Abstract
For the adoptive transfer of tumor-directed T lymphocytes to prove effective, there will probably need to be a match between the chemokines the tumor produces and the chemokine receptors the effector T cells express. The Reed-Stemberg cells of Hodgkin lymphoma (HL) predominantly produce thymus- and activation-regulated chemokine/CC chemokine ligand 17 (TARC/CCL17) and macrophage-derived chemokine (MDC/CCL22), which preferentially attract type 2 T helper (Th2) cells and regulatory T cells (Tregs) that express the TARC/MDC-specific chemokine receptor CCR4, thus generating an immunosuppressed tumor environment. By contrast, effector CD8+ T cells lack CCR4, are nonresponsive to these chemokines and are rarely detected at the tumor site. We now show that forced expression of CCR4 by effector T cells enhances their migration to HL cells. Furthermore, T lymphocytes expressing both CCR4 and a chimeric antigen receptor directed to the HL associated antigen CD30 sustain their cytotoxic function and cytokine secretion in vitro, and produce enhanced tumor control when infused intravenously in mice engrafted with human HL. This approach may be of value in patients affected by HL.
Introduction
Adoptive transfer of Epstein-Barr virus (EBV)–specific cytotoxic T lymphocytes (CTLs) can induce prolonged disease stabilization and complete remissions in patients with EBV-associated Hodgkin lymphoma (HL).1,2 The great majority of HL, however, lack EBV antigens and so cannot benefit from this promising approach.3 CD30 is an alternative target antigen because it is expressed by virtually all Reed-Stemberg cells.4 Adoptive transfer of T lymphocytes that are genetically modified to express a CD30-specific chimeric antigen receptor (CAR-CD30), which combines the antigen-binding domain of a monoclonal CD30 antibody (scFv) with the ζ chain of the CD3/T-cell receptor complex, may therefore be of value in HL that lacks EBV antigens.5
Previous studies have shown that retargeting effector T cells to tumor antigens by the expression of CAR molecules is necessary but not sufficient for clinical responses.6 One additional requirement is that modified T cells should effectively traffic to the tumor sites.7 This aspect may be particularly problematic for HL as the tumor generates a chemokine milieu that significantly influences which T-cell subtypes traffic to and accumulate in the tumor.8,9 For instance, Reed-Stemberg cells produce the chemokines thymus- and activation-regulated chemokine/CC chemokine ligand 17 (TARC/CCL17) and macrophage-derived chemokine (MDC)/CCL22 that attract T helper (Th2) cells and regulatory T cells (Tregs),9,10 which express CCR4, the receptor for these chemokines.9-14 In contrast, CD8+ effector T cells, which lack CCR4 expression, are rarely detected within HL tumors, a result probably attributable to an incompatible match between the chemokines secreted by the tumor cells and the chemokine receptors expressed by the effector T cells.
The abundance of Tregs (and Th2 cells) in tumors including HL can create a hostile immune microenvironment by impairing the antitumor activity of the few cytotoxic-effector T lymphocytes able to reach the tumor site.11 Thus, increasing the number of cytototxic T cells that efficiently reach the tumor site should enhance antitumor responses.
We now show that migration of CAR-CD30-redirected, effector T lymphocytes toward an HL-generated TARC gradient is improved by forced expression of CCR4. Transgenic expression of CCR4 does not impede the functionality of these cells; consequently, their overall antilymphoma activity is enhanced in vivo.
Methods
Tumor cell lines
The HL-derived cell lines HDLM-2, L-428, L-1236, L-540, KM-H2, and the anaplastic large cell lymphoma-derived cell line Karpas-299 were obtained from the German Collection of Cell Cultures (DMSZ, Braunschweig, Germany). These cell lines are CD30+ but lack expression of EBV-associated antigens. All cells were maintained in culture with RPMI 1640 medium (HyClone Laboratories, Logan, UT) containing 10% fetal bovine serum (HyClone Laboratories) and 2 mM l-glutamine (Invitrogen, Carlsbad, CA). Cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C.
