediators like prostaglandins and leukotrienes, have been shown to influence differentiation of monocytes toward either macrophages or DCs.8-12 Therefore, the microenvironment at the site of inflammation shapes protective immune responses in part by determining precursor differentiation.
Oxidized phospholipids (Ox-Pls) are formed during inflammation by reactive oxygen species13,14 but are also present in atherosclerotic lesions and apoptotic cells.15,16 We and others17,18 have shown that Ox-Pls inhibit lipopolysaccharide (LPS)–induced activation of endothelial cells and DCs by blocking ligand–receptor interactions and that they also inhibit the activation of DCs in response to other Toll-like receptor (TLR) ligands such as TLR-3 and TLR-2. However, the authors of several studies found proinflammatory effects of Ox-Pls. Ox-Pls have been shown to be capable of activating endothelial cells, leading to an enhanced recruitment of monocytes.19 This action is believed to play a causative role in atherogenesis20 but also in the resolution phase of an acute inflammation.21
Atherosclerosis-prone mice that lack apolipoprotein E and therefore have a greater burden of (oxidized) lipids,22 display a dysbalanced migratory pattern of DCs, show decreased DC maturation, and have a reduced clearance of pathogens when challenged with bacteria or virus.23-26
In this paper, we investigated the effects of Ox-Pls on monocytic differentiation to immunostimulatory DCs. We report that differentiation to DCs in the presence of Ox-Pls leads to the generation of a cell type lacking the expression of the hallmark differentiation factor CD1a. When these cells are stimulated with ligands of various TLRs, they display a reduced ability to mature phenotypically and have a diminished ability to stimulate T-cell proliferation and to produce IL-12. Analyzing the underlying mechanism, we found that Ox-Pls do not inhibit the pivotal transcription factor nuclear factor (NF)–κB. However, Ox-Pls do inhibit the phosphorylation of histone H3 induced by inflammatory stimuli and the recruitment of NF-κB to the Il-12p40 promoter, whereas recruitment of NF-κB to the IκBα promoter was not affected. These data implicate histone modification in suppressing DC-derived IL-12 production by Ox-Pls. Thus, Ox-Pls are potent regulators of DC function, in part by inhibiting inflammatory stimulus-derived modifications of histones.
Methods
Media and reagents
The cell-culture medium RPMI 1640 (Life Technologies) was supplemented with 2mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal calf serum (Sigma-Aldrich). Recombinant human granulocyte/macrophage colony-stimulating factor (GM-CSF) and IL-4 were kindly provided by Novartis Research Institute. Mannosylated fluorescein isothiocyanate–bovine serum albumin (FITC-BSA), FITC-labeled dextran, and peptidoglycan (PGN) from Staphylococcus aureus were obtained from Sigma-Aldrich. Ultra-pure LPS (serotype 0111:B4) and Poly I:C were from Invivogen. 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (PAPC) was bought at Avanti Polar Lipids. Immobilon-P transfer membranes were products of Millipore. Actinomycin D was obtained from Sigma-Aldrich.
Antibodies
The following murine mAbs were generated in our laboratory: negative control monoclonal antibody (mAb) VIAP (calf intestinal alkaline phosphatase specific), DF272 (B7-H1), 1/47 (major histocompatibility complex [MHC] class II), VIT6b (CD1a), 7C4 (CD1b), 10C3 (CD1c), VIM12 (CD11b), VIM13 (CD14), CD33-4D3 (CD33), 7-188 (MMR), and 5-216 (ICAM-1/CD54). Hybridomas producing mAb W6/32 (MHC class I) and G28-5 (CD40) were obtained from ATCC. The CD14 mAb (MEM18) was kindly provided by An der Grub (Bio Forschungs), and the CD19 mAb (HD37) was a gift from G. Moldenhauer (Department of Molecular Immunology, DFKZ Heidelberg).
The mAbs CD80 DAL-1 (CD80), 3G8 (CD16), HB 15 (CD83), and BU63 (CD86) were purchased from Invitrogen. Anti-CD1d mAbs and anti–DC-SIGN mAbs were purchased from BD Biosciences. Polyclonal antibodies against phosphorylated form of extracellular signal-regulated kinase (ERK) 1/2, p38, as well as their unphosphorylated forms were from Cell Signaling Technology. Polyclonal IκB-α Ab from Santa Cruz Biotechnology. Anti-p65 antibody was from Santa Cruz Biotechnology. Anti–phospho-histone H3 (Ser10) and anti–phospho-acetyl H3 (H3pS10/K14Ac) were from Upstate. Peroxidase-conjugated secondary antibodies were purchased from Amersham Life Science.
Lipid oxidation
PAPC was oxidized (ie, OxPAPC) by exposure of dry lipid to air for 72 hours. The extent of oxidation was monitored by positive ion electrospray mass spectrometry as described previously.19 OxPAPC used for experiments contained less than 50 pg/mL endotoxin as determined by the Limulus amebocyte assay (BioWhittaker).
Cell preparation and stimulation
Peripheral-blood mononuclear cells were isolated from heparinized whole blood of healthy donors by standard density gradient centrifugation with Ficoll-Paque (Pharmacia Biotech). Subsequently, monocytes and T cells were separated by magnetic sorting by use of the MACS technique (Miltenyi Biotec) as described.27 Monocytes were enriched by the use of biotinylated CD14 mAbs VIM13 and MEM18 (purity > 95%). Purified T cells were obtained through negative depletion of CD11b, CD14, CD16, CD19, CD33, and MHC class II–positive cells with the respective mAbs. DCs were generated from CD14+ monocytes cultured in the presence of GM-CSF (50 ng/mL) and IL-4 (100 U/mL) for 6 days. Maturation of DCs was induced by adding 100 ng/mL LPS and 10 μg/mL PGN.
T-cell proliferation assay
For the mixed lymphocyte reaction (MLR), allogenic, purified T cells (105/well) were incubated in 96-well cell-culture plates (Corning-Costar) with graded numbers of DCs for 6 days. The assay was performed in triplicate. Proliferation of T cells was monitored by measuring [methyl-3H] thymidine (ICN Pharmaceuticals) incorporation, added on day 5 of culture. Cells were harvested 18 hours later, and incorporated [methyl-3H] thymidine was detected on a microplate scintillation counter (PerkinElmer).
