Abstract
Recombinant FVIII formulated in PEG-ylated liposomes (rFVIII-PEG-Lip) was reported to increase the bleed-free days from 7 to 13 days (at 35 IU/kg rFVIII) in severe hemophilia A patients. To understand the underlying mechanism, we sought to recapitulate its efficacy in hemophilia A mice. Animals treated with rFVIII-PEG-Lip achieved approximately 30% higher survival relative to rFVIII after tail vein transection inflicted 24 hours after dosing. The efficacy of rFVIII-PEG-Lip represents an approximately 2.5-fold higher “apparent” FVIII activity, which is not accounted for by its modestly increased (13%) half-life. The enhanced efficacy requires complex formation between rFVIII and PEG-Lip before the administration. Furthermore, PEG-Lip associates with the majority of platelets and monocytes in vivo, and results in increased P-selectin surface expression on platelets in response to collagen. Rotational thromboelastometry (ROTEM) analysis of whole blood from rFVIII-PEG-Lip–treated animals at 5 minutes up to 72 hours after dosing recapitulated the 2- to 3-fold higher apparent FVIII activity. The enhanced procoagulant activity is fully retained in plasma unless microparticles are removed by ultracentrifugation. Taken together, the efficacy of rFVIII-PEG-Lip is mediated mainly by its sensitization of platelets and the generation of procoagulant microparticles that may express sustained high-affinity receptors for FVIII.
Introduction
rFVIII-PEG-Lip is a noncovalent complex of recombinant FVIII (rFVIII) formulated in 9% PEGylated liposomes (PEG-Lips). PEG-Lips are composed of POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine) and MPEG-2000-DSPE (N-[Carbonyl-methoxy-poly-ethylene-glycol-2000]-1,2-distearoyl-sn-glycero-3-phosphoethanolamine) at a 9:1 weight-volume or 97:3 molar ratio.1 Five clinical studies have been completed in which a total of 80 patients have received treatment of rFVIII-PEG-Lip. In the Russia I study, rFVIII-PEG-Lip was reported to double the bleed-free days in severe hemophilia A patients from 7 to 13 days (35 IU/kg) and 6 to 11 days (25 IU/kg), compared with the corresponding doses of rFVIII.2 The Russia II study further showed a PEG-Lip–dependent dose response in efficacy. At a fixed dose of 35 IU/kg rFVIII, the number of bleed-free days increased proportionally to increasing concentrations of PEG-Lip.3 However, the plasma pharmacokinetics (PK) of rFVIII, as determined by both activated partial thromboplastin time (aPTT) and chromogenic-based assay (Coatest) in a more recent US phase 1 trial, were comparable in patients who were treated with rFVIII and rFVIII-PEG-Lip.4 Thus, the prolonged efficacy of rFVIII-PEG-Lip observed in earlier clinical studies did not seem to be the result of prolonged plasma PK of rFVIII.
In response to vascular injury, platelets quickly adhere to subendothelial matrix and become activated to form a hemostatic plug at the injury site.5 Activated platelets provide a procoagulant phosphatidylserine surface for the assembly of Xase and prothrombinase complexes to generate a thrombin burst, resulting in fibrin deposition that stabilizes the hemostatic plug.6,7 In addition, P-selectin exposed on activated platelets rapidly recruits TF-bearing microparticles via its interaction with P-selectin glycoprotein ligand-1 during the initial phase of thrombus formation.8 Platelet-derived microparticles have 50- to 100-fold higher specific procoagulant activity than activated platelets.9 Microparticles not only generally provide a phosphatidylserine-enriched surface for the assembly of Xase and prothrombinase, but also express sustained high-affinity receptors for FVIII to promote a thrombin burst on their membranes.10,11 Liposomes have been shown to interact rapidly with blood cells such as platelets via lipid exchange, membrane fusion,12 or sequestration.13,14 Therefore, it was hypothesized that via association with PEG-Lips, a small fraction of rFVIII was deposited to the cellular and subcellular compartments of blood including microparticles, where FVIII would be protected for a prolonged period of time to effectively promote clot formation, and yet evade detection in plasma-based assays.
To understand the pharmacologic mechanism of action of rFVIII-PEG-Lip, we first determined its enhanced efficacy in the tail vein transection (TVT) bleeding model of hemophilia A (HemA) mice. The in vivo efficacy of rFVIII-PEG-Lip was further confirmed and dissected ex vivo by quantitative rotational thromboelastometry (ROTEM). We also examined the interaction of rFVIII-PEG-Lip with blood cells and its effects on the prohemostatic functions of platelets. Our results suggest that the efficacy of rFVIII-PEG-Lip is mediated by multiple pathways mainly through its sensitization of platelets and induction of procoagulant microparticles that sequester FVIII.
Methods
Dose preparation
The rFVIII dose was prepared by diluting 1000 IU/mL rFVIII-FS (Bayer HealthCare LLC) in formulation buffer (2.2% glycine, 1% sucrose, 2.5 mM CaCl2, 30 mM NaCl, 20 mM histidine, and 80 ppm Tween 80) to the final concentration of 20 IU/mL. For rFVIII-PEG-Lip, 1000 IU/mL rFVIII-FS was diluted in 9% of PEG-Lip (Bayer HealthCare LLC) to 20 IU/mL rFVIII and 8.82% PEG-Liposome. Further dilution of reconstituted rFVIII-PEG-Lip was carried out in rFVIII-FS formulation buffer supplemented with 5% albumin. For the PK study with 125I-labeled FVIII, 1.5 IU (2 × 105 cpm) 125I-FVIII was reconstituted in buffer or 9% of PEG-Lip (the final concentration of PEG-Lip was 8.64%). The dosing solutions were inverted gently for 5 minutes at room temperature and used within 2 hours after reconstitution.
Animal procedures
HemA mice with C57Bl/6 background homozygous for FVIII knockout (exon 16)15 were obtained from Haig Kazazian (University of Pennsylvania) and bred on site at Bayer HealthCare LLC. For tail vein transection studies, the littermates of 8- to 10-week-old male HemA mice were assigned to different treatment groups blinded to the operator, and all treatment groups had comparable representations of the weight distribution of the animals. Mice were dosed by tail vein injection at 24 hours before the injury. Mice were anesthetized with a cocktail containing 50 μg/kg ketamine and 1 mg/kg medetomidine by intraperitoneal injection. When mice reached an adequate anesthetic depth showing no response to toe pinch, the tail was marked at an internal diameter of 2.7 mm of a French catheter. Then the animals received 1 mg/kg atipamezole by intraperitoneal injection to reverse the anesthetic effect of medetomidine in approximately 30 minutes. The tail vein at the mark was immediately transected with a no. 11 straight-blade scalpel, and a timer was started. The tail was then submerged into saline (prewarmed at 37°C in a water bath for 30 minutes). When the saline became too opaque to visualize, it was replaced with a new tube until the tail stopped bleeding. The bleeding time was recorded for the purpose of quality control. The animals were excluded from the study if the bleeding time was out of the normal range of 30 to 120 seconds. The mouse was then returned to its individual cage with white paper bedding (Versi-Dri), placed on top of a 4 × 8-inch heating pad. In the following 11 hours and then overnight at 24 hours, several qualitative end points were monitored and recorded hourly, including (1) rebleeding: from no (−) to mild (+), moderate (++), or severe (+++); (2) attitude: from bright, alert, responsive (B) to quiet, alert, responsive (Q) or nonalert, but responsive (R); and (3) activity: from eating/drinking (E/D) to ambulating (A), sleeping (S), or resting (R). An animal was deemed moribund and requiring humane killing if it was rated +++/Q (or R)/R (bleeding/attitude/activity) for 2 consecutive hours, and it failed to turn over when laid on its back in 3 consecutive tests. All mice were killed at 24 hours after tail vein transection. All study protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Bayer HealthCare LLC under the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.16
Biacore analysis
The procedure was reported previously1 with the following modifications. HSA (Plasbumin; Bayer HealthCare LLC; 6.6 μg/mL) and rFVIII-FS (Bayer HealthCare LLC; 4.4 μg/mL) were immobilized at approximately 1200 RU coating density using the adaptive immobilization program in the Biacore 3000 software. The instrument flow rate was set to 15 μL per minute with a 5-minute association phase followed by 5-minute dissociation. The liposome samples were diluted in 50 mM citrate buffer (pH 7.0) to a final concentration of 0.10% (14.6 nM liposome particle suspension). The flow rate was changed to 30 μL per minute during regeneration. The regeneration buffer contained 20 mM n-Octylglucopyranoside, 68.5 mM NaCl, 1.9 mM KCl, 0.2 mM Na2PO4, 12 mM Hepes, pH 7.4, 0.5 mM CaCl2, and 0.5 mM MgCl2. A 15-μL pulse of the regeneration buffer was used between liposome injections. A maximum of 6 injections per chip was possible before surface stability was compromised; the first injection was eliminated from calculations due to high variability. Data were analyzed by calculating the difference in maximal binding between the HSA and rFVIII flow cells at 290 seconds after injection. This time was selected to avoid the data fluctuations found at the end of sample injections (300 seconds). The maximal binding value (RU) for rFVIII was subtracted from HSA and divided by rFVIII maximal binding to arrive at a percentage difference measurement.