Retroviral constructs
Full-length human CCR4 was cloned by reverse-transcribed polymerase chain reaction from OKT3-(Ortho Biotech Products, Bridgewater, NJ) activated CD4+ cells cultured for 5 days with IL-4 (1000 U/mL; R&D Systems, Minneapolis, MN). CCR4 was cloned into the SFG retroviral vector.15 The retroviral vector encoding the CAR-CD30 was kindly provided by Drs H. Abken and A. Hombach and previously described.5,16 For this study, we constructed a second-generation CAR-CD30 in which we included the CD28 endodomain to augment costimulation of the modified T cells after antigen engagement of their chimeric receptor.17,18 We also generated a bicistronic retroviral construct encoding both CCR4 and CAR-CD30 in which CAR-CD30 is driven by an internal ribosome entry site [CCR4(I)CAR-CD30]. The chemokine TARC was cloned by reverse-transcribed polymerase chain reaction from the HDLM-2 cell line and subcloned into a retroviral vector (PL-x-SP) containing the puromycin resistance gene as a selectable marker (PL-TARC-SP). Two additional retroviral vectors encoding eGFP-Firefly-Luciferase (eGFP-FFLuc) and Firefly-Luciferase gene (FFLuc) were used to label T lymphocytes and tumor cells, respectively, for in vivo study as previously described.16,18 Transient retroviral supernatant was produced using 293T cells, as previously described.16,18 Karpas-299 cell line (Karpas/wt) that does not produce the chemokine TARC was genetically modified with PL-TARC-SP and selected using puromycin (Sigma-Aldrich, St Louis, MO) to generate Karpas/TARC cells that produce significant amounts of TARC that could be detected in the supernatant by specific enzyme-linked immunosorbent assay (ELISA).
Generation and transduction of effector cells
Peripheral blood mononuclear cells (PBMCs) were activated in 24-well plates coated with OKT3 and CD28 antibodies (BD Biosciences PharMingen, San Diego, CA) in the presence of recombinant human interleukin-2 (IL-2, 100 U/mL; Proleukin, Chiron, Emeryville, CA). Activated T cells were transduced on day 3 in 24-well plates precoated with recombinant fibronectin fragment (FN CH-296; Retronectin; Takara, Kyoto, Japan) using specific retroviral supernatant and IL-2, as previously described.18,19 T cells were then collected and expanded in T-cell media (50% RPMI, 50% Click [Irvine Scientific, Santa Ana, CA], 10% fetal bovine serum, and 2 mM L-glutamine) using IL-2 (50-100 U/mL) to obtain sufficient number of cells to perform the experiments in vitro and in vivo.
Transwell migration assay
Migration was assessed using 5-μm pore 24-transwell plates (Corning Life Sciences, Acton, MA). The lower chamber was loaded with culture supernatant containing the chemokine of interest. To obtain supernatant, cell lines (HDLM-2, L428, Karpas/wt, and Karpas/TARC) were cultured for 16 hours in serum-free medium (AIM-V, Invitrogen) at a concentration of 106 cells/mL. Supernatant was then collected and centrifuged before being loaded in the lower chamber. Effector cells (105) labeled with 51Cr (100 μCi) were added to the upper chamber. As a positive control, effector cells were placed directly into the lower chamber. As a negative control, medium without chemokine was placed into the lower chamber. Plates were incubated for 4 to 5 hours at 37°C, 5% CO2. At the end of the incubation period, the transwell inserts were removed and the content of the lower compartment carefully recovered. Cells were lysed with Triton-X (Sigma-Aldrich) and chromium release measured using a gamma counter (PerkinElmer Life and Analytical Sciences, Waltham, MA). The percentage of migrating cells was calculated as follows: 100× [cpm from experimental supernatant (cells migrated in the lower chamber) − cpm in the presence of media only (random migration)]/[cpm of positive control − cpm random migration]. To evaluate specific migration, neutralizing antibodies for the chemokine TARC (R&D Systems) or appropriate isotype controls (R&D Systems) were added to lower chamber before the chemotactic assay.
Enzyme-linked immunosorbent assay and cytometric bead array
The production of the chemokines TARC and MDC were quantified by specific ELISA using commercially available kits (R&D Systems, PeproTech, Rocky Hill, NJ). Supernatants tested with ELISA were collected from the cultures used for the chemotactic assay. Supernatants collected 24 hours after activation with OKT3 (100 ng/mL) of transduced or control T cells was tested using the Th1/Th2 CBA assay (BD Biosciences PharMingen).
Phenotypic analysis
Expression of cell surface molecules was determined by flow cytometry using standard methodology. The following monoclonal antibodies conjugated with phycoerythrin, fluorescein isothiocyanate, and/or peridinin chlorophyll protein were used: CD3, CD4, CD8, CD30, CCR4, CD45RA, CD45RO, CCR7, CD62L, CD56, αβT-cell receptor (BD Biosciences PharMingen). Expression of CAR by T cells was detected using Cy-5–conjugated goat anti–human IgG (H+L) antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA), which recognizes the human IgG1-CH2-CH3 component incorporated as a spacer region within the CAR. Samples were analyzed using a FACSCalibur (BD Biosciences PharMingen), and data were analyzed by CellQuest Pro software (BD Biosciences, San Jose, CA). At least 10 000 positive events were measured for each sample.