Immunofluorescence analysis
For membrane staining, cells (5 × 105) were incubated for 30 minutes at 4°C with unlabeled mAbs at a concentration of 20 μg/mL. Staining of FcR-bearing cells was performed in the presence of human IgG Abs (20 mg/mL; Beriglobin; Aventis Behring). After washing cells twice with ice-cold PBS containing 1% BSA, binding of the primary mAb was visualized by the use of Oregon Green–conjugated goat anti–mouse Ab from Molecular Probes. Cells were then washed 3 times with phosphate-buffered saline/BSA. Membrane fluorescence was analyzed on a FACSCalibur flow cytometer (BD Biosciences) supported by CellQuest software (BD Biosciences). The exclusion of dead cells was performed by the addition propidium iodide.
Determination of cytokine production
DCs were treated as indicated, and after 24 hours the supernatants were harvested and analyzed by enzyme-linked immunosorbent assay (ELISA). Cytokines were measured by sandwich ELISAs by the use of matched-pair antibodies. Capture and detection antibodies for human interferon-γ (IFN-γ) and IL-12 p70 were obtained from R&D Systems Inc; for tumor necrosis factor (TNF), from BD PharMingen. Standards consisted of human recombinant material from R&D Systems Inc. Assays were performed in duplicate according to the recommendations of the manufacturers. The lower limit of detection was 20 pg/mL for IL-10, IL-12, and TNF. For T-cell polarization assay, MLR supernatants were harvested at the fourth day of coculture and analyzed for IFN-γ by ELISA as described previously.
Western blotting
After stimulation, DCs were lysed in Laemmli buffer (Bio-Rad) and proteins separated by electrophoresis on 10% sodium dodecyl sulfate-polyacrylamide gels. Proteins were blotted onto polyvinylidene difluoride membrane and, after blocking with 5% dry milk/0.1% Tween-20, incubated with primary antibodies in the same solution. Bound antibodies were detected by anti-IgG conjugated with peroxidase (Amersham Life Science) and subsequent chemiluminescent detection.
RNA isolation, cDNA preparation, and mRNA stability
RNA was isolated by the use of TRI reagent (Sigma-Aldrich) reagent according to the protocol of the manufacturers. A total of 900 ng of total RNA were reverse transcribed with MuLV-RT by the use of Oligo dT16 primers (Finnzymes). Design of primers was as follows: the cDNA sequences of the investigated genes were obtained from GenBank. PCR primers were designed by the use of the PRIMER3 software from the Whitehead Institute for Biomedical Research.
The amplified cDNA regions were chosen to span 1 or more large introns in the genomic sequence, thus avoiding coamplification of genomic DNA under our amplification protocol. The testing of primer specificity included melting point analyses, agarose gel electrophoresis of the polymerase chain reaction (PCR) products, and subsequent DNA sequencing. Primer sequences used were CD83 fwd: AAGGCCCTATTCCCTGAAGA, CD83 rev: CAGGACAATCTCCGCTCTGT; IL-12p40 fwd: GGATGCCGTTCACAAGCTCA, IL-12p40 rev: GCTCTTGCCCTGGACCTGAA; IκBα fwd: AGACCTGGCCTTCCTCAACT, IκBα rev: TGCTCACAGGCAAGGTGTAG; IL-23p19 fwd: AGATGGCTGTGACCCCCAAG, IL-23p19 rev: CTGGCCCACAGGGCTATCAG; and IL-12p35 fwd: TTTGCGGCCGCACCTCCCCGTGGCCACTCC, IL-12p35 rev: TTTGCGGCCGCA-TTCAGATAGCTCATCATCCT.
For mRNA stability experiments, DCs were stimulated with LPS (100 ng/mL) for 1 hour. Actinomycin D (10 μg/mL) was added, and cells were incubated with OxPAPC 10 μg/mL or 50 μg/mL for the indicated times. RNA was isolated and reverse transcribed and mRNA abundance was measured by quantitative PCR (qPCR).
Real-time qPCR
Quantitative reverse-transcription (RT)–PCR was performed by the use of a LightCycler (Roche Molecular Biochemicals) with SYBR Green I detection. The protocol for amplification and quantification has been described previously.28
Transient transfection
Transfections of HEK293 cells with calcium–DNA precipitates were conducted essentially according to a well-established protocol.29 The TLR4/CD4 fusion construct30 was kindly provided by R. Medzhitov (Department of Immunobiology, Yale University School of Medicine) and was cotransfected with a 5xNf-κB-luc construct. pTK-RL (Promega) was used to control for transfection efficiency. Cells were lysed at the indicated time points, and luciferase activity was determined with the Dual Reporter Assay kits (Promega).
Electrophoretic mobility shift assay
Nuclear extracts from DCs were prepared as described.17 Oligonucleotides resembling the consensus binding site for NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′) were purchased from Santa Cruz Biotechnology. The double-stranded oligonucleotides used in all experiments were end-labeled with T4 polynucleotide kinase and [γ-32P]-adenosine triphosphate. After labeling, 5 μg of nuclear extract was incubated with 120 000 cpm-labeled probe in the presence of 3 μg of poly(dI-dC) at room temperature for 30 minutes. This mixture was separated on a 6% polyacrylamide gel in EDTA (ethylenediaminetetraacetic acid) buffer at pH 8.5. Control experiments were performed as described.31 For specific competition, 5 pmol of unlabeled NF-κB oligonucleotide was included, and for nonspecific competition, 5 pmol of double-stranded AP-1 oligonucleotides was used.