Coatest assay
FVIII activity in HemA mouse plasma was measured by the Coatest VIII:C/4 assay from Chromogenix (DiPharma), following the manufacturer's instructions. The standard curve was generated using rFVIII-FS serially diluted from 100 mU/mL to 0.78 mU/mL in buffer containing naive HemA mouse plasma.
125I-labeling of rFVIII
rFVIII-FS was reconstituted in sterile water for injection and dialyzed at 4°C overnight against a buffer containing 20 mM HEPES (pH 8.0); 200 mM NaCl; 0.1% Tween-80; 1% sucrose; and 2.5 mM CaCl2 to get rid of free amine residues. The dialyzed rFVIII was concentrated by centrifugation through Amicon Ultra (10000 molecular weight cutoff) to a final concentration of approximately 6.3 U/μL as determined by Coatest. rFVIII was then labeled with the Bolton-Hunter reagent according to the method described previously.17 Briefly, 31 μL rFVIII (200 IU) was combined with 19 μL 0.1 M borate (pH 8.5). The mixture was then added to the predried 125I-labeled Bolton-Hunter reagent (1000 μCi [37.0 MBq]; Amersham), followed by further incubation for 45 minutes on ice. The 125I-labeled FVIII was separated from Bolton-Hunter reagent using a Zeba Desalt Spin Column, and was collected into 50 μL of the formulation buffer with an estimated specific radioactivity of approximately 0.6 μCi/μg [22.2 kBq μg]. The 125I-rFVIII retained approximately 96% activity compared with unlabeled rFVIII as determined by Coatest.
Pharmacokinetics
HemA mice were injected with approximately 100 IU/kg rFVIII or rFVIII-PEG-Lip via tail vein. At 5 minutes, and 4, 8, 16, 24, 32, and 48 hours after dosing, 5 mice from each treatment group were killed by CO2, followed immediately by blood collection from the vena cava. Plasma FVIII activities were then determined by Coatest. The plasma FVIII activity versus time was plotted and fitted by a noncompartmental model with sparse sampling and linear trapezoidal (linear/log interpolation) method in WinNonLin 5.0.1 (Pharsight). For the PK study with 125I-labeled FVIII, HemA mice received 1.5 IU (2 × 105 cpm) 125I-FVIII reconstituted in buffer or PEG-Lip. At each specified time point, 5 mice from each treatment group were killed for blood collection via the vena cava. Whole blood (150 μL) was used for gamma counting, another 150 μL whole blood was separated into plasma and cell pellet by centrifugation at 1500g for 5 minutes before gamma counting. The 125I-FVIII count versus time was fitted by 2-compartment intravenous-bolus model with first-order elimination and “Gauss-Newton” (Levenberg and Hartley) minimization method to deduce the PK parameters.
ROTEM
Blood was collected from the vena cava of treated HemA mice and mixed with 4% sodium citrate at a 9:1 ratio. Three hundred microliters of citrated whole blood was added into a prewarmed ROTEM cup and the clotting analysis was initiated by the addition of 20 μL 0.2 M CaCl2 within 5 minutes from blood collection. To obtain readings in the linear range of the assay, the samples from treated animals at 5 minutes and 4 hours after dosing were diluted in freshly collected blood from naive HemA mice and then used for ROTEM assays.
Preparation of FITC-labeled PEG-Liposome
Dry lipids POPC (Genzyme), MPEG-2000-DSPE (Genzyme), and FITC-MPEG-2000-DSPE (Avanti Polar Lipids) were weighed and mixed at 90:9:1 weight-volume by dissolving in chloroform (500 μL CHCl3 for ≤ 100 μg lipids). Chloroform was removed using a gaseous nitrogen stream followed by spinning in a SpeedVac (Savant) for 1.5 hours at room temperature. The dry lipid mixture was resuspended to 20 mM in 50 mM citrate buffer (pH 7.0) by repeated pipetting. The lipid suspension was sonicated briefly on ice using a Sonic Dismembrator Model 100 (Fisher Scientific) to break up any large aggregates and to induce liposome formation. The liposome suspension was then extruded 5 times through each of the filters in the following order: 0.2-μm, 0.1-μm, and 0.08-μm filters on a Mini-Extruder (Avanti Polar Lipids). FITC-PEG-Liposome preparations were stored in the dark at 4°C.
Flow cytometry
For evaluating the blood cell association with PEG-Lip ex vivo, HemA mice received tail vein injection of 50 μL 9% PEG-Lip or FITC-PEG-Lip with or without 0.2 IU rFVIII, and 50 μL blood was collected from the retro-orbital venous plexus into an equal volume of 0.8% (wt/vol) sodium citrate at various times after dosing. Citrated whole blood was immediately diluted in modified Tyrode buffer (20 mM HEPES, 5 mM d-glucose, 100 ng/mL PGE1, and 0.35% BSA; Sigma-Aldrich), incubated with monoclonal antibodies specific for surface markers of platelet (CD41-MWReg30 and CD61-2C9.G2; BD Bioscience), or isotype controls (R3-34-IgG1,k; A19-3- Hamster IgG1; BD Bioscience) for 20 minutes at room temperature, fixed with 1% of paraformaldehyde, and analyzed on a FACScan or LSRII flow cytometer (Becton Dickinson Immunocytometry Systems) using CellQuest Pro, FACSDiva (BD Bioscience), and FlowJo (TreeStar) software. All acquisition channels were set on logarithmic scale, except for the leukocyte gates, which were acquired with linear FSC and SSC to maximize cell type–specific size and granular content of each subset. For evaluating the platelet activation ex vivo, citrated whole blood from treated HemA mice was activated with 1 μg/mL collagen (equine type I; Chrono-Log) for 10 minutes at 37°C. The surface markers for platelets (CD41+) and activated platelets (CD41+ and CD62P+) were identified using respective fluorophore-conjugated monoclonal antibodies. To determine the levels of platelet activation in vivo, citrated whole blood from treated animals was immediately stained with CD41- and CD62P-specific antibodies for 10 minutes at room temperature.
Statistics
The survival curves and log-rank test, 2-tailed t test, Kruskal-Wallis test, and Mann-Whitney U test were generated and performed in Prism 4 (GraphPad Inc).
