Abstract 133
PMBCL represents a unique lymphoma type in the WHO classification and shares overlapping histological and gene expression features with both diffuse large B-cell (DLBCL) and classical Hodgkin lymphoma. Similar to DLBCL, PMBCL may show loss of expression of the major histocompatibility class II (MHC II) antigens, which correlates with inferior patient survival (Rimsza, Blood 2006). MHC II proteins are expressed on antigen-presenting cells and are important in eliciting immune responses. In DLBCL, decreased expression of HLA-DR is related to loss of immunosurveillence. Our aim was to validate HLA-DR as an important prognostic biomarker in PMBCL and correlate it with immune response in a cohort of patients treated with multi-agent chemotherapy.
A tissue microarray block was built with duplicate 0.6mm cores of paraffin embedded diagnostic biopsies from 103 patients treated with CHOP/CHOP-like chemotherapy at the BC Cancer Agency (1980-2005). Standard immunohistochemistry was performed with CD20, HLA-DR (IgG2b), CD3, CD4, CD8, CD57, CD68 and cytotoxic markers (TIA1 & Granzyme B). HLA-DR expression was evaluated using a histoscore (intensity of staining and % of positive malignant cells) and correlated with the content (%) of different non-neoplastic infiltrating T-cell subsets and macrophages. Univariate & multivariate analyses were used to characterize overall survival (OS) and progression free survival (PFS).
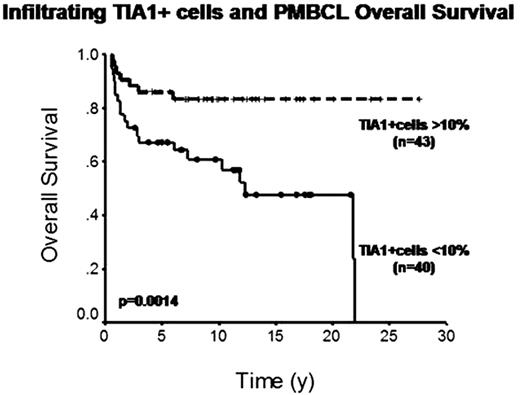
The median follow-up of living patients was 10 years. The IPI predicted OS (p=0.042) but not PFS. Of the 92 cases with interpretable staining, 32 were positive for HLA-DR and 60 negative, with 10-year OS of 86% vs. 61% (p=0.006) and 2-year PFS of 78% vs. 53% (p=0.018), respectively. A Cox multivariate model established both HLA-DR status and IPI as independent predictors of OS (RR=0.3, 95%CI=0.12–0.75, p=0.01; RR=2.9, 95%CI=1.2–6.9, p=0.06, respectively). HLA-DR expression correlated significantly with increased content of all analyzed T cell markers, especially CD3, CD8 and TIA1 (x2, p<0.001), but not with macrophage content (CD68). Of all non-malignant markers, only TIA1+ cell content significantly correlated with survival. Of the 83 cases with interpretable staining, 43 had more than 10% of infiltrating TIA1+ cells and 40 had less, with 10-year OS of 83% vs. 57% (p=0.0014) and 2-year PFS of 76% vs. 50% (p=0.014), respectively. In multivariate analysis, including IPI and HLA-DR and cytotoxic markers, only TIA1 status was an independent predictor of OS (RR=0.3, 95%CI=0.11–0.63, p=0.003).
We validated the negative prognostic importance of loss of HLA-DR expression by neoplastic B cells in PMBCL patients treated with multi-agent chemotherapy in a single institution experience. Loss of HLA-DR expression correlated with decreased numbers of infiltrating benign T cell populations, especially CD8+ and TIA1+ cells, where decreased cytotoxic T cell content correlated independently with inferior survival. This study shows loss of immunogenicity and immunosurveillance are key mechanisms in the response to treatment of PMBCL patients and suggests that specific therapies focused on this pathway may benefit patients.
Connors:Roche Canada (F Hoffmann-La Roche): Research Funding. Gascoyne:Roche Canada, Genentech, Lilly, Millennium : Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal