Abstract
We sought to determine the contributions of protein tyrosine phosphatases (PTPs) to the pathogenesis of B-cell lymphomas. We found that T-cell PTP (TC-PTP) was overexpressed in transformed B cells. We hypothesized that TC-PTP may be a tumor-promoting gene that is regulated by MYC overexpression in B cells. Knockdown of TC-PTP in murine tumors resulted in decreased cell viability in vitro because of an arrest in the G1 phase of the cell cycle. Furthermore, cells with reduced TC-PTP expression were unable to either engraft or expand in vivo. Taken together, these data indicate that TC-PTP is required for B-cell tumor maintenance. Our data also suggested a correlation between TC-PTP expression and MYC overexpression. To investigate this further, we used malignant murine B cells that contain a doxycycline-repressible MYC transgene. We found that repression of MYC overexpression with doxycycline reduced TC-PTP expression. Moreover, enforced expression of TC-PTP showed partial rescue of the expansion of tumor cells after suppression of MYC overexpression. These results suggest that MYC overexpression induces TC-PTP overexpression, which in turn promotes tumor proliferation, implicating TC-PTP as an important effector of the MYC-driven proliferation program in B-cell lymphomas. Thus, TC-PTP may be a suitable molecular target for the treatment of B-cell lymphomas.
Introduction
B-cell lymphomas constitute the majority of non-Hodgkin lymphoma, the fifth most common form of cancer in the United States.1 The World Health Organization classification system identifies at least 15 distinct forms of B-cell lymphomas, which differ substantially in their clinical presentation, underlying biology, and patient prognosis.2 Nonetheless, B-cell lymphomas share 2 common features. First, large B-cell lymphomas express surface B-cell receptor (BCR).3 Second, they have undergone a chromosomal translocation involving an immunoglobulin gene and a proto-oncogene; prominent among these is the MYC oncogene.3 The MYC gene encodes a ubiquitously expressed, short-lived, transcriptionally active protein that regulates cellular proliferation, differentiation, and survival.4 Overexpression of MYC has been implicated in the majority of human lymphoid malignancies.5 MYC overexpression can occur through several mechanisms, including mutations in the promoter region of MYC,6 gene amplification,4 and stabilization,7 or chromosomal translocations.3,8 For example, the t(8;14) chromosomal translocation places the MYC oncogene under the control of the immunoglobulin heavy chain enhancer, resulting in MYC overexpression in the B-cell compartment.
The t(8;14) MYC translocation is modeled in the Eμ-MYC mouse.9 Eμ-MYC mice develop tumors composed of pre-pro B cells that resemble B-cell acute lymphoblastic leukemia.9 We found that we could modify these tumors by the addition of a BCR transgene, BCRHEL. The resultant Eμ-MYC/BCRHEL mice developed tumors resembling a subset of CD5− B-cell lymphocytic leukemia.10 The further addition of cognate antigen, soluble hen egg lysosyme (sHEL), to Eμ-MYC/BCRHEL mice resulted in aggressive lymphomas resembling Burkitt lymphoma (BL) in the Eμ-MYC/BCRHEL/sHEL mice.10
We were interested in the role of protein tyrosine phosphatases (PTPs) in lymphomagenesis. We found that T-cell PTP (TC-PTP) was aberrantly expressed in B-cell lymphomas. TC-PTP is a nonreceptor PTP predominantly expressed as a 45-kDa form that shuttles between the cytoplasm and nucleus. TC-PTP has been shown to negatively regulate cytokine signaling through dephosphorylation of tyrosine residues on Jak and STAT proteins.11-14 TC-PTP can directly act on other substrates, including the epidermal growth factor receptor,15 Src,16 and Shc.17 TC-PTP knockout mice die 3 to 5 weeks after birth of a myeloproliferative disease.18 Before death, knockout mice show a block in B-cell development.19 Recently, TC-PTP was shown to be overexpressed in certain activated B-cell-like diffuse large B-cell lymphoma (ABC-DLBCL) cell lines.20 These observations prompt the hypothesis that TC-PTP is a tumor-promoting gene that may be regulated by MYC overexpression in B cells.
In this study, we report that TC-PTP expression is up-regulated in all of the murine MYC-driven B-cell lymphomas tested, relative to wild-type cells. We found similar expression patterns in both mouse and human transformed B cells. Knockdown of TC-PTP in murine transformed B cells resulted in a reduction of cell viability resulting from delayed progression through the cell cycle. Moreover, tumor cells expressing a small RNA hairpin (shRNA) to TC-PTP failed to either engraft or expand in vivo, suggesting a critical role for TC-PTP in the maintenance of B-cell lymphoma. In addition, we find a correlation between MYC and TC-PTP overexpression. Finally, we show that enforced expression of TC-PTP results in a partial rescue of the expansion of tumor cells after suppression of MYC overexpression. Taken as a whole, our data suggest a model in which TC-PTP may promote the proliferation of B-cell lymphoma downstream of MYC.
Methods
Transgenic mice and cell lines
Eμ-MYC mice were previously described.9 Eμ-MYC/BCRHEL and Eμ-MYC/BCRHEL/sHEL mice and DBL-114, DBL-120, TBL-1, and TBL-8 cell lines were previously described.10 FLL-21, FLL-42, and FLL-44 cell lines were generated from tumors arising in MMTV-rtTA/TRE-MYC/BCRHEL/sHEL mice, in a process identical for the DBL and TBL cell lines.10 BL cell lines, RAJI, RAMOS, and DAUDI, were obtained from Dr John Cambier (National Jewish Health, Denver, CO). All cells were maintained in C10,10 except DAUDI, which were grown in Iscove modified Dulbecco medium with 10% fetal bovine serum and 100 units/mL penicillin/streptavidin. Primary murine splenocytes were obtained from C57BL/6 mice. Primary human B cells were obtained from normal, anonymous human volunteers without any additional clinical data. Approval was obtained from the Institutional Review Board at the National Jewish Medical and Research Center. Informed consent was provided to volunteers according to the Declaration of Helsinki. B cells were positively selected for using pan-CD19 Dynal beads (Invitrogen) according to the manufacturer's instructions.