Results
rFVIII-PEG-Lip demonstrates enhanced efficacy, which requires the specific binding of PEG-Liposome to rFVIII, compared with rFVIII in the tail vein transection bleeding model of HemA mice
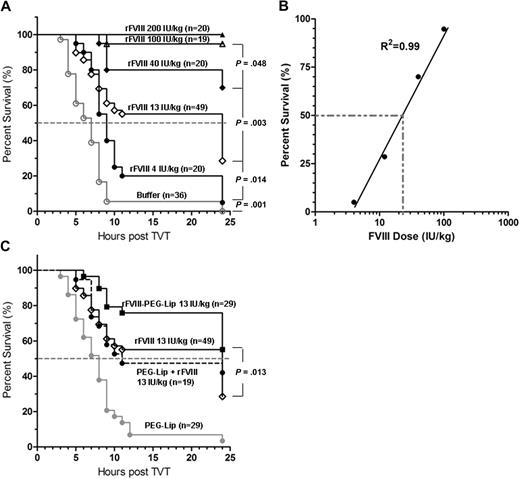
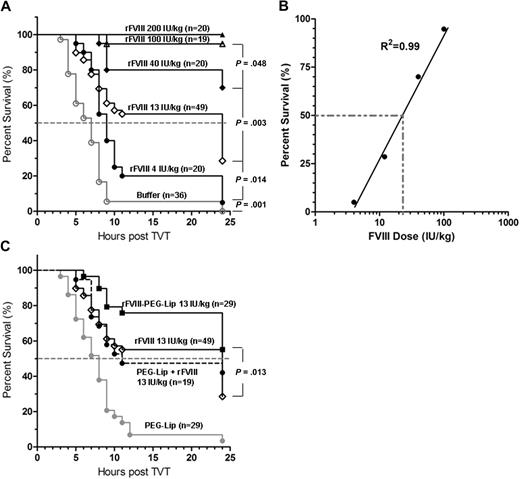
To determine whether we could recapitulate the clinically observed efficacy of rFVIII-PEG-Lip in an animal model, we used a modified tail vein transection (TVT) model of HemA mice originally developed by Broze et al.18 TVT mimics the spontaneous venous bleeding characteristic of severe hemophilic patients and is highly sensitive to the treatment of rFVIII. As shown in Figure 1A-B, the percentage of survival in HemA mice (n = 19-49/dose) after the transection of 1 lateral tail vein correlates linearly with the increasing log[rFVIII dose] from 4 to 100 IU/kg (R2 = 0.99) administered at 24 hours before the injury. The rFVIII dose needed to achieve 50% survival (ED50) was approximately 21 IU/kg (Figure 1B). TVT can distinguish the difference in survival resulting from approximately 2.5- to 3-fold differences in rFVIII dosing with statistical significance (P < .05, log-rank test).
Enhanced efficacy of rFVIII-PEG-Lip after tail vein transection in HemA mice. (A) HemA mice received tail vein injections of 50 μL rFVIII formulation buffer (excipient) or rFVIII at a dose of 4, 13, 40, 100, and 200 IU/kg. Twenty-four hours later, 1 of the lateral tail veins was transected, and the percentage of mice that survived the injury was monitored hourly for the first 11 hours and then at 24 hours. (B) Plot of the rFVIII dose response in percentage of survival after tail vein transaction as shown in panel A. (C) Comparison of the survival curves of HemA mice treated with 13 IU/kg rFVIII formulated in buffer, or PEG-Liposome, or sequential injection of PEG-Liposome followed by rFVIII, or 50 μL PEG-Liposome (excipient). Two-tailed P values were determined by the log-rank test on the respective survival curves.
Enhanced efficacy of rFVIII-PEG-Lip after tail vein transection in HemA mice. (A) HemA mice received tail vein injections of 50 μL rFVIII formulation buffer (excipient) or rFVIII at a dose of 4, 13, 40, 100, and 200 IU/kg. Twenty-four hours later, 1 of the lateral tail veins was transected, and the percentage of mice that survived the injury was monitored hourly for the first 11 hours and then at 24 hours. (B) Plot of the rFVIII dose response in percentage of survival after tail vein transaction as shown in panel A. (C) Comparison of the survival curves of HemA mice treated with 13 IU/kg rFVIII formulated in buffer, or PEG-Liposome, or sequential injection of PEG-Liposome followed by rFVIII, or 50 μL PEG-Liposome (excipient). Two-tailed P values were determined by the log-rank test on the respective survival curves.
In HemA mice (n = 29) treated with 13 IU/kg rFVIII-PEG-Lip, the survival rate was significantly better than that in animals (n = 49) treated with 13 IU/kg rFVIII alone (55% vs 29%, P = .013; Figure 1C). The difference in the survival rate is estimated to be equivalent to an approximately 2.5-fold difference in rFVIII dose based on the dose-response curve (Figure 1B).
To test whether the binding of PEG-Liposome to rFVIII is required for the enhanced efficacy in vivo, HemA mice (n = 19) were injected with PEG-Liposome first followed by the injection of rFVIII separately within 1 to 2 minutes in the tail vein. The resulting survival curve no longer differed from that of rFVIII alone (n = 49; Figure 1C), indicating that the enhanced efficacy of rFVIII-PEG-Lip depends on the preformation ex vivo of a complex of rFVIII and PEG-Liposome.
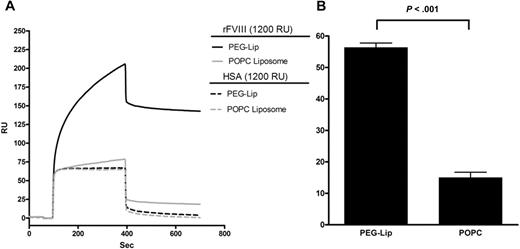
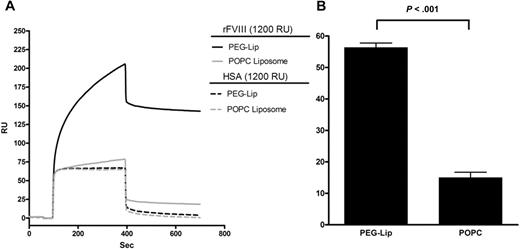
Consistent with this notion, the specific binding of PEG-Liposome to rFVIII was confirmed by surface plasmon resonance measurement (Figure 2A), as previously reported.1 The maximal binding of PEG-Liposome to immobilized rFVIII was 56% (± 1.4%) higher than its nonspecific binding to immobilized human serum albumin (HSA; Figure 2B). By contrast, the lipemic control POPC-Liposome bound to rFVIII only 15% (± 1.7%) higher than that to HSA (P < .001 vs PEG-Lip, 2-tailed t test; Figure 2A-B), and did not enhance the efficacy of rFVIII when coadministered with rFVIII (data not shown).
Specific binding of PEG-Liposome to rFVIII by Biacore analysis. (A) A representative Biacore binding profile of the flow-through PEG-Liposome and the negative control POPC-Liposome to rFVIII or HSA immobilized on a CM5 chip at a coating density of 1200 RU. (B) The percentage of difference in the maximal binding of the respective liposome to immobilized rFVIII or HSA. Results presented are mean ± SD from 4 Biacore measurements on separately derivatized CM5 chips. The binding of PEG-Liposome to rFVIII is significantly different from that by POPC-Liposome (P < .001, 2-tailed t test).
Specific binding of PEG-Liposome to rFVIII by Biacore analysis. (A) A representative Biacore binding profile of the flow-through PEG-Liposome and the negative control POPC-Liposome to rFVIII or HSA immobilized on a CM5 chip at a coating density of 1200 RU. (B) The percentage of difference in the maximal binding of the respective liposome to immobilized rFVIII or HSA. Results presented are mean ± SD from 4 Biacore measurements on separately derivatized CM5 chips. The binding of PEG-Liposome to rFVIII is significantly different from that by POPC-Liposome (P < .001, 2-tailed t test).
