Roumenina and colleagues identify mutations in complement factor B that render it uniquely hyperfunctional, predisposing affected persons to atypical hemolytic uremic syndrome and revealing novel mechanistic insights.
Atypical hemolytic uremic syndrome (aHUS) is a rare but devastating disorder, characterized by thrombocytopenia, microangiopathic hemolytic anemia, and renal dysfunction. More than 50% of patients develop end-stage renal failure and 25% die during the acute phase of the illness.1 Recent studies indicate that the hallmark aHUS-associated microvascular and arteriolar endothelial cell injury is pathogenically linked to excess complement activation via the alternative pathway (AP). This is supported by the finding that approximately 50% of patients have disease-associated, AP complement-activating missense mutations in genes encoding complement regulatory proteins (factor H [FH], factor I, membrane cofactor protein, factor H–related protein, C4b binding protein, thrombomodulin, C3, and factor B [FB]).2,3 Despite these insights, therapies are lacking, and several key questions remain: What is the etiology of the remaining 50% of cases? Why is there variable penetrance of the syndrome? What are the triggers for onset? Why is the endothelium targeted? What precipitates the thrombosis? Why is the kidney most frequently and severely affected?
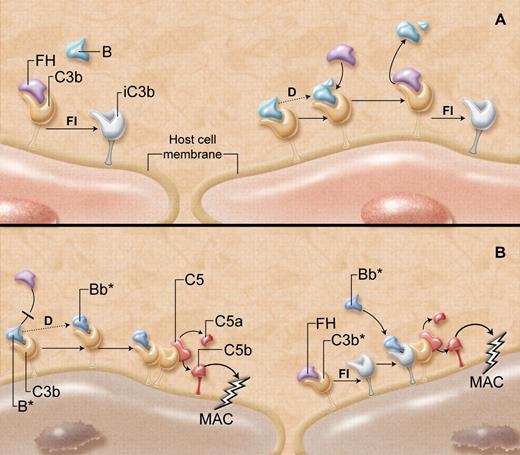
Genetic studies of patients with aHUS, coupled with a greater understanding of the molecular mechanisms of complement regulation, are providing some answers. The AP provides a rapid route for complement activation and amplification on the surfaces of invading pathogens (see figure, panel A).4 Initiation occurs spontaneously with hydrolysis of C3 to form metastable C3(H2O) to which the plasma protein FB may bind in solution. FB is then susceptible to cleavage by the circulating protease, factor D (FD), exposing a serine protease domain on the Bb fragment of FB. The so-formed fluid-phase C3 convertase, C3(H2O)Bb, cleaves additional C3 into C3a and C3b, the latter of which rapidly coats essentially all surfaces in contact with plasma. On pathogens that do not support the accumulation of competing complement regulators such as FH, FB binds to the C3b. The FB is cleaved by FD, and in the presence of Mg2+ ions, yields a C3bBb complex, the AP C3 convertase, which is further stabilized by properdin. Subsequent rapid generation of the AP C5 convertase, C3bBbC3b, leads to C5 cleavage, release of anaphylatoxins, and ultimate assembly of the membrane attack complex, designed to lyse the pathogen.
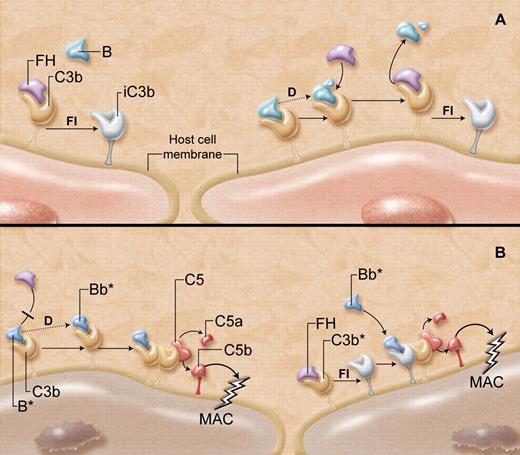
“Super factor B” and its gains-of-function. (A) Host cells, including endothelial cells, have multiple mechanisms to protect against complement-mediated destruction. As C3b is generated in the circulation, it deposits on all exposed cell membrane surfaces. (Left) Factor H (FH) outcompetes factor B (B) for binding to C3b on the host cell, acting as a cofactor for factor I (FI)–mediated generation of inactive C3b (iC3b), thereby suppressing further complement activation. (Right) If factor B does manage to bind to any host cell surface C3b, the B is cleaved and activated (dotted line) by factor D (D) to yield the alternative pathway C3 convertase (C3bBb). This complement-activating C3 convertase, however, is not long-lived, as FH rapidly and effectively destabilizes the C3bBb complex, promoting its dissolution, enhancing further generation of iC3b, and overall protecting the host cell from damage. (B) “Super factor B” (B*) has unique properties that overcome the protective functions of FH. (Left) B* binds more avidly to C3b and thus prevents FH from interacting with C3b. B* is then cleaved by factor D, and the so-formed C3 convertase (C3bBb*), particularly resistant to the normal destabilizing properties of FH, is able to generate more C3 convertase and C5 convertase (C3bBb*C3b). This causes the release of anaphylatoxins C3a and C5a, respectively, and the formation of the membrane attack complex (MAC), which damages or lyses the host cell. (Right) If FH does manage to bind to C3b and facilitate the generation of iC3b, the “super factor B” has the unique capacity to bind to the iC3b, recruiting it as a component of a novel C3 convertase (iC3bBb*). This in turn yields C5 convertase, leading to formation of the MAC that integrates into the host cell membrane. The resultant endothelial cell damage is believed to increase the risk of developing the thrombotic microangiopathy, atypical hemolytic uremic syndrome. Professional illustration by A. Y. Chen.