RT-PCR and PCR detection of genes
A total of 107 cells were pelleted and then resuspended in 1 mL of TRIzol (Invitrogen). Before treatment with TRIzol, FLL cells were treated for 18 hours with 10 μg/mL doxycycline (Clontech; stock = 1 mg/mL in phosphate-buffered saline [PBS]) or, as a control, an equal volume of PBS added directly to the media. The manufacturer's instructions were followed to extract total mRNA, which was suspended in 15 μL of diethyl pyrocarbonate-treated H2O. Reverse-transcribed polymerase chain reaction (RT-PCR) was performed on half of the mRNA (5 × 106 cell equivalents) with 1 μg of oligo (dT; Invitrogen), 3.3mM dithiothreitol (Invitrogen), dNTP mix (Invitrogen), 20 U of Rnase-OUT (Invitrogen), and 300 U of Moloney murine leukemia virus (M-MLV) RT (Invitrogen) for 2 hours at 42°C. The other half of the extracted mRNA was processed without RT. The resulting cDNA was precipitated with 200mM NH4-Oac, pH 4, and 2 volumes of ethanol for 2 hours at −20°C, and then resuspended in 50 μL of water (105 cell equivalents/μL). Gene expression was first detected by either semiquantitative or agarose gel analysis. cDNA was diluted 1:50 (2000 cell equivalents/μL) and PCR was performed using 2 μL of cDNA, 10 pmol of forward oligo, 10 pmol of reverse oligo, 20mM Tris, pH 8.4, 50mM KCl, 4mM MgCl2, and 10 U of Taq. The following oligo pairs were used (Integrated DNA Technologies): mouse TC-PTP: forward ACTTCCGAACACATGCTGCC, reverse TCAAACAACCAGATTCTCTAAC; mouse β-actin: forward CCTAAGGCCAACCGTGAAAAG, reverse TCTTCATGGTGCTAGGAGCCA; human TC-PTP: forward ACTTCCTAACACATGCTGCC, reverse AAGTGTCTACCAGAGAGAAGG; human GAPDH: forward ACCACAGTCCATGCCATCAC, reverse TCCACCACCCTGTTGCTGTA. Results were analyzed on 2% agarose gels using a Gel-Doc XR (Bio-Rad).
Quantitative PCRs were set up with 2 μL of cDNA(4000 cell equivalents), 10 pmol of forward oligo, 10 pmol of reverse oligo, and SYBR GREEN PCR Master Mix (Applied Biosystems). Oligos for quantitative PCR were designed using Primer Express (Applied Biosystems): mouse TC-PTP: forward GGAGGAGAGCAGTGAGAGCATT, reverse GCCGTCGTAGCCTTTCTATCC; mouse GAPDH: forward CATGGCCTTCCGTGTTCCTA, reverse GCGGCACGTCAGATCCA; mouse ornithine decarboxylase (ODC): forward GCCAAAAAAACCGTGTGGAA, reverse TGTTCATTTGACTCATCTTCATCGT; mouse CD45: forward CCTGCTCGCACCACTGAA, reverse TGGATGATATGTGGTCTCTGAAGAG; human TC-PTP: forward TGAGAGAATCTGGCTCCTTGAAC, reverse TGCCTGCACTACAGTGGATCA; human GAPDH: forward CAAGGCTGTGGGCAAGGT, reverse GGAAGGCCATGCCAGTGA; human MYC: forward AGGGTCAAGTTGGACAGTGTCA, reverse TGGTGCATTTTCGGTTGTTG. Analysis was performed on an ABI 7300 (Applied Biosystems) using a standard curve and following the manufacturer's instructions.
Western blot analysis
Cells were lysed in 1% Triton X-100 (TX-100) and subjected to Western blot, as previously described.21 Antibodies used include: murine TC-PTP was detected with rabbit anti–TC-PTP (6F3) obtained from Medimabs, human TC-PTP was detected with mouse anti–TC-PTP (CF4-1D) obtained from EMD, Myc was detected with rabbit anti-Myc (N-262) from Santa Cruz Biotechnology, and β-actin mouse antibody (8226) was obtained from Abcam. Secondary horseradish peroxidase–linked anti–rabbit and anti–mouse were purchased from Cell Signaling Technologies.
Disruption of gene expression with shRNAs
The generation and cloning of shRNAs into lentiviral vectors were previously described.10 Target sequences are as follows: shLuc, CTTACGCTGAGTACTTCGA; shTC-PTP.1, TGATCACAGTCGTGTTAAA; shTC-PTP.2, ACAGAGAAATGGTGTTTAA. Lentiviral infection protocols were previously described.10 To validate knockdown, infected cells were sorted to more than 95% green fluorescent protein (GFP)+ cells and then lysed in 1% TX-100 and subjected to Western blot, as described in “Western blot analysis.” Quantitation of Western blots was performed as previously described.21 The in vitro competition assay was described previously, as was the adoptive transfer of infected tumor cells into mice.10 Carboxyfluorescein succinimidyl ester (CFSE) labeling was performed as previously described.22 Anti-Thy1.1–Alexa 647 (OX7) was obtained from BioLegend and was used to label transduced cells expressing the Thy1.1 reporter construct as previously reported.21
Ectopic expression of TC-PTP
Human TC-PTP was amplified from clone 3872164 (Open Biosystems) using the following primers: forward ATTGAATTCGCAGCCATGCCCACCACCATTGAA, reverse ATTCTCGAGGGTGTCTGTCAATCTTGG. The PCR product was cloned in pMIG using a 5′ EcoRI and a 3′ Xho1 site. pMIG-MYC has been previously described.23 Retrovirus was generated as previously described22 to transduce FLL-44 cells. To validate that TC-PTP was expressed, transduced FLL-44 cells were first treated with 10 μg/mL doxycycline and then lysed in 1% TX-100 and subjected to Western blot, as previously described.21 Anti–human TC-PTP clone CF4 was used to detect human TC-PTP (EMC). Transduced cells were treated with 5 μg/mL doxycycline or an equal volume of PBS and followed for 96 hours. Media and doxycycline were replaced every 48 hours. Fluorescence-activated cell sorter (FACS) analysis was performed on a FACSCalibur to detect changes in the frequency of GFP+ cells, as previously described.10
Results
TC-PTP is overexpressed in B-cell lymphomas
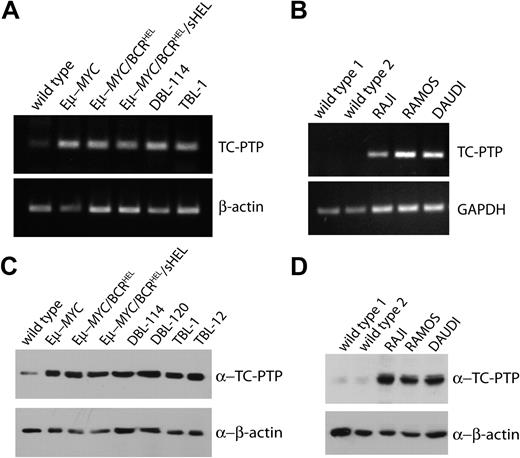
We sought to understand the contribution of PTP genes to the maintenance of B-cell neoplasia. To do so, we analyzed the expression patterns of more than 40 PTPs in wild-type splenocytes and primary B-cell malignancies obtained from spleens in Eμ-MYC, Eμ-MYC/BCRHEL, or Eμ-MYC/BCRHEL/sHEL transgenic mice (supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). We also examined 2 cell lines, DBL-114 and TBL-1, derived from the primary mouse tumors. We found that TC-PTP expression was higher in all transformed B cells tested, whereas levels of the β-actin control mRNA were equivalent (Figure 1A). To validate this result, we examined TC-PTP mRNA expression in the MYC-driven human BL tumor cell lines, RAJI, RAMOS, and DAUDI, relative to wild-type human peripheral B cells. We found that human TC-PTP expression was higher in the malignant B cells than in the wild-type peripheral B cells compared with levels of the housekeeping gene GAPDH (Figure 1B). In addition, we found that TC-PTP protein expression was also substantially higher in both murine primary tumors and tumor cell lines (Figure 1C) and in human B-cell lymphoma cell lines (Figure 1D), compared with purified peripheral B cells. These results mirror the mRNA expression patterns we observed in Figure 1A and B. Although levels of TC-PTP expression can vary between different subsets of B cells, these differences are relatively small and cannot account for the dramatic increase in TC-PTP observed in the B-cell lymphomas (GEO profiles, accession GDS1695). The increased levels of TC-PTP mRNA in transformed cells led us to hypothesize that TC-PTP may be important in the maintenance of B-cell lymphomas.