PEG-Liposome only modestly prolongs the circulating half-life of rFVIII in HemA mice
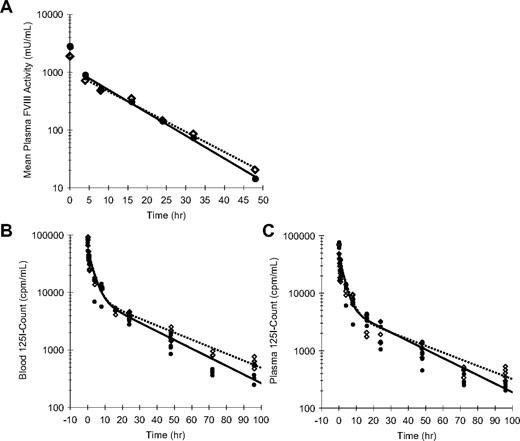
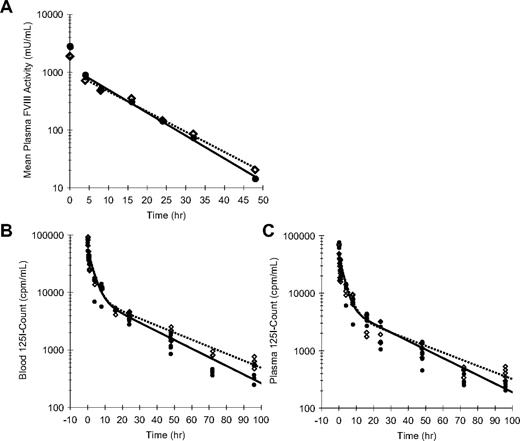
To assess whether enhanced efficacy of rFVIII-PEG-Lip could be accounted for by an extended PK, we measured the decay of plasma FVIII activity in rFVIII-PEG-Lip– or rFVIII-treated HemA mice (n = 5 mice/time point per treatment; Figure 3A). The noncompartment modeling in WinNonLin estimated only a modest 13% increase in the terminal half-life (t1/2) of rFVIII-PEG-Lip from 7.8 (± 0.4) hours (n = 4 studies) to 8.8 (± 0.2) hours (n = 2 studies; P = .024, 2-tailed t test), and a 31% increase in the mean residence time (MRT) from 8.7 (± 1.1) hours to 11.4 (± 0.6) hours (P = .039). None of the other PK parameters was significantly different between rFVIII-PEG-Lip and rFVIII.
Pharmacokinetics of rFVIII and rFVIII-PEG-Lip in HemA mice. (A) A representative plasma PK profile. In this experiment, HemA mice received either 116 IU/kg rFVIII (●) or 70 IU/kg rFVIII-PEG-Lip ( ). Citrated blood was collected at 5 minutes, and 4, 8, 16, 24, 32, and 48 hours after tail vein injection. Plasma FVIII activities were measured by Coatest. Results presented are mean from 5 mice/treatment at each time point. The decay curves were fitted by noncompartmental model with sparse sampling in WinNonLin. (B-C) HemA mice were dosed with 1.5 IU (2 × 105 cpm)/mouse of 125I-rFVIII reconstituted in buffer (●) or PEG-Liposome (
). Citrated blood was collected at 5 minutes, and 4, 8, 16, 24, 32, and 48 hours after tail vein injection. Plasma FVIII activities were measured by Coatest. Results presented are mean from 5 mice/treatment at each time point. The decay curves were fitted by noncompartmental model with sparse sampling in WinNonLin. (B-C) HemA mice were dosed with 1.5 IU (2 × 105 cpm)/mouse of 125I-rFVIII reconstituted in buffer (●) or PEG-Liposome ( ). The decay of 125I-FVIII in both whole blood (B) and platelet-poor plasma (C) was monitored at 5 minutes, 30 minutes, and 1, 4, 8, 16, 24, 48, 72, and 96 hours. Results presented are individual gamma count from 5 mice at each time point for each treatment. The decay curves were fitted using 2-compartmental model in WinNonLin.
). The decay of 125I-FVIII in both whole blood (B) and platelet-poor plasma (C) was monitored at 5 minutes, 30 minutes, and 1, 4, 8, 16, 24, 48, 72, and 96 hours. Results presented are individual gamma count from 5 mice at each time point for each treatment. The decay curves were fitted using 2-compartmental model in WinNonLin.
Pharmacokinetics of rFVIII and rFVIII-PEG-Lip in HemA mice. (A) A representative plasma PK profile. In this experiment, HemA mice received either 116 IU/kg rFVIII (●) or 70 IU/kg rFVIII-PEG-Lip ( ). Citrated blood was collected at 5 minutes, and 4, 8, 16, 24, 32, and 48 hours after tail vein injection. Plasma FVIII activities were measured by Coatest. Results presented are mean from 5 mice/treatment at each time point. The decay curves were fitted by noncompartmental model with sparse sampling in WinNonLin. (B-C) HemA mice were dosed with 1.5 IU (2 × 105 cpm)/mouse of 125I-rFVIII reconstituted in buffer (●) or PEG-Liposome (
). Citrated blood was collected at 5 minutes, and 4, 8, 16, 24, 32, and 48 hours after tail vein injection. Plasma FVIII activities were measured by Coatest. Results presented are mean from 5 mice/treatment at each time point. The decay curves were fitted by noncompartmental model with sparse sampling in WinNonLin. (B-C) HemA mice were dosed with 1.5 IU (2 × 105 cpm)/mouse of 125I-rFVIII reconstituted in buffer (●) or PEG-Liposome ( ). The decay of 125I-FVIII in both whole blood (B) and platelet-poor plasma (C) was monitored at 5 minutes, 30 minutes, and 1, 4, 8, 16, 24, 48, 72, and 96 hours. Results presented are individual gamma count from 5 mice at each time point for each treatment. The decay curves were fitted using 2-compartmental model in WinNonLin.
). The decay of 125I-FVIII in both whole blood (B) and platelet-poor plasma (C) was monitored at 5 minutes, 30 minutes, and 1, 4, 8, 16, 24, 48, 72, and 96 hours. Results presented are individual gamma count from 5 mice at each time point for each treatment. The decay curves were fitted using 2-compartmental model in WinNonLin.
To investigate the possibility that rFVIII-PEG-Lip preferentially deposits FVIII to the cellular compartment of blood via PEG-Liposome, we further determined the distribution of FVIII in blood and plasma over time using 125I-Bolton-Hunter–labeled rFVIII that retained 96% of rFVIII activity as well as the capacity to bind PEG-Liposome (data not shown). HemA mice were dosed with 1.5 IU (2 × 105 cpm)/mouse of 125I-FVIII reconstituted in either buffer or PEG-Liposome, and the decay of 125I-FVIII in both whole blood and platelet-poor plasma (PPP) was monitored over 4 days (n = 5 mice/time point per treatment). In whole blood, the distribution phase of FVIII was identical between rFVIII-PEG-Lip and rFVIII, but rFVIII-PEG-Lip showed a 23% increase in the terminal t1/2 compared with rFVIII (24.6 vs 20.0 hours) and a 28% increase in MRT (24.5 vs 19.1 hours; Figure 3B). In plasma, rFVIII-PEG-Lip appeared to have a 27% longer distribution t1/2 (23.7 vs 18.7 hours), a 28% longer terminal t1/2 (26.0 vs 20.3 hours), and overall a 22% longer MRT (23.1 vs 18.9 hours) in comparison with rFVIII (Figure 3C).