Chromatin immunoprecipitation
Chromatin immunoprecipitation was performed as previously described32 with some modifications. DCs were either unstimulated or stimulated with LPS for 1 hour or pretreated for 20 minutes with 10 μg/mL OxPAPC and subsequently stimulated with LPS for 1 hour were isolated and purified as described previously. Cells were resuspended in PBS and cross-linked in 1% formaldehyde for 10 minutes at 37°C. Cross-linking was stopped by adding glycine to final concentration of 125mM, and cells were washed, lysed, and shared as described.32 Lysed chromatin (5 × 106 cells/immunoprecipitation) was diluted 1:10 in dilution buffer and precleared and precipitated overnight with the respective antibodies. On the next day chromatin–antibody complexes were harvested by incubation with 30 μL of protein A-Sepharose beads (50% slurry, 100 μg/mL salmon sperm DNA, 500 μg/mL BSA) while rocking at 4°C for 2 hours. The beads were washed, and chromatin–antibody complexes were eluted from the protein A–Sepharose beads by the addition of 2% sodium dodecyl sulfate, 0.1M NaHCO3, and 10mM dithiothreitol. After decross-linking and proteinase K digestion, the DNA was extracted with phenol-chloroform, precipitated with ethanol, and dissolved in water. The abundance of distinct DNA fragments after chromatin immunoprecipitation from was quantified by the use of real-time PCR (iCycler; Bio-Rad). The following promoter-specific primers were used IL-12p40: sense 5′-GCCTTTGCATATATCAGACAG-3′, antisense 5′-GCATACAGTTGTTCCATCC-3′; IkBa sense 5′-GACGACCCCAATTCAAATCG, antisense 5′-TCAGGCTCGGGGAATTTCC-3′; and CD83: sense 5′-GAGCAAGCCACCTTCACCT-3′, antisense 5′-CTCCAGCTTCTGCTCCTGA.
Results
Marker profile of monocyte-derived DCs generated in the presence of Ox-Pls
Blood monocytes cultured for 6 days in the presence of GM-CSF and IL-4 progressively exhibited a typical phenotype of immature DCs, especially up-regulation of CD1a and down-regulation of CD14. DCs cultured in the presence of OxPAPC, a well-described mixture of Ox-Pls,19 lost CD14 but failed to up-regulate CD1a, a typical DC differentiation marker.27 DCs differentiated in the presence of OxPAPC (termed Ox-DCs) displayed comparable expression of the costimulatory molecules CD40 and CD80 (B7-1) of the inhibitory receptor B7-H1 as well as of MHC I. The expression of CD86 (B7-2) and MHC class II was slightly enhanced (Figure 1). Neither DCs nor Ox-DCs showed significant CD83 expression, a marker for mature DCs. Induction of the DC-specific molecule DC-SIGN (CD209) and the mannose receptor (CD206) was not affected by OxPAPC. The expression of the Fcγ-receptors CD16, CD32, and CD64 (not shown) were comparable with untreated DCs. Morphologically Ox-DCs were similar to DCs with respect to cell size and the expression of dendrites. These data show that Ox-DCs selectively fail to up-regulate CD1 family members but express a repertoire of costimulatory molecules and differentiation markers comparable with unstimulated DCs.
Effects of the presence of OxPAPC during DC differentiation. Human peripheral blood monocytes were cultured for 6 days in GM-CSF and IL-4 to receive immature monocyte-derived DCs (clear histograms) or with the addition of 25 μg/mL OxPAPC added at day of differentiation (gray histograms). Cells were harvested, and the surface expression level of the indicated markers was measured by flow cytometry. The dotted line in the CD1a histogram represents VIAP staining (isotype control). Results are representative of at least 5 independent experiments.
Effects of the presence of OxPAPC during DC differentiation. Human peripheral blood monocytes were cultured for 6 days in GM-CSF and IL-4 to receive immature monocyte-derived DCs (clear histograms) or with the addition of 25 μg/mL OxPAPC added at day of differentiation (gray histograms). Cells were harvested, and the surface expression level of the indicated markers was measured by flow cytometry. The dotted line in the CD1a histogram represents VIAP staining (isotype control). Results are representative of at least 5 independent experiments.
Ox-DCs are immune-stimulatory cells with potent antigen-uptake capacities
Next, we assessed the capacity of Ox-DCs to stimulate T-cell proliferation in an allogenic MLR. Like untreated DCs, Ox-DCs showed strong T cell–stimulating capacity in an allogenic setting (Figure 2A). Ox-DCs showed no reduction in the uptake of FITC-labeled dextran or FITC-labeled mannose-BSA, suggesting that neither macropinocytosis nor receptor-mediated endocytosis were decreased compared with DCs (Figure 2B-C). Ox-DCs therefore have characteristic DC functions.
Ox-DCs display typical DC functions. (A) Purified T cells were stimulated with graded numbers of allogenic immature DCs or DCs differentiated in the presence of 10, 25, or 40 μg/mL OxPAPC, and the proliferation of T cells was monitored on day 5 of culture by adding [methyl-3H]thymidine followed by measuring [methyl-3H]thymidine incorporation 18 hours later. Data are presented as percent of immature DCs and represent mean values of 3 experiments ± SD. Immature DCs or DCs differentiated in the presence of 10, 25, or 40 μg/mL OxPAPC were incubated with FITC-Dextran (1 mg/mL; B) or mannosylated BSA–FITC (10 μg/mL; C) for 60 minutes and uptake of the fluorescent dyes was measured by flow cytometry. Data are presented as percent of immature DCs and represent mean values of 5 experiments ± SD.
Ox-DCs display typical DC functions. (A) Purified T cells were stimulated with graded numbers of allogenic immature DCs or DCs differentiated in the presence of 10, 25, or 40 μg/mL OxPAPC, and the proliferation of T cells was monitored on day 5 of culture by adding [methyl-3H]thymidine followed by measuring [methyl-3H]thymidine incorporation 18 hours later. Data are presented as percent of immature DCs and represent mean values of 3 experiments ± SD. Immature DCs or DCs differentiated in the presence of 10, 25, or 40 μg/mL OxPAPC were incubated with FITC-Dextran (1 mg/mL; B) or mannosylated BSA–FITC (10 μg/mL; C) for 60 minutes and uptake of the fluorescent dyes was measured by flow cytometry. Data are presented as percent of immature DCs and represent mean values of 5 experiments ± SD.
Stimulation of Ox-DCs with LPS or PGN does not increase the T-cell stimulatory capacity
During inflammatory conditions, DCs are activated and matured by signals derived from the invading pathogens.33 When we treated Ox-DCs with LPS or PGN, we observed that Ox-DCs reacted differently from regular DCs to these stimuli. Upon activation, they displayed a fibroblast-like, spindle-shaped morphology with strong adherence to the tissue-culture well, which was hardly detectable in normally differentiated DCs (not shown). Furthermore, Ox-DCs displayed a reduced up-regulation of costimulatory molecules, which was most pronounced for CD40 but also noted for CD80 and CD86 as well as MHC class II in response to LPS or PGN (Figure 3A-B). The induction of CD83 was not significantly inhibited nor was up-regulation of B7-H1. To test the accessory function of TLR-stimulated Ox-DCs we performed allogenic MLRs. Treatment of DCs with TLR ligands substantially increased their allostimulatory function, which was significantly inhibited in a concentration-dependent manner in Ox-DCs (Figure 3C-D).