TC-PTP is overexpressed in mouse and human B-cell lymphomas. (A) TC-PTP mRNA expression in murine model lymphomas. Total mRNA was extracted from wild-type splenocytes, 3 different primary MYC-driven B-cell lymphomas, and DBL-114 and TBL-1 mouse B-cell lymphoma cell lines. cDNA was prepared and PCR was performed using primers specific to TC-PTP or β-actin, as a control. (B) TC-PTP expression in human BL cell lines. As in panels A and B, total mRNA was extracted from wild-type human B cells and 3 different BL cell lines: RAJI, RAMOS, and DAUDI. PCRs for human TC-PTP and GAPDH were then performed and analyzed by gel. Representative gels of at least 3 repeats. (C) TC-PTP protein expression in murine model lymphomas. TX-100 detergent lysates from purified splenic B cells, primary MYC-driven mouse B-cell lymphomas, and mouse B-cell lymphoma cell lines (DBL-114, DBL-120, TBL-1, and TBL-12) were probed with 6F3 anti-TCPTP and anti–β-actin. Representative blot; each sample was blotted 4 times. (D) TC-PTP protein expression in human BL cell lines. As in panel C, TX-100 protein lysates of wild-type human B cells and 3 different BL cell lines (RAJI, RAMOS, and DAUDI) were probed with CF4-1D anti–human TCPTP and anti–β-actin. Representative blot from a pool of 4.
TC-PTP is overexpressed in mouse and human B-cell lymphomas. (A) TC-PTP mRNA expression in murine model lymphomas. Total mRNA was extracted from wild-type splenocytes, 3 different primary MYC-driven B-cell lymphomas, and DBL-114 and TBL-1 mouse B-cell lymphoma cell lines. cDNA was prepared and PCR was performed using primers specific to TC-PTP or β-actin, as a control. (B) TC-PTP expression in human BL cell lines. As in panels A and B, total mRNA was extracted from wild-type human B cells and 3 different BL cell lines: RAJI, RAMOS, and DAUDI. PCRs for human TC-PTP and GAPDH were then performed and analyzed by gel. Representative gels of at least 3 repeats. (C) TC-PTP protein expression in murine model lymphomas. TX-100 detergent lysates from purified splenic B cells, primary MYC-driven mouse B-cell lymphomas, and mouse B-cell lymphoma cell lines (DBL-114, DBL-120, TBL-1, and TBL-12) were probed with 6F3 anti-TCPTP and anti–β-actin. Representative blot; each sample was blotted 4 times. (D) TC-PTP protein expression in human BL cell lines. As in panel C, TX-100 protein lysates of wild-type human B cells and 3 different BL cell lines (RAJI, RAMOS, and DAUDI) were probed with CF4-1D anti–human TCPTP and anti–β-actin. Representative blot from a pool of 4.
Disruption of TC-PTP expression results in cell-cycle arrest in B-cell lymphomas
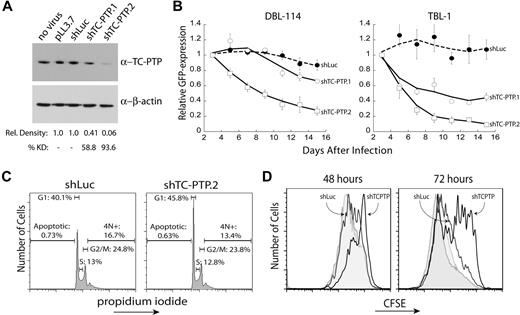
To understand the contribution of TC-PTP to the maintenance of B-cell lymphomas, we pursued a knockdown approach. We generated 2 shRNAs to murine TC-PTP, which were then cloned into a lentiviral vector that coexpresses an shRNA and a GFP reporter.24 A control shRNA against firefly luciferase (shLuc) and the 2 specific shRNAs to TC-PTP (shTC-PTP.1 and shTC-PTP.2) were lentivirally transduced into DBL-114 tumor B cells derived from Eμ-MYC/BCRHEL tumors.10 Knockdown was validated through Western blot analysis of TC-PTP protein levels relative to those of β-actin (Figure 2A). Semiquantitative Western blot analysis of 3 separate blots indicated that both shRNAs reduced TC-PTP protein expression, with shTC-PTP.1 reducing expression by 58.8% and shTC-PTP.2 reducing TC-PTP expression by 93.6% (Figure 2A).