Taken together, PEG-Liposome slightly prolongs (< 30%) the terminal t1/2 of rFVIII in circulation. However, the modestly prolonged t1/2 of rFVIII-PEG-Lip is not sufficient to account for its significantly improved survival relative to rFVIII after TVT in HemA mice.
Enhanced activity of rFVIII-PEG-Lip in HemA Mice is recapitulated in ex vivo whole blood ROTEM
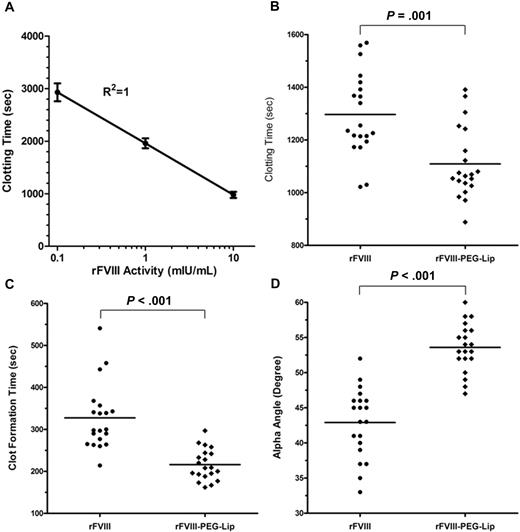
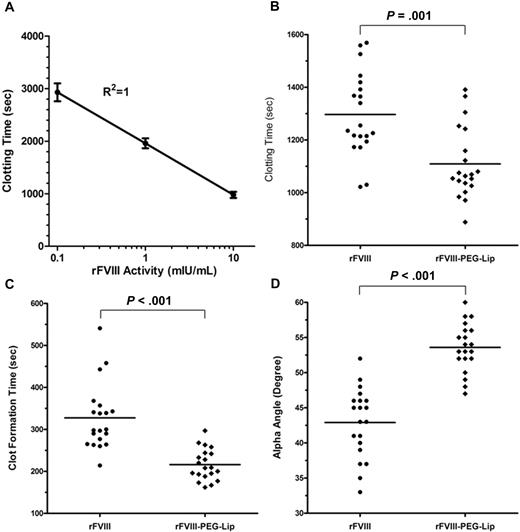
To quantify the difference in FVIII activities by ROTEM, we first established a calibration curve by spiking increasing amounts of rFVIII into freshly collected naive HemA mouse blood (n = 6 mice/dose). Figure 4A shows a linear-log relationship between the clotting time (CT) and the FVIII activity (log[FVIII activity]) in the range of 0.1 to 10 mIU/mL FVIII (R2 = 1). This curve was later used to estimate “apparent” FVIII activity levels in blood samples based on clotting times.
rFVIII-PEG-Lip induces more robust clot formation in whole blood ex vivo. (A) Calibration curve of the clotting time in ROTEM versus levels of rFVIII spiked in freshly collected naive HemA mouse blood. Results presented are mean ± SD from 6 individual blood samples for each rFVIII dose. (B-D) ROTEM of whole blood collected from the vena cava of HemA mice at 24 hours after dosing with 8 IU/kg rFVIII or rFVIII-PEG-Lip (n = 20 mice/treatment). Within 5 minutes from collection, 300 μL blood was recalcified and immediately analyzed by ROTEM. Results presented are (B) clotting time, (C) clot formation time, and (D) alpha angle from each individual blood sample. Bar represents the median in each treatment group. Two-tailed P values were generated by Mann-Whitney test.
rFVIII-PEG-Lip induces more robust clot formation in whole blood ex vivo. (A) Calibration curve of the clotting time in ROTEM versus levels of rFVIII spiked in freshly collected naive HemA mouse blood. Results presented are mean ± SD from 6 individual blood samples for each rFVIII dose. (B-D) ROTEM of whole blood collected from the vena cava of HemA mice at 24 hours after dosing with 8 IU/kg rFVIII or rFVIII-PEG-Lip (n = 20 mice/treatment). Within 5 minutes from collection, 300 μL blood was recalcified and immediately analyzed by ROTEM. Results presented are (B) clotting time, (C) clot formation time, and (D) alpha angle from each individual blood sample. Bar represents the median in each treatment group. Two-tailed P values were generated by Mann-Whitney test.
We then used ROTEM to assess clotting in whole blood collected from HemA mice at 24 hours after dosing with 8 IU/kg rFVIII or rFVIII-PEG-Lip (n = 20 mice/treatment). rFVIII-PEG-Lip resulted in significantly improved clotting parameters relative to rFVIII. The median CT was shortened from 1245 seconds to 1066 seconds (P = .001, Mann-Whitney test; Figure 4B), the median clot formation time was reduced from 299 seconds to 209 seconds (P < .001; Figure 4C), and the median alpha angle was increased from 44 to 54 degrees (P < .001; Figure 4D). Based on the rFVIII calibration curve, the same dose of rFVIII-PEG-Lip achieved an efficacy equivalent to that of approximately 2.5-fold higher levels of rFVIII. Thus, ex vivo ROTEM fully recapitulates the in vivo efficacy.
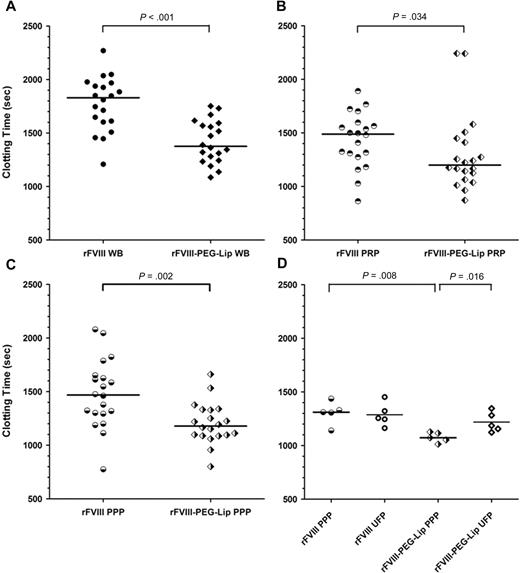
We also used ROTEM to monitor whole blood clotting at 5 minutes, and 4, 48, and 72 hours after dosing, and found that rFVIII-PEG-Lip–treated animals consistently displayed a significantly shorter CT at all time points sampled (Figure 5A and data not shown). The difference in clotting times was equivalent to approximately 2-fold higher apparent FVIII activity in rFVIII-PEG-Lip–treated mice at each of these time points.
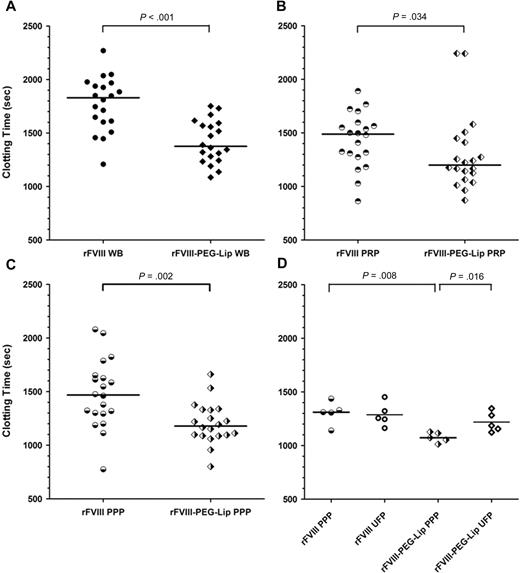
Enhanced coagulation activity of rFVIII-PEG-Lip is removed from PPP by ultracentrifugation. HemA mice were dosed with 8 IU/kg rFVIII (n = 20) or rFVIII-PEG-Lip (n = 20). At 5 minutes after dosing, blood was collected from the vena cava and tested individually by ROTEM. (A) Whole blood (3 μL) was added to 300 μL blood collected from naive HemA mouse. (B) Blood from each mouse was centrifuged at 300g for 5 minutes and 3 μL of the PRP fraction (supernatant) was added to 300 μL freshly collected naive HemA mouse blood. (C) Immediately after the collection of the 3 μL PRP from the low-speed spin, the blood from each mouse was further centrifuged at 1500g for 5 minutes. Three microliters from the PPP fraction (supernatant) was added to 300 μL freshly collected naive HemA mouse blood. (D) Thawed plasma was subjected to ultracentrifugation at 100000g for 20 minutes and 3 μL of the supernatant was added to 300 μL freshly collected naive HemA mouse blood. Two-tailed P values were determined by Mann-Whitney test. P > .05 for PPP versus UFP from rFVIII-treated animals; P = .016 for PPP versus UFP from rFVIII-PEG-Lip–treated animals; P = .008 for PPP from rFVIII- versus rFVIII-PEG-Lip–treated animals; and P > .05 for UFP from rFVIII- versus rFVIII-PEG-Lip–treated animals.
