Abstract
Abstract 134
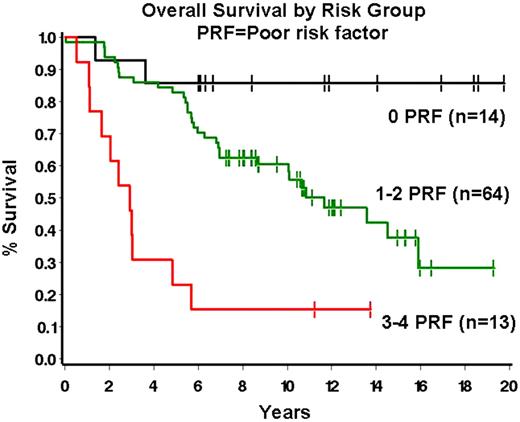
The tumor microenvironment has been recognized as important in the pathobiology of follicular lymphoma (FL). Indeed, the role of extrafollicular lymphoma-associated macrophages (ef-LAMs) and FOXP3+ regulatory T-cells (Tregs) as predictors of overall survival in FL was previously reported by our group and others. We sought to further evaluate the role of specific T-cell subsets in our patient series. To this end we enumerated PD-1+ follicular helper T-cells (Tfhs), a potentially important regulator of immune response, in the tumor microenvironment in a series of 91 newly diagnosed FLs. Initial biopsies from 91 FL patients diagnosed between 9/1/85 and 12/13/02 were evaluated. FL grade 3B cases were excluded. Clinical data was obtained for gender, age, FLIPI risk group, presence of bulky disease, presence of B-symptoms and overall survival (OS). Immunohistochemical staining for PD-1 was performed on tissue microarray sections and the mean number of follicular PD-1+ cells per 9 high power fields (1000x, 3 follicles with 3 fields per follicle) was quantified. Recursive partitioning analysis was then performed to determine an optimal break-point for PD-1. BCL2 expression, ef-LAMs and FOXP3+ Tregs were evaluated as reported by us previously. The Cox proportional hazards model was used to determine significant univariate and multivariate prognostic risk factors, using P≤ 0.10 as the criterion for inclusion and retention in the multivariate analysis. The Kaplan-Meier method was used to summarize OS. 54% of patients were male with a median age at diagnosis of 58 years (range 24–89 years). The distribution of FLIPI scores was 35% low, 27% intermediate, and 37% high. The number of PD-1+ Tfhs was variable (median number of PD-1+ cells = 35.6, range 4.4 – 91.2). Number of PD-1+ cells correlated with number of Tregs (P=.01) and marginally with lack of BCL2 (P=.07) but not with other clinical or pathologic variables. On univariate analysis, age ≥ 55 (P=0.004), increasing FLIPI risk group (P=0.03), bulky disease (>10 cm; P=0.05), lack of BCL2 (P=.01), high ef-LAMs (>16.8/hpf, P=.003), and high PD-1+ cells (>35.6/hpf, P=0.10) were all associated with decreased OS. On multivariate analysis, independent poor prognostic risk factors were: age ≥55 (hazard ratio[HR]=2.76, 95% confidence interval [CI] = 1.33–5.76, P=.007), high ef-LAMs (HR=2.13, 95%CI = 1.11–4.07, P=.02) bulky disease (HR=2.10, 95% CI 0.91–4.83, P=.08), and high PD-1+ cells (HR 2.04, 95% CI=1.11–3.75, P=.02). A prognostic model based on the number of risk factors present can be constructed with patients having 0, 1-2 or 3-4 risk factors defining 3 distinct prognostic groups (Figure 1). In summary, cellular constituents of the FL microenvironment play an important role in the biology of FL. A combination of standard clinical and biologic factors that include Tfhs and ef-LAMs appear to predict OS in FL. Given the putative role for Tfhs in down-regulating the immune response, these data support strategies aimed at modulating Tfh function in FL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.