TC-PTP knockdown results in reduced proliferation of murine B-cell lymphoma cells. (A) Validation of TC-PTP knockdown. DBL-114 cells were lentivirally transduced with either a control shRNA against firefly luciferase or 1 of 2 shRNAs specific to murine TC-PTP expressed in vector pLL3.7. Cells were sorted for less than 95% transduction and sorted cells were lysed in 1% TX-100. A total of 5 μg of each lysate was subjected to semiquantitative Western blot analysis with 6F3 anti–TC-PTP and anti–β-actin as a loading control. A representative Western blot is shown along with quantitation of 3 different blots. (B) TC-PTP knockdown confers a competitive growth disadvantage to mouse B-cell lymphoma cells relative to cells expressing normal levels of TC-PTP. DBL-114 cells (left panel) or TBL-1 (right panel) were lentivirally transduced with the listed shRNAs, resulting in a mixed population of transduced (GFP+) and nontransduced (GFP−) cells. The frequency of GFP+ cells (relative to an empty vector control) for each construct was then monitored for 2 weeks after infection. (C) TC-PTP knockdown results in an accumulation of cells in the G1 phase of the cell cycle. DBL-114 cells sorted for control shRNA (shLuc) or TC-PTP–specific shRNA (shTC-PTP.2) were fixed with cold ethanol and stained with propidium iodide to examine total cellular DNA content. Cells were subjected to FACS analysis, and 20 000 events were collected for each shRNA. The cell-cycle data are as follows (mean ± SD): shLuc; apoptotic, 0.73% ± 0.09%; G1, 40.1% ± 0.46%; S, 13.0% ± 0.25%; G2/M, 24.8% ± 0.80%; 4N+, 16.7% ± 3.40%; shTC-PTP, apoptotic, 0.63% ± 0.22%; G1, 45.8% ± 1.31%; S, 12.8% ± 0.74%; G2/M, 23.8% ± 1.46%; 13.4% ± 4.69%; n = 3. (D) TC-PTP knockdown retards cellular proliferation. DBL-114 cells were transduced to express the control shRNA (shLuc) or shTC-PTP.2, and a thy1.1 reporter. Cells were labeled with CFSE 3 days after infection and evaluated at 48 and 72 hours later. Cells were counterstained with OX7 anti–Thy1.1–Alexa 647 to differentiate shRNA expressing cells and analyzed by flow cytometry. The data are shown for both transduced cells (solid line, labeled) and untransduced cells (shaded, labeled) from each population. Shown are representative plots from 3 different datasets.
TC-PTP knockdown results in reduced proliferation of murine B-cell lymphoma cells. (A) Validation of TC-PTP knockdown. DBL-114 cells were lentivirally transduced with either a control shRNA against firefly luciferase or 1 of 2 shRNAs specific to murine TC-PTP expressed in vector pLL3.7. Cells were sorted for less than 95% transduction and sorted cells were lysed in 1% TX-100. A total of 5 μg of each lysate was subjected to semiquantitative Western blot analysis with 6F3 anti–TC-PTP and anti–β-actin as a loading control. A representative Western blot is shown along with quantitation of 3 different blots. (B) TC-PTP knockdown confers a competitive growth disadvantage to mouse B-cell lymphoma cells relative to cells expressing normal levels of TC-PTP. DBL-114 cells (left panel) or TBL-1 (right panel) were lentivirally transduced with the listed shRNAs, resulting in a mixed population of transduced (GFP+) and nontransduced (GFP−) cells. The frequency of GFP+ cells (relative to an empty vector control) for each construct was then monitored for 2 weeks after infection. (C) TC-PTP knockdown results in an accumulation of cells in the G1 phase of the cell cycle. DBL-114 cells sorted for control shRNA (shLuc) or TC-PTP–specific shRNA (shTC-PTP.2) were fixed with cold ethanol and stained with propidium iodide to examine total cellular DNA content. Cells were subjected to FACS analysis, and 20 000 events were collected for each shRNA. The cell-cycle data are as follows (mean ± SD): shLuc; apoptotic, 0.73% ± 0.09%; G1, 40.1% ± 0.46%; S, 13.0% ± 0.25%; G2/M, 24.8% ± 0.80%; 4N+, 16.7% ± 3.40%; shTC-PTP, apoptotic, 0.63% ± 0.22%; G1, 45.8% ± 1.31%; S, 12.8% ± 0.74%; G2/M, 23.8% ± 1.46%; 13.4% ± 4.69%; n = 3. (D) TC-PTP knockdown retards cellular proliferation. DBL-114 cells were transduced to express the control shRNA (shLuc) or shTC-PTP.2, and a thy1.1 reporter. Cells were labeled with CFSE 3 days after infection and evaluated at 48 and 72 hours later. Cells were counterstained with OX7 anti–Thy1.1–Alexa 647 to differentiate shRNA expressing cells and analyzed by flow cytometry. The data are shown for both transduced cells (solid line, labeled) and untransduced cells (shaded, labeled) from each population. Shown are representative plots from 3 different datasets.
We next performed an in vitro competition assay to determine the effect of TC-PTP knockdown on cell viability. DBL-114 cells were transduced with empty vector, control shRNA (shLuc), or one of the 2 specific shRNAs against TC-PTP. After transduction, we obtained a mixture of GFP+ (transduced) and GFP− (nontransduced) cells. The frequency of GFP+ cells, relative to empty vector, was monitored by flow cytometry for 2 weeks, as we have previously done for other targets.10,21 The frequency of GFP+ cells infected with shRNAs specific to TC-PTP decreased over time, suggesting that knockdown of TC-PTP conferred a competitive disadvantage on these cells compared with nontransduced cells in the same well, as we have described previously (Figure 2B left panel).10,21 Moreover, this phenotype correlated with the levels of knockdown observed in Figure 2A. In contrast, expression of the control shRNA to firefly luciferase had no effect on the frequency of GFP over time (Figure 2B). We observed similar results in another EμMYC/BCRHEL cell line, DBL-120 (data not shown) and in 2 cell lines derived from EμMYC/BCRHEL/sHEL tumors, TBL-1 cells (Figure 2B right panel) and TBL-8 cells (data not shown).
Results from the in vitro competition assay indicated that disruption of TC-PTP expression in malignant B cells reduced cell viability by either the induction of cell death or decreased cellular proliferation. To differentiate between these 2 possibilities, we examined the total cellular DNA content by propidium iodide staining. DBL-114 cells were transduced with either shLuc or shTC-PTP.2 and sorted to more than 95% GFP+ cells. TBL cells were not used because they are difficult to transduce at levels suitable for cell sorting.10 Sorted cells were then fixed with cold ethanol and stained with propidium iodide. FACS analysis showed no increase in apoptosis, characterized by subdiploid DNA content, in cells transduced with shTC-PTP.2 versus shLuc (Figure 2C left panel). We did, however, observe an accumulation of cells in the G1 phase of the cell cycle, as characterized by diploid DNA content, in cells infected with shTC-PTP.2 compared with shLuc (Figure 2C right panel).