Ox-DCs have a limited ability to mature. Immature DCs (thin black line) or DCs differentiated in the presence of 40 μg/mL OxPAPC (gray histogram) were stimulated with LPS (100 ng/mL; A) or PGN (1 μg/mL; B, thick black line) for 24 hours, harvested, and stained for the indicated markers and measured by flow cytometry. Purified T cells were stimulated with graded numbers of allogenic immature DCs or DCs differentiated in the presence of 40 μg/mL OxPAPC (left) or the indicated amounts of OxPAPC (right) and stimulated with LPS (C) or PGN (D), and the proliferation of T cells was monitored on day 5 of culture by adding [methyl-3H]thymidine followed by measuring [methyl-3H]thymidine incorporation 18 hours later.
Ox-DCs have a limited ability to mature. Immature DCs (thin black line) or DCs differentiated in the presence of 40 μg/mL OxPAPC (gray histogram) were stimulated with LPS (100 ng/mL; A) or PGN (1 μg/mL; B, thick black line) for 24 hours, harvested, and stained for the indicated markers and measured by flow cytometry. Purified T cells were stimulated with graded numbers of allogenic immature DCs or DCs differentiated in the presence of 40 μg/mL OxPAPC (left) or the indicated amounts of OxPAPC (right) and stimulated with LPS (C) or PGN (D), and the proliferation of T cells was monitored on day 5 of culture by adding [methyl-3H]thymidine followed by measuring [methyl-3H]thymidine incorporation 18 hours later.
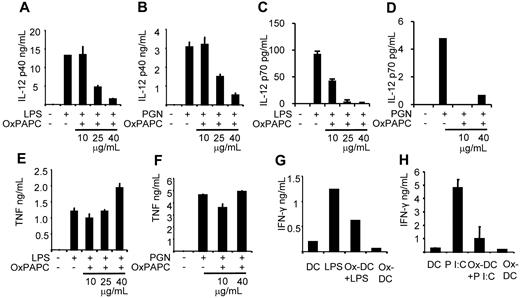
IL-12 production induced by LPS or PGN is inhibited in Ox-DCs
When we evaluated the cytokine profile of TLR-stimulated Ox-DCs, we found a strong decrease of IL-12p40 as well as IL-12p70 production regardless of the stimulus used (Figure 4A-D). In contrast, TNF production of DCs and Ox-DCs was comparable when LPS and PGN were used to stimulate the cells (Figure 4E-F). Ox-DCs themselves without any stimulus did not produce either cytokine (not shown). There was also neither increased production of IL-10 nor was there a clear tendency in the modulation of IL-10, mostly because IL-10 was not or only slightly induced by either stimulus in our experiments (not shown).
Ox-DCs produce less IL-12. (A-F) Immature DCs or DCs differentiated in the presence of 10, 25, or 40 μg/mL OxPAPC were stimulated with LPS or PGN. At 24 hours later the supernatants were harvested and analyzed for IL-12p40, IL-12p70, and TNF by ELISA. Data are representative of 3 independent experiments. (G-H) Supernatant of MLR where DCs and Ox-DCs stimulated with LPS or Poly I:C were used as stimulators was taken and analyzed for IFN-γ by ELISA. Data are representative of 2 independent experiments.
Ox-DCs produce less IL-12. (A-F) Immature DCs or DCs differentiated in the presence of 10, 25, or 40 μg/mL OxPAPC were stimulated with LPS or PGN. At 24 hours later the supernatants were harvested and analyzed for IL-12p40, IL-12p70, and TNF by ELISA. Data are representative of 3 independent experiments. (G-H) Supernatant of MLR where DCs and Ox-DCs stimulated with LPS or Poly I:C were used as stimulators was taken and analyzed for IFN-γ by ELISA. Data are representative of 2 independent experiments.
Because IL-12 is one of the best-described T cell–polarizing factors, we tested the ability of Ox-DCs matured with TLR-ligands to initiate a Th1-type of immune response, measured by production of IFN-γ by T cells in an MLR. As depicted in Figure 4G and H, Ox-DCs had a strongly diminished capacity to elicit IFN-γ production in T cells.
The NF-κB signaling pathway is intact in Ox-DCs
NF-κB is a pivotal transcription factor in the activation of DCs.34 We therefore tested whether this pathway was operating in Ox-DCs in response to LPS and PGN. We found that the activation of NF-κB was not significantly impaired as shown by electrophoretic mobility shift assay (Figure 5A). We have demonstrated previously that OxPAPC inhibits LPS-mediated activation of endothelial cells and DCs via a mechanism involving blocking of the extracellular recognition of LPS.17,18 To test whether OxPAPC had any direct effects on intracellular signaling events leading to NF-κB activation, we performed reporter assays using a TLR-4 mutant construct engineered to be constitutively active, therefore allowing us to assess the impact of OxPAPC on TLR4-mediated intracellular signaling events leading to activation of NF-κB. In addition, the effect of OxPAPC on the trans-activating capacity of NF-κB could be tested. We found that OxPAPC did not inhibit NF-κB–driven luciferase production. Therefore, we concluded that OxPAPC does not inhibit intracellular signaling events leading to the activation of NF-κB initiated by TLR-4, nor does it interfere with transactivation of NF-κB (Figure 5B).
OxPAPC does not inhibit NF-κB activation. (A) Immature DCs or DCs differentiated in the presence of 10 (Ox 10) or 25 (Ox 25) μg/mL OxPAPC were stimulated with LPS or PGN. After 70 minutes, total nucleoprotein was extracted. 32P-labeled oligonucleotides containing a NF-κB consensus sequence were incubated at room temperature with 5 μg of nuclear extracts, followed by nondenaturating gel electrophoresis. Similar results were obtained in 2 independent experiments. (B) HEK 293 cells were transfected with a constitutively active TLR4 and a 5xNF-κB firefly luciferase reporter construct. A Renilla reporter plasmid was cotransfected. Firefly luciferase activity with or without addition of OxPAPC (50 μg/mL) was determined after the indicated time points and normalized to Renilla luciferase. Data are presented as mean values ± SD of 3 independent experiments.