Enhanced coagulation activity of rFVIII-PEG-Lip is removed from PPP by ultracentrifugation. HemA mice were dosed with 8 IU/kg rFVIII (n = 20) or rFVIII-PEG-Lip (n = 20). At 5 minutes after dosing, blood was collected from the vena cava and tested individually by ROTEM. (A) Whole blood (3 μL) was added to 300 μL blood collected from naive HemA mouse. (B) Blood from each mouse was centrifuged at 300g for 5 minutes and 3 μL of the PRP fraction (supernatant) was added to 300 μL freshly collected naive HemA mouse blood. (C) Immediately after the collection of the 3 μL PRP from the low-speed spin, the blood from each mouse was further centrifuged at 1500g for 5 minutes. Three microliters from the PPP fraction (supernatant) was added to 300 μL freshly collected naive HemA mouse blood. (D) Thawed plasma was subjected to ultracentrifugation at 100000g for 20 minutes and 3 μL of the supernatant was added to 300 μL freshly collected naive HemA mouse blood. Two-tailed P values were determined by Mann-Whitney test. P > .05 for PPP versus UFP from rFVIII-treated animals; P = .016 for PPP versus UFP from rFVIII-PEG-Lip–treated animals; P = .008 for PPP from rFVIII- versus rFVIII-PEG-Lip–treated animals; and P > .05 for UFP from rFVIII- versus rFVIII-PEG-Lip–treated animals.
Microparticles are required for enhanced blood clotting
To identify the blood component(s) that contribute to the PK-independent efficacy of rFVIII-PEG-Lip, whole blood of HemA mice collected at 5 minutes after treatment with 8 IU/kg rFVIII or rFVIII-PEG-Lip (n = 20 mice/treatment) was analyzed by ROTEM. The median CT in the rFVIII-PEG-Lip group was significantly shorter than that in the rFVIII group (1376 seconds vs 1830 seconds; P < .001, Mann-Whitney test; Figure 5A), representing an approximately 2.4-fold higher apparent FVIII activity in rFVIII-PEG-Lip–treated animals. The approximately 2-fold activity enhancement remained in the platelet-rich plasma (PRP) of rFVIII-PEG-Lip–treated animals, which showed a median CT of 1200 seconds compared with the 1489 seconds in the PRP from rFVIII-treated animals (P = .034; Figure 5B). Further separation of PRP into platelet-poor plasma (PPP) did not eliminate the 2-fold difference in activity between rFVIII-PEG-Lip and rFVIII groups (median CT, 1179 seconds vs 1469 seconds; P = .002; Figure 5C). However, after PPP was further fractionated by high-speed centrifugation at 100000g for 20 minutes to remove microparticles and cell membrane remnants, the enhancing activity in the plasma from rFVIII-PEG-Lip–treated animals was lost and the clotting activity was very close to that of rFVIII-treated animals (n = 5 mice/treatment; Figure 5D). Control experiments showed that rFVIII premixed with PEG-Liposomes and added to naive HemA plasma had no enhanced procoagulant activity compared with rFVIII alone by Coatest, aPTT, or ROTEM, and the FVIII activity remained in the supernatant fraction after centrifugation at 100000g (data not shown). Thus, it appears that the activity of rFVIII-PEG-Lip in vivo may be enhanced by procoagulant microparticles generated rapidly within 5 minutes after rFVIII-PEG-Lip administration.
PEG-Liposomes associate with the majority of platelets and monocytes and promote platelet sensitization
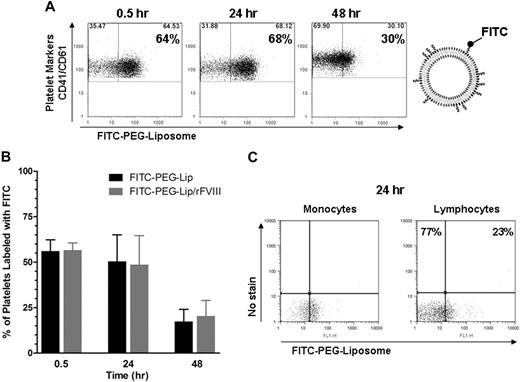
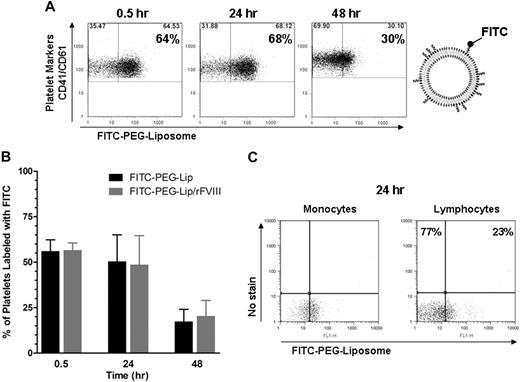
To investigate how rFVIII-PEG-Lip might promote the formation of procoagulant microparticles, we determined whether PEG-Liposomes can directly bind to platelets or monocytes. After HemA mice were injected with FITC-labeled PEG-Liposomes either alone or in complex with rFVIII, the majority of CD41+ and CD61+ platelets in blood were readily labeled by FITC-PEG-Lip at 30 minutes, and largely remained in association through 48 hours (Figure 6A). Furthermore, the interaction of platelets with PEG-Liposomes was independent of rFVIII. With or without premixing FITC-PEG-Lip with rFVIII, the percentage of platelets associated with FITC-PEG-Liposomes in each treatment group (7 mice/group) was comparable (Figure 6B). In addition to platelets, approximately 51% of the monocytes as well as 23% of the lymphocytes were associated with FITC-PEG-Liposomes at 24 hours after treatment (Figure 6C).
The majority of platelets and monocytes are associated with PEG-Liposome. HemA mice received tail vein injection of PEG-Liposome or FITC-labeled PEG-Liposomes (FITC-PEG-Lip) either alone or formulated with 0.2 IU rFVIII (FITC-PEG-Lip/rFVIII). Fifty microliters of blood was sampled at 0.5, 24, and 48 hours and analyzed by flow cytometry. (A) Fluorescence-activated cell sorting profile on blood samples from a representative FITC-PEG-Lip–treated HemA mouse. Platelets were stained with fluorophore-conjugated monoclonal antibodies against mouse CD41 and CD61. A threshold for FITC fluorescence level was defined as below which fell 99% of all platelet events from blood injected with unlabeled PEG-Liposomes. (B) Percentage of CD41+, CD61+ platelets labeled by FITC-PEG-Liposomes in HemA mice treated with FITC-PEG-Lip alone or FITC-PEG-Lip formulated with rFVIII. Results presented are mean ± SD from 7 mice at each time point. (C) Fluorescence-activated cell sorting of blood from 1 FITC-PEG-Lip–treated HemA mouse, in which monocytes and lymphocytes were identified by characteristic light scatter profile. Blood from unlabeled PEG-Liposome–injected animals was used to determine FITC (FL1)–negative (x-axis) and no stain (FL4)–negative (y-axis) threshold of each cell type to correct for cell type–dependent autofluorescence. Upon FITC-PEG-Lip treatment, cell-associated FITC signal (x-axis) increased but unstained fluorescence signal (y-axis) remained constant.