We next examined cell proliferation directly. DBL-114 cells were transduced with either a control shRNA (shLuc) or shTC-PTP.2 that coexpressed a Thy1.1 reporter gene that was visualized by antibody staining. Transduced cells were labeled with CFSE 2 days after the last day of transduction. Cell division can be tracked after the dilution of CFSE over time using flow cytometry; the dye is diluted among the daughter cells after cell division. Analysis of CFSE-labeled cells at 48 and 72 hours later revealed that cells expressing the TC-PTP–specific shRNA had proliferated substantially less than cells expressing the control shLuc shRNA, or the nontransduced population from either sample (Figure 2D). Taken as a whole, these data suggest a role for TC-PTP in promoting tumor cell proliferation by facilitating the transition of cells from G1 to S phase in the cell cycle.
TC-PTP expression is required for tumor maintenance in vivo
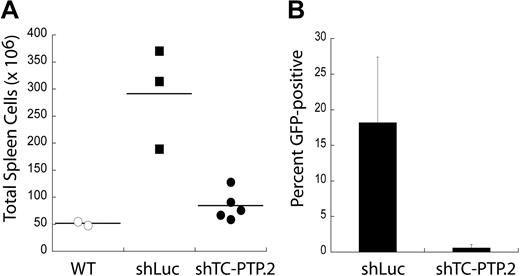
Murine BL cell lines expressing shRNAs to TC-PTP can still proliferate in vitro, albeit slower than cells expressing control shRNA. To determine whether TC-PTP was required for tumor maintenance in vivo, we used a system amenable to transplantation of syngeneic tumors. DBL-114 cells were transduced with lentivirus encoding either shLuc or shTC-PTP.2 and sorted to more than 95% GFP+ (infected) cells. Sorted cells were transplanted into cohorts of syngeneic, age- and sex-matched mice, which were then monitored until the first mice developed visible clinical signs of disease, as described previously.10,21,22 At this point, the mice were killed and their tumors examined. We found that mice receiving murine lymphoma B cells with the control shLuc shRNA developed lymphomas, as demonstrated by higher cell numbers in the spleen compared with wild-type control mice that did not receive tumor transplantations (Figure 3A). In contrast, mice that received murine lymphoma B cells expressing shTC-PTP.2 only had slightly higher cell numbers in their spleens than control mice that did not receive tumor transplantations (Figure 3B). Moreover, FACS analysis of tumor masses in mice receiving shTC-PTP.2 expressing tumor cells found almost no GFP fluorescence (0.58% ± 0.49% GFP+), indicating that any tumors that developed in these mice were derived from the approximately 5% of cells not expressing the shRNA targeting TC-PTP. Tumors in mice injected with cells expressing the control shRNA maintained higher levels of GFP (18.2% ± 9.2% GFP+). The tumor masses resulting from the shLuc-transduced cells do not maintain their original frequency of GFP expression because of several factors, including loss of GFP and shRNA expression using the lentiviral expression system. These results suggest that TC-PTP expression is required for the maintenance of these B-cell lymphomas in vivo.
TC-PTP expression is required for tumor maintenance in vivo. (A) TC-PTP knockdown tumors are not maintained in vivo. DBL-114 cells were transduced with either a control shRNA (shLuc) or an shRNA specific to TC-PTP (shTC-PTP.2). Transduced cells were then sorted to > 95% GFP+ (infected), and 105 of these cells were adoptively transferred into cohorts of age- and sex-matched C57BL6 mice. These mice were allowed to develop externally palpable tumors, at which point they were killed and their tumors examined for cell numbers and GFP fluorescence. Tumorous spleens were harvested, and single-cell suspensions were generated and then enumerated. Wild-type spleen counts are shown for comparison. (B). Tumors that do develop in mice injected with TC-PTP knockdown tumor cells are GFP−. Tumors were harvested from mice injected with either shLuc or shTC-PTP.2 tumor cells and subjected to FACS analysis to determine the frequency of GFP+ cells.
TC-PTP expression is required for tumor maintenance in vivo. (A) TC-PTP knockdown tumors are not maintained in vivo. DBL-114 cells were transduced with either a control shRNA (shLuc) or an shRNA specific to TC-PTP (shTC-PTP.2). Transduced cells were then sorted to > 95% GFP+ (infected), and 105 of these cells were adoptively transferred into cohorts of age- and sex-matched C57BL6 mice. These mice were allowed to develop externally palpable tumors, at which point they were killed and their tumors examined for cell numbers and GFP fluorescence. Tumorous spleens were harvested, and single-cell suspensions were generated and then enumerated. Wild-type spleen counts are shown for comparison. (B). Tumors that do develop in mice injected with TC-PTP knockdown tumor cells are GFP−. Tumors were harvested from mice injected with either shLuc or shTC-PTP.2 tumor cells and subjected to FACS analysis to determine the frequency of GFP+ cells.
MYC overexpression correlates to TC-PTP expression
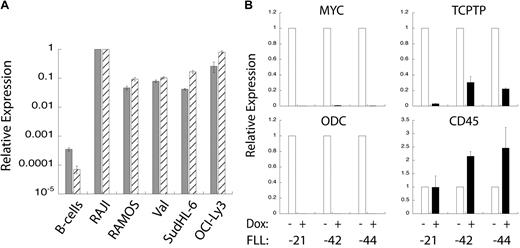
The mRNA and protein expression patterns shown in Figure 1 suggested that TC-PTP overexpression might correlate to the overexpression of MYC in murine lymphoma B cells. To examine this more closely, we performed quantitative PCR analysis of MYC and TC-PTP expression in wild-type human B cells and BL and DLBCL cell lines. We found that both MYC and TC-PTP expression is substantially higher in all of the BL and DLBCL cell lines compared with wild-type B cells. Moreover, there is a correlation between the expression levels of these 2 genes in either wild-type or tumor cells. We subsequently investigated how the repression of MYC overexpression in tumor cells affects TC-PTP expression. To do this, we used B-cell lymphoma cell lines derived from a model mouse tumor in MMTV-rtTA/TRE-MYC/BCRHEL/sHEL mice,10 referred to herein as FLL cell lines. These murine lymphoma B cells have a doxycycline-repressible human MYC transgene; the addition of doxycycline to these cells represses overexpression of the MYC transgene. Three different FLL cell lines (FLL-21, -42, and -44) were treated with 10 μg/mL doxycycline or an equal volume of PBS for 18 hours, at which point mRNA was extracted and cDNA generated. Quantitative PCR analysis showed that doxycycline treatment reduced levels of human MYC mRNA relative to GAPDH mRNA. This was reflected in the expression levels of ODC, which is a MYC-responsive gene (Figure 4B).23 TC-PTP mRNA expression levels were also reduced in the doxycycline-treated cells, correlating to the levels of human MYC expression (Figure 4B). In contrast, CD45 mRNA expression levels were either unchanged or increased on doxycycline treatment, indicating that not all mRNA expression levels were reduced relative to GAPDH. Taken together, these data indicate a direct correlation between MYC and TC-PTP overexpression in the case of a surfeit of MYC.