“Super factor B” and its gains-of-function. (A) Host cells, including endothelial cells, have multiple mechanisms to protect against complement-mediated destruction. As C3b is generated in the circulation, it deposits on all exposed cell membrane surfaces. (Left) Factor H (FH) outcompetes factor B (B) for binding to C3b on the host cell, acting as a cofactor for factor I (FI)–mediated generation of inactive C3b (iC3b), thereby suppressing further complement activation. (Right) If factor B does manage to bind to any host cell surface C3b, the B is cleaved and activated (dotted line) by factor D (D) to yield the alternative pathway C3 convertase (C3bBb). This complement-activating C3 convertase, however, is not long-lived, as FH rapidly and effectively destabilizes the C3bBb complex, promoting its dissolution, enhancing further generation of iC3b, and overall protecting the host cell from damage. (B) “Super factor B” (B*) has unique properties that overcome the protective functions of FH. (Left) B* binds more avidly to C3b and thus prevents FH from interacting with C3b. B* is then cleaved by factor D, and the so-formed C3 convertase (C3bBb*), particularly resistant to the normal destabilizing properties of FH, is able to generate more C3 convertase and C5 convertase (C3bBb*C3b). This causes the release of anaphylatoxins C3a and C5a, respectively, and the formation of the membrane attack complex (MAC), which damages or lyses the host cell. (Right) If FH does manage to bind to C3b and facilitate the generation of iC3b, the “super factor B” has the unique capacity to bind to the iC3b, recruiting it as a component of a novel C3 convertase (iC3bBb*). This in turn yields C5 convertase, leading to formation of the MAC that integrates into the host cell membrane. The resultant endothelial cell damage is believed to increase the risk of developing the thrombotic microangiopathy, atypical hemolytic uremic syndrome. Professional illustration by A. Y. Chen.
An array of negative regulatory pathways normally protect host cells from complement-mediated destruction. One crucial system involves the plasma protein FH, which prevents FB binding to C3b on host cells, promotes dissociation of any C3bBb complexes that might have formed, and acts as a cofactor for factor I (FI)–mediated inactivation of C3b to iC3b. Several mutant forms of FH account for 30% to 40% of the cases of aHUS.5,6 However, the protective properties of FH might conceivably also be abrogated by the presence of a “super FB.”
Indeed, this is precisely what Roumenina and colleagues report in this issue of Blood.7 They describe 3 patients with aHUS, with 2 different missense mutations in FB that profoundly augment its powers. K325N and D254G amino acid substitutions both reside within the serine protease Bb fragment, near the so-called Mg2+ adhesion site of FB. These result in increased affinity of FB for C3(H2O) and C3b, likely outcompeting FH for binding to C3b (see figure, panel B). Thus, the rate of formation and amount of fluid-phase and membrane-bound C3 convertase is increased, causing enhanced C3b deposition on all cell surfaces including those of host cells. With less access to FH, there is also less FI-mediated generation of iC3b. This hyperfunctional C3 convertase is also more stable with resistance to spontaneous and FH-induced decay.
But that is not all. The authors provide evidence that the mutant FB acquires the unique ability to bind to iC3b, yielding an entirely new and functional iC3bBb C3 convertase. Thus, even if host cells manage to recruit FI to inactivate surface deposits of C3b, the variant FB will use the iC3b to further activate complement leading to cellular damage.
How relevant are these findings to the loss of endothelial integrity that occurs in aHUS? Probably very relevant. When exposed to serum containing “super FB,” quiescent endothelial cells, and even more so, endothelial cells of renal glomerular origin, are especially susceptible to complement-mediated damage.7 Although not shown, it is likely that these cells then exhibit a prothrombotic, proinflammatory phenotype in line with the renal microvascular thrombosis seen in aHUS.
Given the prominent complement-activating properties of the “super FB” mutants, it is remarkable that the 2 family members with the D254G FB mutation presented with aHUS only as adults. Did these persons develop compensatory mechanisms for protection that were finally overcome after 30 or more years? Conversely, why in the patient with the other apparently similar FB mutation did that mutation reveal itself at 1 month of age? The critical modifying epigenetic, environmental, and genetic factors that determine the time of onset and the severity of the syndrome are still a mystery waiting to be solved.
The acquisition of several AP complement-activating properties by the “super FB” mutants highlights the challenges of designing effective treatments for aHUS. Overcoming the mechanisms by which these variants promote complement activation may require targeting multiple sites on FB, or intervening in downstream complement cascade pathways. Any strategy must account for the relative contribution of the different acquired properties of the variant FB, normalize complement activation, and prevent endothelial cell damage. The findings also impel us to consider, in a similar “out-of-the-box” approach as used by Roumenina et al, whether the formation of other unique complement-activating complexes might also underlie some of the hitherto unexplained cases of aHUS.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■