The majority of platelets and monocytes are associated with PEG-Liposome. HemA mice received tail vein injection of PEG-Liposome or FITC-labeled PEG-Liposomes (FITC-PEG-Lip) either alone or formulated with 0.2 IU rFVIII (FITC-PEG-Lip/rFVIII). Fifty microliters of blood was sampled at 0.5, 24, and 48 hours and analyzed by flow cytometry. (A) Fluorescence-activated cell sorting profile on blood samples from a representative FITC-PEG-Lip–treated HemA mouse. Platelets were stained with fluorophore-conjugated monoclonal antibodies against mouse CD41 and CD61. A threshold for FITC fluorescence level was defined as below which fell 99% of all platelet events from blood injected with unlabeled PEG-Liposomes. (B) Percentage of CD41+, CD61+ platelets labeled by FITC-PEG-Liposomes in HemA mice treated with FITC-PEG-Lip alone or FITC-PEG-Lip formulated with rFVIII. Results presented are mean ± SD from 7 mice at each time point. (C) Fluorescence-activated cell sorting of blood from 1 FITC-PEG-Lip–treated HemA mouse, in which monocytes and lymphocytes were identified by characteristic light scatter profile. Blood from unlabeled PEG-Liposome–injected animals was used to determine FITC (FL1)–negative (x-axis) and no stain (FL4)–negative (y-axis) threshold of each cell type to correct for cell type–dependent autofluorescence. Upon FITC-PEG-Lip treatment, cell-associated FITC signal (x-axis) increased but unstained fluorescence signal (y-axis) remained constant.
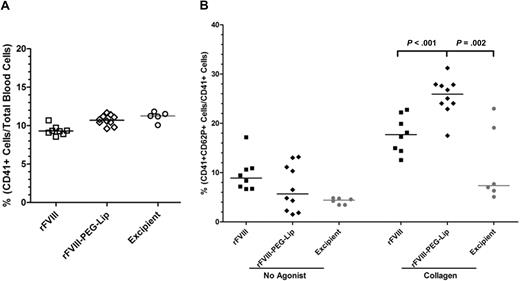
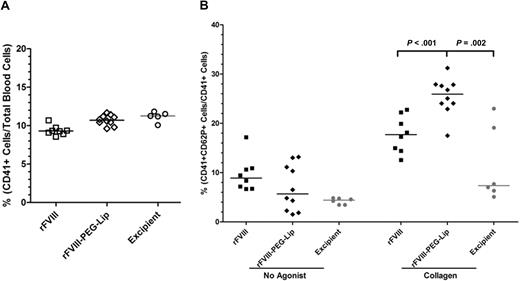
We further determined whether the interactions with PEG-Liposomes alter platelet functions. Blood from HemA mice sampled at 24 hours after dosing with 8 IU/kg rFVIII (n = 8), rFVIII-PEG-Lip (n = 10), or excipient (n = 5) showed similar levels of activated platelets (CD62P+, CD41+) at less than 1% of the circulating platelets (CD41+; data not shown), which make up approximately 10% of the total blood cells (Figure 7A). We also observed similar patterns in platelet aggregation and ADP release in whole blood upon stimulation by collagen (1 μg/mL) between rFVIII-PEG-Lip– and rFVIII-treated groups. However, the surface-associated P-selectin was detected on 26% (± 1%) of platelets in blood from rFVIII-PEG-Lip–treated animals, which is significantly higher than 18% (± 1%) in blood from rFVIII-treated animals (P < .001, Mann-Whitney test).
Platelet activation in response to collagen ex vivo. HemA mice were dosed with 8 IU/kg rFVIII (n = 8), 8 IU/kg rFVIII-PEG-Lip (n = 10), or excipient (n = 5). Blood was collected at 24 hours after dosing. (A) Percentage of circulating platelets (CD41+) in total blood cells was measured by flow cytometry. (B) Percentage of CD41+ platelets expressing surface-associated P-selectin (CD62P+) before and after stimulation with 1 μg/mL collagen. Results presented are values from individual animals, with the bar representing median in each treatment group. Two-tailed P values were determined by Mann-Whitney test.
Platelet activation in response to collagen ex vivo. HemA mice were dosed with 8 IU/kg rFVIII (n = 8), 8 IU/kg rFVIII-PEG-Lip (n = 10), or excipient (n = 5). Blood was collected at 24 hours after dosing. (A) Percentage of circulating platelets (CD41+) in total blood cells was measured by flow cytometry. (B) Percentage of CD41+ platelets expressing surface-associated P-selectin (CD62P+) before and after stimulation with 1 μg/mL collagen. Results presented are values from individual animals, with the bar representing median in each treatment group. Two-tailed P values were determined by Mann-Whitney test.
Discussion
In this report, we have evaluated the efficacy of rFVIII-PEG-Lip in the tail vein transection bleeding model of HemA mice that mimics the venous bleeding characteristic of severe hemophilia A patients. In this model, prophylactic treatment with rFVIII-PEG-Lip at 24 hours before injury resulted in a significantly higher rate of survival, corresponding to that of animals treated with approximately 2.5-fold higher dose of rFVIII (Figure 1). The in vivo efficacy was corroborated and extended by ROTEM, which demonstrated improved clot formation in whole blood from rFVIII-PEG-Lip–treated HemA mice at 5 minutes, and 4, 24, 48, and 72 hours after dosing (Figures 4–5, and data not shown). At each time point, rFVIII-PEG-Lip consistently demonstrated a 2- to 2.5-fold higher apparent FVIII activity by ROTEM.
Previously, PEG-Liposome was reported to significantly extend the circulating half-life of rFVIII that corresponded to the prolonged survival time after repeated tail clips in HemA mice.1 However, the accuracy of the PK measurement was compromised by the double-labeling of PEG-Liposome with 111In and rFVIII with 125I because both are gamma-emitting isotopes. In this report, using highly sensitive assays with a broader linear range, we have observed a much less profound extension in the PK and efficacy of rFVIII-PEG-Lip relative to rFVIII in HemA mice. Furthermore, the limited increase in FVIII half-life, that is, 13% by plasma FVIII activity and 22% to 28% by 125I-rFVIII decay in blood and plasma (Figure 3), is not sufficient to achieve more than 2-fold higher plasma FVIII activity required for rFVIII-PEG-Lip efficacy at 24 hours (Figure 1). More importantly, although the recovery of FVIII activity at 5 minutes is identical between rFVIII and rFVIII-PEG-Lip by aPTT and Coatest measurements (data not shown), the clotting activity in rFVIII-PEG-Lip–treated animals is already markedly increased by ex vivo ROTEM analysis (Figure 5) and in a tail clip bleeding model of HemA mice (data not shown).