Cytotoxicity assay
The cytotoxic activity of transduced effector cells was evaluated using a 4-hour 51Cr release assay as previously described.16 Target cells were: Daudi cell line (CD30+ < 0.1%), HDLM-2 (CD30+ = 100%), and Karpas-299 (CD30+ = 100%). 51Cr labeled target cells incubated in complete medium or 1% Triton X-100 were used to determine spontaneous and maximal 51Cr release, respectively. After 4 hours, supernatants were collected and radioactivity was measured on a gamma counter. The mean percentage of specific lysis of triplicate wells was calculated as 100 × (experimental release − spontaneous release)/(maximal release − spontaneous release).
Inhibition assay
PBMCs were labeled with carboxyfluorescein succinimidyl ester (CFSE, Invitrogen) following the manufacturer's instructions. PBMCs were then stimulated with irradiated (40 Gy) allogeneic PBMCs (at a 4:1 effector/target ratio) and OKT3 0.5 μg/mL. Control or transduced T cells were added to the culture (at a 1:1 ratio) to evaluate inhibition of proliferation. As positive control, naturally occurring Treg cells (CD4+CD25bright), were isolated from PBMCs using immunomagnetic selection with CD25 magnetic beads (2 μL/107 cells), followed by selection of CD4+, according to Godfrey et al.20 On day 6, cells were labeled with CD4 peridinin chlorophyll protein and CD8 phycoerythrin and CFSE dilution analyzed using a FACSCalibur to assess cell division.
In vivo experiments
All mouse experiments were performed in accordance with Baylor College of Medicine Animal Husbandry and Institutional Animal Care and Use Committee guidelines and were approved by the Baylor College of Medicine's Institutional Review Board.
Homing
To assess homing and localization of control and genetically modified T cells in vivo, we used a severe combined immunodeficiency (SCID) mouse model and the In Vivo Imaging System (IVIS) imaging system (Xenogen, Caliper Life Sciences, Hopkinton, MA). Six- to 8-week-old CB17/SCID mice (Harlan, Indianapolis, IN) were sublethally irradiated (230 cGy) and engrafted subcutaneously with 5 × 106 CD30+ tumor cells (Karpas/wt) on the left side and with the same tumor cells engineered to secret TARC (Karpas/TARC) on the contralateral side. Five to 7 days later, the mice received intravenous injection of 10 × 106 control or genetically modified T cells and IL-2 (Teceleukin, Fisher Bioservices, Rockville, MD) intraperitoneally (500 U/mouse) 3 times a week. To track the cells in vivo, both control and genetically modified cells were transduced with the eGFP-FFLuc vector. T-cell migration was evaluated using the IVIS imaging system. Briefly, a constant region of interest was drawn over the tumor regions and the intensity of the signal measured as total photon/sec/cm2/sr (p/s/cm2/sr) as previously described.16,18,21
Antitumor activity
Because there are few reproducible systemic models of HL, we assessed the antitumor activity of T cells transduced with the bicistronic vector CCR4(I)CAR-CD30 using NOG/SCID/γcnull mice that reproducibly allow subcutaneous engraftment of CD30+TARC+ HL cell lines, including HDLM-2.22 To measure the in vivo growth of these cells, HDLM-2 were transduced with the FFLuc vector and selected in puromicin. FFLuc+ HDLM-2 cells (3 × 106 cells/mice) were resuspended in Matrigel (BD Biosciences, San Jose, CA) and injected subcutaneously. Three to 5 days later, when the light emission of the tumor was consistently measurable, mice received intravenous control or genetically modified T cells (10 × 106 cells/mouse) and IL-2 intraperitoneally (500 U/mice) twice a week. To monitor tumor growth, the IVIS imaging system (Xenogen) was used as described in “Homing.”
Statistical analysis
All in vitro data are presented as average plus or minus SD. Student t test was used to determine the statistical significance of differences between samples, and P values less than .05 were accepted as indicating a significant difference. For the bioluminescence experiments, the homing of T cells to the tumor site was expressed as fold expansion of the light intensity and compared between light intensity at Karpas/wt and Karpas/TARC tumor sites. Changes in intensity of signal from baseline at each time point were calculated and compared using paired t tests or Wilcoxon signed-ranks test.