TC-PTP expression correlates to MYC overexpression. (A) TC-PTP and MYC overexpression in human BL cell lines. Quantitative PCR analysis of MYC ( ) and TC-PTP (
) and TC-PTP ( ) expression relative to GAPDH expression. Shown are wild-type B cells, RAJI and RAMOS BL cell lines, Val and SudHL-6 GC-like DLBCL cell lines, and OCI-Ly3 ABC-like DLBCL cell line. Error bars represent SD for 2 replicates; n = 3 or more. (B). TC-PTP expression is reduced after loss of MYC overexpression. FLL cell lines, derived from MMTV-rtTA/TRE-MYC/BCRHEL/sHEL mice, have a doxycycline-repressible MYC promoter. Three different FLL cell lines (-21, -42, and -44) were treated with doxycycline to turn off MYC overexpression, after which mRNA was harvested and cDNA produced. Quantitative PCR analysis was performed relative to GAPDH for the following genes: MYC (top left panel), ODC (bottom left panel), TC-PTP (top right panel), and CD45 (bottom right panel) expression relative to GAPDH and normalized to untreated cells. Error bars show SD for 2 replicates; n = 4 for MYC and GAPDH, and n = 2 for ODC and CD45.
) expression relative to GAPDH expression. Shown are wild-type B cells, RAJI and RAMOS BL cell lines, Val and SudHL-6 GC-like DLBCL cell lines, and OCI-Ly3 ABC-like DLBCL cell line. Error bars represent SD for 2 replicates; n = 3 or more. (B). TC-PTP expression is reduced after loss of MYC overexpression. FLL cell lines, derived from MMTV-rtTA/TRE-MYC/BCRHEL/sHEL mice, have a doxycycline-repressible MYC promoter. Three different FLL cell lines (-21, -42, and -44) were treated with doxycycline to turn off MYC overexpression, after which mRNA was harvested and cDNA produced. Quantitative PCR analysis was performed relative to GAPDH for the following genes: MYC (top left panel), ODC (bottom left panel), TC-PTP (top right panel), and CD45 (bottom right panel) expression relative to GAPDH and normalized to untreated cells. Error bars show SD for 2 replicates; n = 4 for MYC and GAPDH, and n = 2 for ODC and CD45.
TC-PTP expression correlates to MYC overexpression. (A) TC-PTP and MYC overexpression in human BL cell lines. Quantitative PCR analysis of MYC ( ) and TC-PTP (
) and TC-PTP ( ) expression relative to GAPDH expression. Shown are wild-type B cells, RAJI and RAMOS BL cell lines, Val and SudHL-6 GC-like DLBCL cell lines, and OCI-Ly3 ABC-like DLBCL cell line. Error bars represent SD for 2 replicates; n = 3 or more. (B). TC-PTP expression is reduced after loss of MYC overexpression. FLL cell lines, derived from MMTV-rtTA/TRE-MYC/BCRHEL/sHEL mice, have a doxycycline-repressible MYC promoter. Three different FLL cell lines (-21, -42, and -44) were treated with doxycycline to turn off MYC overexpression, after which mRNA was harvested and cDNA produced. Quantitative PCR analysis was performed relative to GAPDH for the following genes: MYC (top left panel), ODC (bottom left panel), TC-PTP (top right panel), and CD45 (bottom right panel) expression relative to GAPDH and normalized to untreated cells. Error bars show SD for 2 replicates; n = 4 for MYC and GAPDH, and n = 2 for ODC and CD45.
) expression relative to GAPDH expression. Shown are wild-type B cells, RAJI and RAMOS BL cell lines, Val and SudHL-6 GC-like DLBCL cell lines, and OCI-Ly3 ABC-like DLBCL cell line. Error bars represent SD for 2 replicates; n = 3 or more. (B). TC-PTP expression is reduced after loss of MYC overexpression. FLL cell lines, derived from MMTV-rtTA/TRE-MYC/BCRHEL/sHEL mice, have a doxycycline-repressible MYC promoter. Three different FLL cell lines (-21, -42, and -44) were treated with doxycycline to turn off MYC overexpression, after which mRNA was harvested and cDNA produced. Quantitative PCR analysis was performed relative to GAPDH for the following genes: MYC (top left panel), ODC (bottom left panel), TC-PTP (top right panel), and CD45 (bottom right panel) expression relative to GAPDH and normalized to untreated cells. Error bars show SD for 2 replicates; n = 4 for MYC and GAPDH, and n = 2 for ODC and CD45.
Ectopic TC-PTP overexpression can partially substitute for MYC overexpression
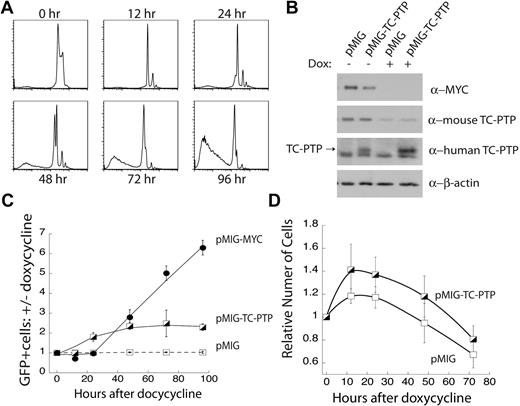
We next sought to test whether enforced overexpression of TC-PTP was able to substitute for MYC-driven proliferation and survival of B-cell lymphoma cells. We found that TC-PTP expression is necessary for the MYC-dependent proliferation of transformed B cells (Figure 2), and we observed a correlation between the overexpression of MYC and TC-PTP in B-cell lymphomas (Figure 4). Together, these data suggest the possibility that TC-PTP is an effector of MYC-dependent proliferation. We used the FLL-44 cell line, where MYC transgene expression can be abolished with the addition of doxycycline. We first used propidium iodide staining of total cellular DNA content to track the cellular changes on MYC repression. Within 12 hours after 5 μg/mL doxycycline treatment, we observed cell-cycle arrest (Figure 5A). This was followed by apoptosis starting at 48 hours, with near-complete cell death by 96 hours (Figure 5A). This result demonstrated that MYC was required for proliferation and survival in FLL-44 cells.