OxPAPC does not inhibit NF-κB activation. (A) Immature DCs or DCs differentiated in the presence of 10 (Ox 10) or 25 (Ox 25) μg/mL OxPAPC were stimulated with LPS or PGN. After 70 minutes, total nucleoprotein was extracted. 32P-labeled oligonucleotides containing a NF-κB consensus sequence were incubated at room temperature with 5 μg of nuclear extracts, followed by nondenaturating gel electrophoresis. Similar results were obtained in 2 independent experiments. (B) HEK 293 cells were transfected with a constitutively active TLR4 and a 5xNF-κB firefly luciferase reporter construct. A Renilla reporter plasmid was cotransfected. Firefly luciferase activity with or without addition of OxPAPC (50 μg/mL) was determined after the indicated time points and normalized to Renilla luciferase. Data are presented as mean values ± SD of 3 independent experiments.
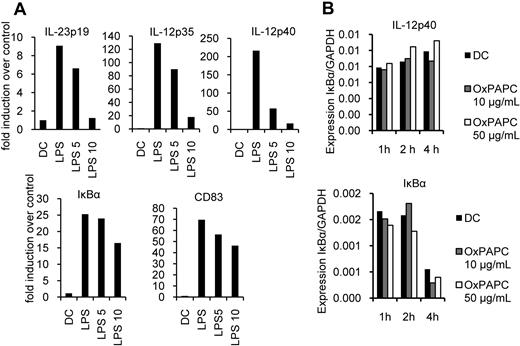
To investigate the mechanism of the selective IL-12 inhibition, we analyzed IL-23p19, IL-12p35, and IL-12p40 mRNA induction after LPS stimulation in the presence of OxPAPC. We found that OxPAPC was able to almost completely inhibit the transcription of IL-23p19, IL-12p35, as well as IL-12p40 mRNA, whereas induction of CD83 as well as IκBα mRNA was hardly altered (Figure 6A). Furthermore, we tested whether OxPAPC influenced mRNA decay rates of the genes analyzed. We therefore stimulated DCs with LPS for 1 hour, added actinomycin D to block transcription of new mRNA, and measured the amount of target mRNA over glyceraldehyde-3-phosphate dehydrogenase. We found IκBα to be degraded during the period analyzed (Figure 6B). In contrast, IL-12p40 mRNA was stable during the period analyzed. However, in all instances we were not able to detect significant differences in mRNA stability of IL-12p40 and IκBα in the presence of OxPAPC compared with untreated cells.
OxPAPC differentially inhibits induction of activation-associated genes. DCs were stimulated with LPS (100 ng/mL) with or without pretreatment for 20 minutes with the indicated amounts of OxPAPC (5 or 10 μg/mL, LPS 5 and LPS 10, respectively) for 1 hour, and RNA was isolated and (A) IL-23p19, IL-12p35, IL-12p40, IκBα, and CD83 mRNA expression was determined by RT-PCR. (B) DCs were stimulated with LPS for 1 hour and subsequently treated with actinomycin D. They were then stimulated with the indicated amounts of OxPAPC for 1, 2, and 4 hours, and the abundance of the indicated mRNAs/glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were determined by RT-PCR. Data are representative of 2 independent experiments.
OxPAPC differentially inhibits induction of activation-associated genes. DCs were stimulated with LPS (100 ng/mL) with or without pretreatment for 20 minutes with the indicated amounts of OxPAPC (5 or 10 μg/mL, LPS 5 and LPS 10, respectively) for 1 hour, and RNA was isolated and (A) IL-23p19, IL-12p35, IL-12p40, IκBα, and CD83 mRNA expression was determined by RT-PCR. (B) DCs were stimulated with LPS for 1 hour and subsequently treated with actinomycin D. They were then stimulated with the indicated amounts of OxPAPC for 1, 2, and 4 hours, and the abundance of the indicated mRNAs/glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were determined by RT-PCR. Data are representative of 2 independent experiments.
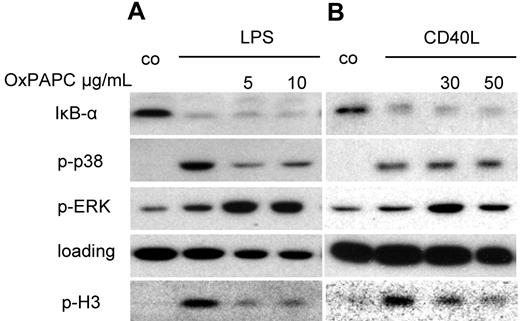
Phosphorylation of histone H3 is strongly reduced in Ox-DCs
We next analyzed whether OxPAPC was able to selectively interfere with specific signaling events initiated by LPS. As shown in Figure 7, OxPAPC did not change IκBα degradation in response to LPS or CD40L, which was used in our experiments to control for NF-κB activation within the experiment. Phosphorylation of ERK mitogen-activated protein (MAP) kinase was enhanced compared with stimulation with LPS alone, which could also be noted when CD40L was used as stimulus. LPS-induced phosphorylation of p38 MAP kinase, however, was strongly diminished in the presence of OxPAPC, which was not the case in CD40L-stimulated cells. However, phosphorylation of the histone H3 at the serine 10 position was strongly inhibited, an event, which has been shown to be important in the induction of IL-12p40 transcription.35 Inhibition of histone H3 phosphorylation was not restricted to LPS-stimulated DCs because CD40L-induced phosphorylation of histone H3 also was inhibited in the presence of OxPAPC (Figure 7B).
Differential inhibition of LPS-induced signaling events by OxPAPC. DCs were stimulated for 15 minutes with LPS (A) or CD40L (B) with or without 20 minutes of pretreatment with the indicated amounts of OxPAPC, harvested, and analyzed by Western blot for IκB-α, phosphorylated p38 MAP-kinase, and phosphorylated ERK1/2 and phosphorylated histone H3 (serine 10). Results are representative of 3 independent experiments.