Results
HL cell lines secrete TARC and MDC chemokines but activated T lymphocytes lack CCR4 expression
Identification of cell lines producing TARC and MDC chemokines.
We first evaluated the production of TARC and MDC by HL-derived cell lines (HDLM-2, L428, L540, KH-M2, and L1236) and confirmed that all lines released significant amounts of TARC (ranging from 700 pg/mL/106 cells to > 2000 pg/mL/106 cells) and 3 lines (HDLM-2, L540, and L1236) also released MDC (ranging from 200 pg/mL/106 cells to 700 pg/mL/106 cells; Table 1). We selected 2 cell lines, HDLM-2, producing both chemokines, and L428, producing only TARC, in which to evaluate the effects of TARC/MDC and CCR4 interaction. In addition, we used Karpas-299, an anaplastic large cell lymphoma cell line that expresses the target antigen CD30 but does not secrete TARC or MDC, as a negative control (Table 1).
Expression of CCR4.
We next determined the expression of CCR4 by freshly isolated and recently activated T cells. Only a small fraction of freshly isolated CD3+ cells expressed CCR4 (5.6% ± 3.2%). Cells expressing CCR4 were mainly CD4+ (5.0% ± 3.4%), and expression was almost undetectable in CD8+ cells (0.7% ± 0.8%; Table 2). Five days after activation with OKT3 and CD28 antibodies, T lymphocytes showed a modest increase of CCR4 expression on CD4+ cells (13.5% ± 3.8%), but CD8+ cells continued to have low or undetectable levels of the chemokine receptor (3.0% ± 3.8%; Table 2).
Hence, TARC production is a consistent biologic property of HL cells, and the lack of CCR4 expression by activated CD8+ T lymphocytes may impede their migration to sites of disease.
Functional expression of CCR4 can be obtained in activated T lymphocytes using gene transfer.
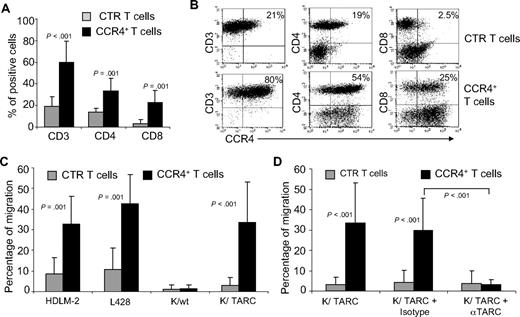
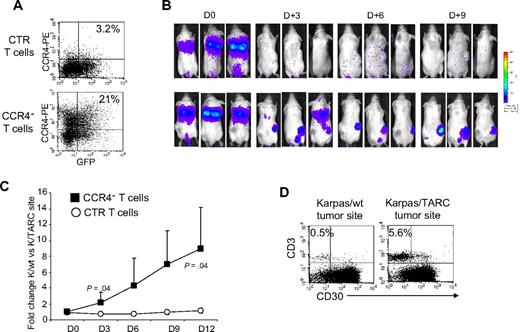
PBMCs isolated from 7 healthy donors were activated and then transduced with a retroviral vector encoding CCR4. After transduction, CCR4 expression increased from 19% plus or minus 9% to 60% plus or minus 19% (P < .001; Figure 1A,B), as assessed by FACS analysis. In the transgenic population, CCR4 was expressed by both CD4+ (33% ± 12%) and CD8+ T lymphocytes (23% ± 12%). Receptor expression was stable over 4 weeks of culture (data not shown). To discover whether transgenic CCR4 was functional, we compared the migration of control and CCR4-transduced (CCR4+) T lymphocytes toward TARC gradients using a transwell migration assay. As shown in Figure 1C, CCR4+ T lymphocytes had significantly improved migration toward culture supernatant collected from HL cell lines that naturally secrete TARC (percentage migration, 32% ± 13% and 43% ± 14% toward HDLM-2 and L-428 supernatants, respectively) compared with control T cells (percentage migration, 8% ± 6% and 11% ± 10% toward HDLM-2 and L-428 supernatants, respectively; both P = .001). To further confirm that the migration was exclusively mediated by TARC/CCR4 interaction, we compared the migration activity of control and CCR4+ T cells toward the supernatants collected from the wild-type Karpas tumor cell line (Karpas/wt, TARC negative) and its counterpart that had been genetically modified to produce TARC (Karpas/TARC; TARC > 2000 pg/mL/106 cells in the supernatant, as assessed by ELISA; Table 1). Both control and CCR4+ T cells had negligible migration toward Karpas/wt supernatant (percentage migration, 1.2% ± 2.2% and 1.3% ± 2%, respectively; Figure 1C). In contrast, we observed significant migration of CCR4+ T cells (percentage migration, 34% ± 20%), but not of control T cells (percentage migration, 4% ± 4%; P < .001), toward Karpas/TARC supernatant (Figure 1C). Importantly, migration of CCR4+ T cells was inhibited by the addition of a TARC-blocking antibody (percentage migration, 3% ± 3%) but not by an isotype control (percentage migration, 30% ± 16%; P < .001; Figure 1D).