Ectopic expression of TC-PTP partially rescues loss of MYC expression. (A) Doxycycline-induced repression of the MYC transgene results in cell-cycle arrest followed by cell death. FLL-44 cells were treated with 5 μg/mL doxycycline, and changes to total cellular DNA content were tracked by propidium iodide staining. Representative plots are shown; n = 4. (B) Western blot analysis of ectopic TC-PTP expression. FLL-44 cells were transduced with either empty vector pMIG or pMIG-TC-PTP. Transduced and sorted cells were either left untreated or treated for 48 hours with doxycycline to repress the MYC transgene, after which the cells were lysed with 1% TX-100 and lysates were analyzed by Western blot with anti-Myc, anti–TC-PTP (6F3), anti–human TC-PTP (CF4-1D), and anti–β-actin. Blots were stripped in between each Western blot. (C) Ectopic TC-PTP expression is positively selected for following the repression of MYC overexpression. Transduced FLL-44 cells were treated with 5 μg/mL doxycycline, to turn off expression of the MYC transgene, or an equal volume of PBS vehicle as a control. The frequency of GFP+ cells was tracked by FACS analysis, and is presented as the ratio of doxycycline-treated to vehicle-treated cells for each set of transductions. Error bars represent SE; n = 4 or more experiments. (D) Ectopic TC-PTP expression promotes limited proliferation in the absence of the MYC transgene. Proliferation was monitored by cell counting in transduced and sorted FLL-44 cells. Results are normalized to cell counts at 0 hours; n = 4.
Ectopic expression of TC-PTP partially rescues loss of MYC expression. (A) Doxycycline-induced repression of the MYC transgene results in cell-cycle arrest followed by cell death. FLL-44 cells were treated with 5 μg/mL doxycycline, and changes to total cellular DNA content were tracked by propidium iodide staining. Representative plots are shown; n = 4. (B) Western blot analysis of ectopic TC-PTP expression. FLL-44 cells were transduced with either empty vector pMIG or pMIG-TC-PTP. Transduced and sorted cells were either left untreated or treated for 48 hours with doxycycline to repress the MYC transgene, after which the cells were lysed with 1% TX-100 and lysates were analyzed by Western blot with anti-Myc, anti–TC-PTP (6F3), anti–human TC-PTP (CF4-1D), and anti–β-actin. Blots were stripped in between each Western blot. (C) Ectopic TC-PTP expression is positively selected for following the repression of MYC overexpression. Transduced FLL-44 cells were treated with 5 μg/mL doxycycline, to turn off expression of the MYC transgene, or an equal volume of PBS vehicle as a control. The frequency of GFP+ cells was tracked by FACS analysis, and is presented as the ratio of doxycycline-treated to vehicle-treated cells for each set of transductions. Error bars represent SE; n = 4 or more experiments. (D) Ectopic TC-PTP expression promotes limited proliferation in the absence of the MYC transgene. Proliferation was monitored by cell counting in transduced and sorted FLL-44 cells. Results are normalized to cell counts at 0 hours; n = 4.
We next ectopically expressed TC-PTP in FLL-44 cells. These cells were transduced with a retrovirus expressing human TC-PTP and a GFP reporter gene (pMIG-TC-PTP), or with empty vector (pMIG) alone. Western blot analysis of transduced FLL-44 cells sorted for more than 95% GFP expression confirmed that 48 hours of 5 μg/mL doxycycline treatment ablated MYC protein expression and substantially reduced mouse TC-PTP protein expression in the FLL-44 cells (Figure 5B). Furthermore, Western blotting with an antibody specific for human TC-PTP confirmed that human TC-PTP was expressed independently of MYC transgene expression in FLL-44 cells transduced with pMIG-TC-PTP, but not with pMIG alone (Figure 5B).
We tested whether ectopic TC-PTP expression could confer a competitive advantage to FLL-44 cells treated with doxycycline to repress expression of the MYC trangene. FLL-44 cells were singly transduced with pMIG, pMIG-TC-PTP, or pMIG-MYC as a positive control. Initial pMIG-TC-PTP and pMIG-MYC transduction efficiencies were kept below 10%, and pMIG transduction efficiencies below 40%, to allow for an increase in the frequency of GFP. Transduced cells were treated with either 5 μg/mL doxycycline, to repress MYC overexpression, or an equivalent volume of PBS. The frequency of GFP+ cells in the live fraction, as determined by forward and side scattering, was monitored for 96 hours and is presented as the ratio of doxycycline-treated cells versus untreated control cells (Figure 5C). We observed that empty vector, pMIG, alone did not compensate for the repression of MYC expression, as evidenced by the lack of change in the frequency of GFP+ cells over time. In contrast, the frequency of cells expressing pMIG-MYC increased substantially over the duration of the assay, indicating that retroviral expression of MYC could replace MYC transgene expression and rescue the cells from the doxycycline-induced extinction of the transgene (Figure 5C). Furthermore, doxycycline-treated FLL-44 cells transduced with pMIG-MYC continued to persist in culture beyond 96 hours, suggesting that they were resistant to cell death induced by repression of the MYC transgene (data not shown). Cells expressing pMIG-TC-PTP had an intermediate phenotype. The frequency of cells expressing TC-PTP increased over time, more than doubling over the time course (Figure 5C), before the cells in doxycycline-treated wells died (data not shown). When examined in the context of the propidium iodide stains shown in Figure 5A, these results suggest that ectopic TC-PTP expression only provides a competitive advantage in the initial phases of MYC repression, characterized by a block in cellular proliferation, and not in the later phases, characterized by cell death.
In addition, we also directly examined the effect of ectopic TC-PTP expression on cell viability. FLL-44 cells were transduced with either pMIG or pMIG-TC-PTP and sorted to a population of more than 95% GFP+ (transduced) cells. These cells were then passed in triplicate into 24-well plates at 5 × 105 cells per well with an initial dose of 5 μg/mL doxycycline, and proliferation was monitored for 72 hours by counting cells with a hemocytometer. Figure 5D shows that FLL-44 cells expressing pMIG-TC-PTP expand more rapidly than FLL-44 cells expressing the vector control (pMIG) initially, but both populations decline by 72 hours. These results corroborate the in vitro competition results shown in Figure 5C. Taken as a whole, these data indicate that TC-PTP expression is selected on the repression of MYC overexpression, but that it is not sufficient to replace MYC expression by itself in transformed B cells. These results cannot differentiate between the possibilities that TC-PTP is acting downstream of MYC to promote proliferation or that TC-PTP expression can maintain cell viability in the absence of MYC transgene expression.