Differential inhibition of LPS-induced signaling events by OxPAPC. DCs were stimulated for 15 minutes with LPS (A) or CD40L (B) with or without 20 minutes of pretreatment with the indicated amounts of OxPAPC, harvested, and analyzed by Western blot for IκB-α, phosphorylated p38 MAP-kinase, and phosphorylated ERK1/2 and phosphorylated histone H3 (serine 10). Results are representative of 3 independent experiments.
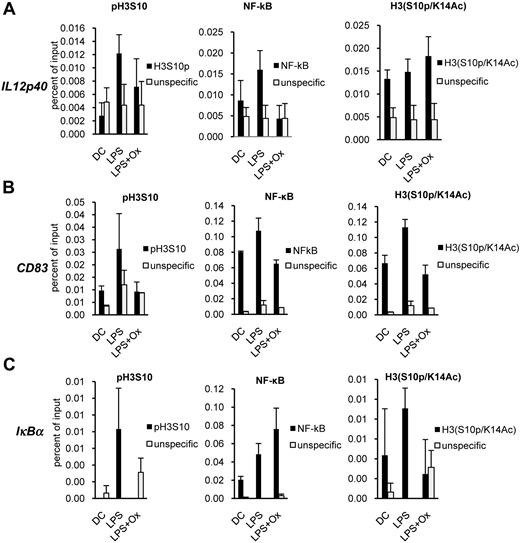
OxPAPC impacts LPS-induced promoter modifications
To test whether indeed the addition of OxPAPC reduced histone H3 phosphorylation at the IL-12p40 promoter in LPS-treated DCs, chromatin immunoprecipitations were performed by the use of an antibody directed against histone H3 phosphorylated on serine 10 (H3pS10) and subsequent PCR with IL-12p40 promoter-specific primers. Treatment of DCs with OxPAPC reduced the amount of IL-12p40 promoter sequences in the precipitate (Figure 8A). In addition, OxPAPC also prevented LPS-induced recruitment of the NF-κB subunit p65 to the IL-12p40 promoter (Figure 8A). Interestingly, when we analyzed the amount of phosphoacetylated histone H3 (phosphorylated at serine 10 and acetylated at K14, H3pS10/K14Ac), we found that LPS increased this modification at the IL-12p40 promoter and that the addition of OxPAPC did not inhibit or even slightly augment this modification. We also analyzed the promoters of CD83 as well as IκBα, 2 genes that are not inhibited by OxPAPC on the mRNA level, with respect to these modifications. We found that LPS induces recruitment of p65 to the promoter of CD83, which was inhibited by OxPAPC (Figure 8B). This finding was surprising because CD83 has been identified as target of NF-κB.36 LPS also induced phosphorylation and phosphoacetylation of histone H3, and both modifications were inhibited by OxPAPC (Figure 8B). At the IκBα promoter, we found recruitment of p65 induced by LPS, which was not inhibited by OxPAPC (Figure 8C). Phosphorylation as well as phosphoacetylation, which were also induced by LPS, was reduced by OxPAPC (Figure 8C). We therefore concluded that OxPAPC modulates LPS-induced histone modifications, which might contribute to the differential inhibition of activation associated genes during DC maturation.
OxPAPC has an impact on LPS-induced promoter modifications. DCs pretreated for 20 minutes with 10 μg/mL OxPAPC were stimulated with LPS (100 ng/mL) for 60 minutes and fixed with formaldehyde. Chromatin immunoprecipitation was performed with the use of an α-H3pS10, α-NF-κB p65, α-H3pS10/K14Ac, or an irrelevant antibody a control (unspecific). Immunoprecipitated DNA was analyzed by qPCR with promoter-specific primers for IL-12p40 (A), CD83 (B), and IκBα (C) and plotted as percent of input. Data are representative of 2 independent experiments.
OxPAPC has an impact on LPS-induced promoter modifications. DCs pretreated for 20 minutes with 10 μg/mL OxPAPC were stimulated with LPS (100 ng/mL) for 60 minutes and fixed with formaldehyde. Chromatin immunoprecipitation was performed with the use of an α-H3pS10, α-NF-κB p65, α-H3pS10/K14Ac, or an irrelevant antibody a control (unspecific). Immunoprecipitated DNA was analyzed by qPCR with promoter-specific primers for IL-12p40 (A), CD83 (B), and IκBα (C) and plotted as percent of input. Data are representative of 2 independent experiments.
Discussion
Heterogeneity and plasticity are hallmarks of cells of the monocytes–macrophage lineage.37,38 In response to microbial stimuli and cytokines, mononuclear phagocytes acquire distinct specialized and polarized functional properties.6,10,39 There is ample evidence that monocytes represent precursors for immune-stimulatory DCs under inflammatory conditions.3,4,38 Ox-Pls are formed by free oxygen radicals in inflammatory processes.13,14,40 Here, we demonstrate that such Ox-Pls selectively regulate the functional differentiation of monocytes into DCs. We observed that Ox-DCs have a diminished ability to produce IL-12 and to stimulate T-cell proliferation upon stimulation via TLR engagement. When we analyzed the underlying mechanism, we found that Ox-Pls inhibit the phosphorylation of histone H3 and consequently the accessibility of the Il-12 gene, leaving other signaling events like activation of NF-κB intact. These data implicate histone modification in suppressing DC-derived IL-12 by Ox-Pls and suggest that Ox-Pls might contribute to avoid overwhelming or even detrimental Th1-driven immune responses at sites of inflammation via epigenetic mechanisms.