Improved migration of activated T lymphocytes genetically modified to overexpress CCR4. (A) The expression of CCR4 in control (CTR) T cells and in T lymphocytes transduced with a retroviral vector encoding CCR4. Surface expression of CCR4 was evaluated by FACS analysis of CD3+, CD4+, and CD8+ T lymphocytes.  represent the mean ± SD of control T cells; ■, mean ± SD of CCR4+ T cells. The data summarize the results of T-cell lines generated from 7 healthy donors. (B) A representative phenotypic analysis. (C) The migration of CTR (
represent the mean ± SD of control T cells; ■, mean ± SD of CCR4+ T cells. The data summarize the results of T-cell lines generated from 7 healthy donors. (B) A representative phenotypic analysis. (C) The migration of CTR ( ) and CCR4+ (■) T cells toward TARC gradients, using a transwell migration assay. T-cell migration was evaluated using culture supernatants collected from 2 HL-derived cell lines (HDLM-2 and L428) that physiologically produce high amounts of TARC, and against the Karpas-299 cell line genetically modified to produce TARC (K/TARC). K/wt was used as a control. The panel indicates that migration toward TARC is significantly improved if T cells are genetically modified to overexpress CCR4. The data are the mean ± SD for T-cell lines generated from 7 healthy donors. (D) The improved migration of CCR4+ T cells (■) is TARC mediated as it is inhibited by addition of anti-TARC antibodies but not by the addition of an isotype control.
) and CCR4+ (■) T cells toward TARC gradients, using a transwell migration assay. T-cell migration was evaluated using culture supernatants collected from 2 HL-derived cell lines (HDLM-2 and L428) that physiologically produce high amounts of TARC, and against the Karpas-299 cell line genetically modified to produce TARC (K/TARC). K/wt was used as a control. The panel indicates that migration toward TARC is significantly improved if T cells are genetically modified to overexpress CCR4. The data are the mean ± SD for T-cell lines generated from 7 healthy donors. (D) The improved migration of CCR4+ T cells (■) is TARC mediated as it is inhibited by addition of anti-TARC antibodies but not by the addition of an isotype control.
Improved migration of activated T lymphocytes genetically modified to overexpress CCR4. (A) The expression of CCR4 in control (CTR) T cells and in T lymphocytes transduced with a retroviral vector encoding CCR4. Surface expression of CCR4 was evaluated by FACS analysis of CD3+, CD4+, and CD8+ T lymphocytes.  represent the mean ± SD of control T cells; ■, mean ± SD of CCR4+ T cells. The data summarize the results of T-cell lines generated from 7 healthy donors. (B) A representative phenotypic analysis. (C) The migration of CTR (
represent the mean ± SD of control T cells; ■, mean ± SD of CCR4+ T cells. The data summarize the results of T-cell lines generated from 7 healthy donors. (B) A representative phenotypic analysis. (C) The migration of CTR ( ) and CCR4+ (■) T cells toward TARC gradients, using a transwell migration assay. T-cell migration was evaluated using culture supernatants collected from 2 HL-derived cell lines (HDLM-2 and L428) that physiologically produce high amounts of TARC, and against the Karpas-299 cell line genetically modified to produce TARC (K/TARC). K/wt was used as a control. The panel indicates that migration toward TARC is significantly improved if T cells are genetically modified to overexpress CCR4. The data are the mean ± SD for T-cell lines generated from 7 healthy donors. (D) The improved migration of CCR4+ T cells (■) is TARC mediated as it is inhibited by addition of anti-TARC antibodies but not by the addition of an isotype control.