Discussion
The results we present here identify TC-PTP as a tumor-promoting gene in MYC-driven B-cell lymphomas. We found that TC-PTP is overexpressed in all mouse and human B-cell lymphomas we examined. Knockdown of TC-PTP in mouse B-cell lymphomas results in decreased cell viability in vitro because of a failure to transition from the G1 to S phase of the cell cycle, resulting in reduced proliferation. In addition, MYC and TC-PTP expression levels correlate with each other, suggesting that MYC may regulate TC-PTP expression. Finally, we show that ectopic expression of TC-PTP can partially substitute for the loss of MYC transgene expression in transformed B cells.
In light of these data, we propose a preliminary model in which MYC overexpression in B-cell lymphomas results in TC-PTP overexpression. This in turn facilitates transition from the G1 to S phase of the cell cycle in transformed B cells. Our data are in agreement with a previous report describing that mouse embryonic fibroblasts (MEFs) from TC-PTP−/− mice proliferate slower than wild-type MEFs because of delayed progression through the G1 phase.25 However, a recent report identifies TC-PTP as a negative regulator of protein tyrosine kinase signaling pathways that promotes bypass of S-phase cell-cycle checkpoints after DNA replication stress in MEFs.26 These results from MEFs imply that TC-PTP can either promote or disrupt cellular proliferation, suggesting different functions for TC-PTP in physiologic conditions or in cells subjected to genotoxic stress. Generally, TC-PTP is thought to negatively regulate proliferation in hematopoietic cells.27 This occurs through suppression of Jak and STAT signaling downstream of cytokine receptors, which are required for proliferation in these cells.11-14 Therefore, one would expect that TC-PTP expression would suppress proliferation in lymphoid tumors; however, we find that this is not the case. One insight may be related to our previous observation that MYC overexpression is able to break tolerance in B cells because these cells lose their dependence on cytokine signaling.22 Therefore, we propose that overexpression of MYC both leads to increased levels of TC-PTP expression and allows TC-PTP to function in some other capacity, independent of inhibiting cytokine-dependent Jak and STAT signaling. TC-PTP can interact with several other signaling molecules (“Introduction”); as such, there are many possible pathways that TC-PTP could modulate in the cell. We are currently exploring this issue in more detail.
Of interest is the correlation between MYC and TC-PTP expression. Our experiments cannot differentiate between the possibilities that MYC is directly regulating the transcription of TC-PTP or that TC-PTP expression is being indirectly regulated by MYC as part of global alterations in the cell-cycle machinery after the changes in MYC expression. Previous studies have demonstrated that TC-PTP mRNA expression levels are modulated by the cell cycle, with the highest levels occurring in the G1 phase and the lowest levels during S phase.28 These results also suggest a correlation between MYC and TC-PTP expression but do not indicate whether MYC can directly regulate TC-PTP expression. However, promoter analysis of TC-PTP revealed 2 c-myc–responsive domains,29 suggesting the possibility that MYC directly regulates TC-PTP transcription. Yet, because of the pleiotropic effect of MYC on the transcriptome, it is not yet possible to determine whether MYC is having an indirect effect on TC-PTP expression.
A recent report found that TC-PTP was overexpressed in certain DLBCL cell lines matching an ABC-like phenotype.20 The authors proposed that TC-PTP overexpression inhibited IL-4–induced STAT6 signaling, although they did not evaluate the effect of TC-PTP expression on tumor maintenance. Their model would be in line with previous results indicating that TC-PTP is a negative regulator of Jak and STAT signaling downstream of cytokine receptors in primary hematopoietic cells.11-14 It is not clear whether a similar mechanism is in place in models of BL or other MYC-driven tumors. Moreover, MYC is not commonly involved in the primary transformation of DLBCL, but it can often be overexpressed as a result of a secondary genetic lesion.8 The expression of MYC in these tumors often correlates to more aggressive forms of DLBCL, such as ABC-DLBCLs.30,31 There may be a correlation between the amount of MYC expression and levels of TC-PTP expression. Our data in Figure 4A seem to support this notion, where we found higher levels of both MYC and TC-PTP mRNA expression in the ABC-DLBCL cell line, OCI-Ly3.
The partial rescue of MYC repression by ectopic TC-PTP overexpression in transformed B cells raises some questions. We observed that TC-PTP expression alone was unable to compensate for the loss of the MYC overexpression. The effects of MYC overexpression are notoriously pleiotropic. Therefore, if TC-PTP is directly downstream of MYC, it is probable that it is only one of the effectors downstream of MYC that may regulate cellular proliferation. Thus, TC-PTP expression alone would not be expected to fully substitute for the loss of MYC overexpression. It is also possible that a rare population of FLL-44 cells may harbor additional mutations, which, in combination with ectopic TC-PTP expression, can overcome the repression of the MYC transgene. Still, the possibility that TC-PTP is used by MYC to drive cellular proliferation is an exciting notion that may have implications in other types of MYC-driven tumors.
Our results point to the possibility that TC-PTP could be a therapeutic target in the treatment of B-cell lymphomas. Recently, considerable effort has gone into developing inhibitors to PTP1B, a close relative of TC-PTP, for the treatment of diabetes and obesity.32 One problem with developing inhibitors to PTP1B is the potential for cross-reactivity with the highly homologous TC-PTP.32 We propose that such inhibitors should be tested for the treatment of MYC-driven B-cell lymphomas.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dianne Ashby and the Biological Resource Center at National Jewish Medical and Research Center for care and maintenance of the mice used in this study and Eiko Browning for editing the manuscript.
R.M.Y. was supported by a Translational Research Award from the Leukemia & Lymphoma Society. Y.R. was supported by the National Cancer Institute (US Public Health Service grant CA-117802) and the Leukemia & Lymphoma Society (Translational Research Award).
National Institutes of Health
Authorship
Contribution: R.M.Y. designed and performed research, contributed vital new reagents, collected data, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; A.P. performed research; and Y.R. designed and performed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yosef Refaeli, Departments of Dermatology and Immunology, Charles C. Gates Regenerative Medicine and Stem Cell Program, University of Colorado Denver, Mail Stop 8320, P18-8127, 12800 E 19th Ave, Aurora, CO 80045; e-mail: Yosef.Refaeli@ucdenver.edu.