Accumulating evidence suggests that Ox-Pls are not merely byproducts of the inflammatory response but can actively regulate inflammation. Most studies focused on the proinflammatory effects of Ox-Pls, which are thought to play a role in initiating and maintaining chronic inflammation such as atherosclerosis.16,20 However, it is increasingly recognized that Ox-Pls at the same time possess potent anti-inflammatory properties, which include the direct antagonism of LPS recognition by cells of the innate immune system.18,41 Indeed, Ox-Pls effectively inhibit the interaction of LPS with LPS-binding protein (LBP), CD14, and TLR4. We have recently demonstrated that oxidation products derived from PAPC are specific regulators of DC activation. We could show that OxPAPC does not activate DCs but efficiently prevents LPS-induced activation of DCs, thereby limiting their capacity to stimulate T cells. Inhibition was not restricted to LPS because OxPAPC also inhibited TLR-2– and TLR-3–mediated DC maturation but did not affect phenotypic DC maturation induced via CD40–CD40L interactions. The complete inhibitory capacity of OxPAPC on LPS-induced signaling cascades, on up-regulation of DC activation markers (CD83, CD86), as well as on cytokine production implicated that OxPAPC acted primarily through blocking of LPS binding to its receptor TLR-4. However, we did notice that IL-12 was more susceptible to the inhibitory effects of OxPAPC than, for example, CD83.17 Under the experimental conditions described in this paper, OxPAPC is now no longer capable of blocking LPS-induced signaling (ie, NF-κB activation, phosphorylation of ERK MAP-kinase) and up-regulation of DC activation markers such as CD83. We also show that OxPAPC does not interfere with the transactivation of NF-κB because NF-κB–mediated transcription of luciferase, initiated by a constitutively active TLR-4, is not inhibited.
Nevertheless, LPS-induced expression of IL-12 is still inhibited in DCs by OxPAPC. Moreover, the ability of LPS-treated DCs to polarize T cells in an MLR toward IFN-γ–producing Th1 cells is abrogated. These results demonstrated that, in addition to its ability to interfere with LPS/TLR-4 interaction, OxPAPC modulates responsiveness of DCs for LPS by targeting the Th1-driving capacity. This phenomenon also has been noticed in dyslipidemic mice lacking the apolipoprotein E gene in vivo.26 BecauseOxPAPC is a known active principle in hyperlipidemia, our studies suggest that OxPAPC might be responsible for the observed effects.
Recently, accumulation of Ox-Pls has been reported in human leprosy lesions.42 In line with our results, the authors show that Ox-Pls inhibited up-regulation of CD1b, thereby diminishing activation of CD1b restricted Mycobacterium leprae–specific T cells.
Furthermore, we identified the inhibition of histone H3 phosphorylation of the IL-12p40 promoter as a possible mechanism of how Ox-Pls can regulate DC function. It has long been observed that histone H3 is phosphorylated on its serine 10 residue in condensed chromosomes during mitosis, leading to the use of phosphorylated Ser10 (p-Ser10) as a mitotic marker.43 However, Nowak and Corces44 have demonstrated that phosphorylation of histone H3 correlates with transcriptionally active genomic loci. Furthermore, diverse stimuli rapidly induce phosphorylation on Ser10 of histone H3, which is associated controlling expression of genes related to inflammation.45 It has been shown that efficient transcription of IL-12p40 is dependent on inducible histone modifications, leading to increased accessibility of the promoter regions to the transcription factor NF-κB.35,46 In our studies, we found that OxPAPC interfered with LPS-induced histone modifications at the IL-12p40, the CD83 as well as the IκBα promoter. However, the extent of histone modifications in human monocyte DCs, especially at the IL-12 promoter, was rather low in our hands. In addition, we did not find a reduction of double modified histone H3 (H3pS10/K14Ac), although OxPAPC completely prevented LPS-induced phosphorylation of p38, which has been implicated in regulation of both modifications.35
Inhibition of histone H3 phosphorylation at serine 10 has been observed in other instances, eg, studying OspF, a Shigella flexneri–derived protein that serves as virulence factor by inhibiting innate immune responses via interference.47
During sepsis an epigenetic inhibition of IL-12 production in splenic DCs was reported.48,49 The authors observed a decreased ratio between H3K4me3 and H3K27me2 at the IL-12 promoter in splenic DCs in a mouse model of sepsis, which correlated with down-regulation of IL-12 gene expression, providing an explanation for DC dysfunction of postseptic patients. The exact stimulus inducing these epigenetic changes has not been identified. We propose that Ox-Pls might play a role in this context. Ox-Pls have been shown to protect from endotoxin-induced septic shock, but have been shown detrimental in an Escherichia coli model of sepsis.50
In cancer, a failure of mounting strong adaptive immune responses to cancer antigens is regarded as a major pathogenic principle in the progression of tumors. Modulation of DCs function toward a tolerogenic state is increasingly acknowledged to be mediated by soluble factors, some of which have been identified.51 Reactive oxygen species have also been implicated in this process.52 Therefore, the high levels of reactive oxygen species in tumors would favor generation of Ox-Pls, which would then add to the immunosuppressive microenvironment.
Therefore, in chronic inflammatory processes Ox-Pls could act by skewing differentiation of DCs toward less immunostimulatory DCs that can still be activated by TLR agonists but which do not support full-blown T-cell activation, especially with respect to their Th1 driving capacity. In addition, OxPAPC modulates LPS-induced histone modifications, which might contribute to the differential inhibition of activation associated genes during DC maturation. Therefore, Ox-Pls may act as inflammation-derived epigenetic regulators of DC activation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Margarethe Merio, Claus Wenhardt, and Petra Cejka for expert technical assistance.
This work was supported by grants from the Austrian Science Fund (SFB2307 and APP20266FW). C.S. was supported by the GEN-AU project “Epigenetic Plasticity of the Mammalian Genome” (Austrian Ministry of Science and Research, BM:WF).
Authorship
Contribution: S.B., G.Z., C.S., S.K., M.S., K.S., and G.J.Z. planned and performed experiments; S.B., G.Z., C.S., V.N.B., O.M., and J.S. analyzed results; V.N.B. and O.M. provided critical reagents; and S.B. and J.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current address for S.B. is the Department of Rheumatology/KIM III, Medical University of Vienna, Vienna, Austria.
Correspondence: Stephan Blüml, Institute of Immunology, Medical University of Vienna, Borschkegasse 8a, A-1090 Vienna, Austria; e-mail: stephan.blueml@meduniwien.ac.at.