Considering the rapid and significant association between PEG-Liposome and platelets (Figure 6 and Mordon et al14 ), delivery of FVIII to platelets mediated by the liposomes could be a plausible mechanism for PK-independent enhancement in efficacy. Platelets normally circulate near the vessel wall, and after injury to endothelium they immediately adhere to the exposed subendothelial surface and release their highly localized concentration of granule contents that mediate platelet aggregation and clot formation.19,20 Platelets and megakaryocytes have a capacity to uptake circulating proteins into secretory granules.21 Thus plasma proteins important for hemostasis, inflammation, and wound healing can be concentrated in platelet secretory granules and delivered to the injury site.21 In addition, platelets have been shown to sequester or uptake liposomes and PEGylated liposomes.13,14 It is conceivable that FVIII, via its association with PEG-Liposome, may also be sequestered and retained in platelets and released upon platelet activation at injury sites. FVIII ectopically expressed in platelets is highly effective in correcting the bleeding phenotype of HemA mice despite undetectable levels of FVIII in the plasma.22,23 However, if platelet-associated FVIII was the primary mode for enhanced efficacy of rFVIII-PEG-Lip, we would not expect to see an increase in clotting activity in platelet-poor plasma by ROTEM (Figure 5), and we would have detected an increase in platelet-associated FVIII by flow cytometry using Cy5-labeled FVIII or biotinylated anti-FVIII antibody or in our 125I-FVIII experiments. On the contrary, we did not detect any appreciable increase in platelet-associated FVIII in the presence of rFVIII-PEG-Lip relative to rFVIII or rFVIII/POPC-liposome either in vitro or ex vivo from treated HemA mice (data not shown). Furthermore, in HemA mice treated with 125I-labeled FVIII alone or formulated in PEG-Liposome, the majority of 125I-FVIII was recovered in the platelet-poor plasma, and the distribution of 125I-FVIII in whole blood, plasma, and cell pellets is comparable between the 2 treatments (Figure 3 and data not shown). Taking into account the comparable incremental recovery, clearance, and volume of distribution between rFVIII-PEG-Lip and rFVIII in HemA mice (Figure 3), it is unlikely that substantial amounts of FVIII have been deposited into platelets or other cellular compartments of the blood by PEG-Liposomes.
On the other hand, the observation that the enhanced procoagulant activity of rFVIII-PEG-Lip in platelet-poor plasma can be removed only by ultracentrifugation (Figure 5) strongly suggests the involvement of circulating subcellular components such as procoagulant microparticles. Highly procoagulant microparticles can arise from many cell types, including platelets,24-27 leukocytes,10,28,29 and endothelial cells.30,31 Platelet-derived microparticles express sustained high-affinity receptors for FVIII and display 50- to 100-fold higher specific procoagulant activity than activated platelets.9-11 Furthermore, leukocyte-derived microparticles were shown to bear TF, which was reported at a concentration of 100 to 150 pg/mL in peripheral blood.32-34 The TF on leukocyte-derived microparticles becomes active upon association with activated platelets via P-selectin and P-selectin glycoprotein ligand-1 interaction.35,36 The in vitro spiking of rFVIII-PEG-Lip into whole blood does not result in higher coagulation activity than rFVIII by ROTEM (data not shown), whereas the enhanced efficacy of rFVIII-PEG-Lip is observed ex vivo 5 minutes after administration, suggesting that the procoagulant microparticles must be induced rapidly via interactions between PEG-Liposome and blood cells in vivo. Because the binding of PEG-Liposome to rFVIII is a prerequisite for its efficacy in vivo (Figures 1–2), it also suggests that FVIII must be sequestered to microparticles during its induction by rFVIII-PEG-Lip. Finally, the increased activity of rFVIII-PEG-Lip is detectable only by ex vivo ROTEM, but not by aPTT and Coatest. The difference is most likely because ROTEM is a highly sensitive assay for global hemostasis, whereas aPTT and Coatest are relatively insensitive and limited to the quantification of FVIII or FVIIIa.
Interestingly, although rFVIII-PEG-Lip does not directly activate platelets in circulation, platelets from rFVIII-PEG-Lip–treated animals are more prone to activation because they displayed higher levels of surface-associated P-selectin in response to collagen compared with those from rFVIII-treated animals (Figure 7). However, no significant difference was found in other platelet activation biomarkers upon collagen stimulation, such as GP IIb-IIIa (αIIbβ3) for fibrinogen binding37 and platelet aggregation and ADP released from α-granules of activated platelets38 (data not shown). P-selectin may be a more sensitive marker for platelet activation. Furthermore, P-selectin plays critical roles in hemostasis and thrombosis.39 P-selectin expressed on activated platelets rapidly recruits TF-bearing microparticles during the initial phase of thrombus formation.8 Thus the increased levels of surface-associated P-selectin upon platelet activation may partly contribute to the enhanced efficacy of rFVIII-PEG-Lip in vivo.
We should point out that microparticles are highly heterogeneous, ranging in size from 0.05 to 1.5 μm40 ; it is technically difficult to accurately identify and quantify TF- and FVIII-bearing microparticles smaller than the wavelength of the laser light in flow cytometry (< 0.5 μm). To further test the hypothesis that immediately after the administration, FVIII may be transferred onto procoagulant microparticles derived from either platelets or monocytes via their interactions with PEG-Liposome transiently in complex with rFVIII, future efforts may rely on functional analysis of real-time thrombus formation by intravital microscopy in HemA mice transfused with microparticle-FVIII complex from rFVIII-PEG-Lip–treated animals.
In summary, rFVIII-PEG-Lip was developed as an effort to improve prophylaxis regimens for hemophilia A patients by reducing the frequent injection of rFVIII necessitated by its short half-life.41 rFVIII-PEG-Lip was reported to double the efficacy duration of rFVIII in early clinical studies. However, its mode of action has evaded the conventional wisdom of proportionally extended plasma pharmacokinetics. Our results shed light on alternative mechanisms including the sensitization of platelets for increased expression of P-selectin on platelet surfaces in response to activation, and the induction of procoagulant microparticles. The prolonged efficacy via microparticle induction merits further investigation and may provide a novel therapeutic approach for hemophilia treatment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors acknowledge funding of early work by the Israel-US Binational Industrial Research and Development Foundation.
Authorship
Contribution: J.P., T.L., J.-Y.K., C.P., and H.J. designed and performed research, analyzed data, and wrote the paper; D.Z., Z.-H.C., X.Z., J.O.N., A.R., and D.C. performed research and analyzed data; G.J. designed and performed research and analyzed data; and G.F.P. and J.M.S. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: All of the authors were employed by Bayer HealthCare LLC when research was conducted.
The current address for G.J. is Talecris Biotherapeutics, 85 T. W. Alexander Dr, Research Triangle Park, NC 27709. The current address for G.F.P. is Biogen Idec Hemophilia Unit, 9 Fourth Ave, Waltham, MA 02451.
Correspondence: Haiyan Jiang, Applied Research, Bayer HealthCare LLC, 2600 Hilltop Dr, Richmond, CA 94806; e-mail: haiyan.jiang.b@bayer.com.


 ). Citrated blood was collected at 5 minutes, and 4, 8, 16, 24, 32, and 48 hours after tail vein injection. Plasma FVIII activities were measured by Coatest. Results presented are mean from 5 mice/treatment at each time point. The decay curves were fitted by noncompartmental model with sparse sampling in WinNonLin. (B-C) HemA mice were dosed with 1.5 IU (2 × 105 cpm)/mouse of 125I-rFVIII reconstituted in buffer (●) or PEG-Liposome (
). Citrated blood was collected at 5 minutes, and 4, 8, 16, 24, 32, and 48 hours after tail vein injection. Plasma FVIII activities were measured by Coatest. Results presented are mean from 5 mice/treatment at each time point. The decay curves were fitted by noncompartmental model with sparse sampling in WinNonLin. (B-C) HemA mice were dosed with 1.5 IU (2 × 105 cpm)/mouse of 125I-rFVIII reconstituted in buffer (●) or PEG-Liposome (