![Figure 2. Ox-DCs display typical DC functions. (A) Purified T cells were stimulated with graded numbers of allogenic immature DCs or DCs differentiated in the presence of 10, 25, or 40 μg/mL OxPAPC, and the proliferation of T cells was monitored on day 5 of culture by adding [methyl-3H]thymidine followed by measuring [methyl-3H]thymidine incorporation 18 hours later. Data are presented as percent of immature DCs and represent mean values of 3 experiments ± SD. Immature DCs or DCs differentiated in the presence of 10, 25, or 40 μg/mL OxPAPC were incubated with FITC-Dextran (1 mg/mL; B) or mannosylated BSA–FITC (10 μg/mL; C) for 60 minutes and uptake of the fluorescent dyes was measured by flow cytometry. Data are presented as percent of immature DCs and represent mean values of 5 experiments ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/27/10.1182_blood-2008-11-191429/4/m_zh89990946270002.jpeg?Expires=1766020125&Signature=J-sobVGAJwghxCT84oM57zgxrbaHLV1ArxsTbzRDn4-E1ezo7VaZIcMFsunu9bYVIbyDFD50hm~nKSrRqEkwiHsUeOcbS3W-KaesgEtukibEBd0osJ3-epFUsnlccX~K8dS-8v2nvi91c4vGAJ8x241m5PmFsxlKODRCmfYsRI4TPu2RXJG419fb4FIMSpB5iUSB4AdzcawF~uX~yEQKlfueCakgPBsc6Ri4rZ~yS6v4BunyqaIQ-vPgKSqiYFPKJoocGbMER1D7v5VAuphYE5ZJ8g1UdzZgScsASMh51thow9yAOZ9k1sojDDCkWPDjyJHFJbTLlk1gmFjH7oJI0w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Ox-DCs have a limited ability to mature. Immature DCs (thin black line) or DCs differentiated in the presence of 40 μg/mL OxPAPC (gray histogram) were stimulated with LPS (100 ng/mL; A) or PGN (1 μg/mL; B, thick black line) for 24 hours, harvested, and stained for the indicated markers and measured by flow cytometry. Purified T cells were stimulated with graded numbers of allogenic immature DCs or DCs differentiated in the presence of 40 μg/mL OxPAPC (left) or the indicated amounts of OxPAPC (right) and stimulated with LPS (C) or PGN (D), and the proliferation of T cells was monitored on day 5 of culture by adding [methyl-3H]thymidine followed by measuring [methyl-3H]thymidine incorporation 18 hours later.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/27/10.1182_blood-2008-11-191429/4/m_zh89990946270003.jpeg?Expires=1766020125&Signature=B2obpXplXlvdtz-iqXRo3W7ZJsoXa0dZosfipgnJnyJm8SecpqrOU3iCcbRaZoiFNJpnAGeGMv74DgF4uJHLPp19Avh~5vqERcE9EDPTRhSn881BJBBbGtUEyZO0byTcqr-jYJwAvo~tX80fIJ4xls31k065kI6deGeWufG5wKn2byyEYTBdNC8S09D~cwsz8IZXOgg8s6dlhr94SiZgdAY9dyRxU2HiDeuXAg8TiBa-84aKnuP1MvTS8ML2B5Vja3wSKK-0IMFlvDJyDj0vKjh0j9OuzXlNVpWivX-gLzHTRBjwIdH0~RaWNjMnTv30itab-vxUG7yvwWyWnO7feA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





![Figure 2. Ox-DCs display typical DC functions. (A) Purified T cells were stimulated with graded numbers of allogenic immature DCs or DCs differentiated in the presence of 10, 25, or 40 μg/mL OxPAPC, and the proliferation of T cells was monitored on day 5 of culture by adding [methyl-3H]thymidine followed by measuring [methyl-3H]thymidine incorporation 18 hours later. Data are presented as percent of immature DCs and represent mean values of 3 experiments ± SD. Immature DCs or DCs differentiated in the presence of 10, 25, or 40 μg/mL OxPAPC were incubated with FITC-Dextran (1 mg/mL; B) or mannosylated BSA–FITC (10 μg/mL; C) for 60 minutes and uptake of the fluorescent dyes was measured by flow cytometry. Data are presented as percent of immature DCs and represent mean values of 5 experiments ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/27/10.1182_blood-2008-11-191429/4/m_zh89990946270002.jpeg?Expires=1766020126&Signature=QJwWByZls3Zpv3ef6xIzkZsjRRJwx~pN1uKDO1LyVok4Vo-fUmYEVbUTXHJZZAYBuEaNv10OoYun-KhOlzMAHicGbiGjCo4q5o28F9aE35wh8r8~d8DEOlyTuF4Ayj1trqqz7MxlOyRCF5jsaNKJgzUiY~LcVUommi8K1Lmc~Q8pCVM1cy~gDSFL~46gZzyJJOtCra6y0I5AnZvy8cFQ5lvwm~UMknRTp9d18PLNacfvlWWNy03ZfI4Y313qi7jAsVvCI3xjOBAYmWApt7Oe8rJjdJCzBTb5hC-9Yl5b77LdvLfU8drnksSKnsr6emuT7LGA~Issgb23DQuyCCAw3g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Ox-DCs have a limited ability to mature. Immature DCs (thin black line) or DCs differentiated in the presence of 40 μg/mL OxPAPC (gray histogram) were stimulated with LPS (100 ng/mL; A) or PGN (1 μg/mL; B, thick black line) for 24 hours, harvested, and stained for the indicated markers and measured by flow cytometry. Purified T cells were stimulated with graded numbers of allogenic immature DCs or DCs differentiated in the presence of 40 μg/mL OxPAPC (left) or the indicated amounts of OxPAPC (right) and stimulated with LPS (C) or PGN (D), and the proliferation of T cells was monitored on day 5 of culture by adding [methyl-3H]thymidine followed by measuring [methyl-3H]thymidine incorporation 18 hours later.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/27/10.1182_blood-2008-11-191429/4/m_zh89990946270003.jpeg?Expires=1766020126&Signature=qmhJiVAvLhDnSHWDJb6r7HSQ-CGrhZz5tscqRrNBy7PH6Cmt0YnDtwUnJlPzeHNMtUFGZoimA3Cs7phP1cGqdSN2dEe54eE78lfQvWE2MkHVqWgIERfLUa8Ph3Xw2JikGH1EKuXlIQV4lob5jaCiwrqujC83JTRryS~KfrpDHVgAvNRG9kkbLzAUVm0MR4VFDEm7v0Ieh4MHAWkS-OJY8S~gJo094G0ublaA18ryUiYEuqLuzbeil2-kOaAyxZXJVVz6QB-GwdwnbZ-kWA2xtN~Gg~adZInaRNRTwJsrio-3sObokoqX-mAtWzPuoloDAw3kNSXmi-4vzjP53Saz~w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)