) and CCR4+ (■) T cells toward TARC gradients, using a transwell migration assay. T-cell migration was evaluated using culture supernatants collected from 2 HL-derived cell lines (HDLM-2 and L428) that physiologically produce high amounts of TARC, and against the Karpas-299 cell line genetically modified to produce TARC (K/TARC). K/wt was used as a control. The panel indicates that migration toward TARC is significantly improved if T cells are genetically modified to overexpress CCR4. The data are the mean ± SD for T-cell lines generated from 7 healthy donors. (D) The improved migration of CCR4+ T cells (■) is TARC mediated as it is inhibited by addition of anti-TARC antibodies but not by the addition of an isotype control.
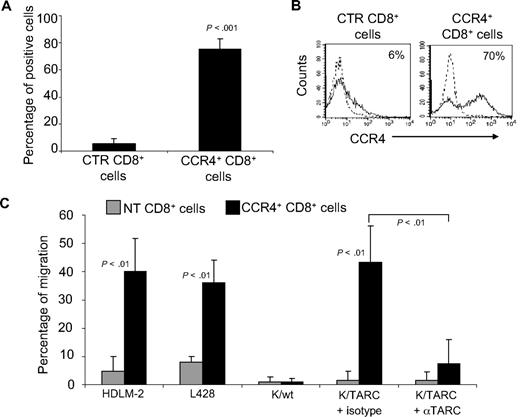
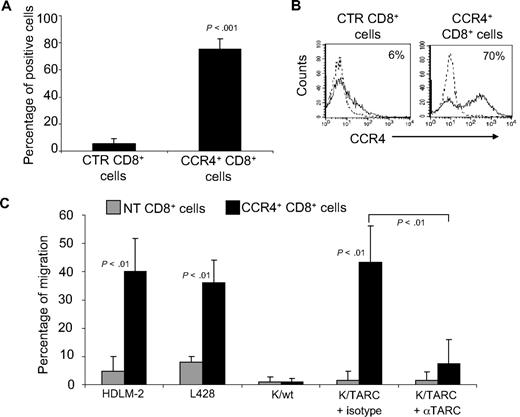
Because control T cells showed some migration when incubated with supernatant collected from HL cell lines, which is probably mediated by the fraction of CD4+ T cells that physiologically express CCR4, we repeated the migration assays using only CD8+ T lymphocytes, which lack CCR4 (5% ± 4% by FACS analysis; Figure 2A,B), and compared them with the migration of CD8+ cells genetically modified to express CCR4 (75% ± 7% by FACS analysis; Figure 2A,B). We found that TARC-mediated migration of CD8+CCR4+ cells toward supernatant collected from HDLM-2 and L428 cells was significantly increased (40% ± 12% and 36% ± 15%, respectively) compared with control CD8+ T cells (4.7% ± 5% and 8% ± 2%; P < .01; Figure 2C). Similarly, migration of CD8+ CCR4+ T cells toward Karpas/TARC (43% ± 13%) was improved compared with Karpas/wt (1% ± 1%; P < .001). In addition, this migration was specifically inhibited by a TARC-blocking antibody (7% ± 8%) but not by an isotype control (43% ± 13%; P = .001). As expected, no significant migration was observed for control CD8+ T cells toward Karpas/wt (1% ± 2%) or Karpas/TARC (3% ± 4%; Figure 2C).
Improved migration of CD8+ T lymphocytes genetically modified to overexpress CCR4. (A) The expression of CCR4 by CTR and transduced CD8+ T cells, using FACS analysis. The data summarize the results of T-cell lines generated from 4 healthy persons. (B) A representative phenotypic analysis. Dotted lines indicate isotype control. (C) The migration of CTR ( ) and CCR4+ (■) CD8+ T cells toward TARC gradients, using a transwell migration assay. The panel indicates that migration toward TARC is significantly increased when CD8+ T cells overexpress CCR4. The data are the mean ± SD for CD8+ T-cell lines generated from 4 healthy donors.
) and CCR4+ (■) CD8+ T cells toward TARC gradients, using a transwell migration assay. The panel indicates that migration toward TARC is significantly increased when CD8+ T cells overexpress CCR4. The data are the mean ± SD for CD8+ T-cell lines generated from 4 healthy donors.
Improved migration of CD8+ T lymphocytes genetically modified to overexpress CCR4. (A) The expression of CCR4 by CTR and transduced CD8+ T cells, using FACS analysis. The data summarize the results of T-cell lines generated from 4 healthy persons. (B) A representative phenotypic analysis. Dotted lines indicate isotype control. (C) The migration of CTR ( ) and CCR4+ (■) CD8+ T cells toward TARC gradients, using a transwell migration assay. The panel indicates that migration toward TARC is significantly increased when CD8+ T cells overexpress CCR4. The data are the mean ± SD for CD8+ T-cell lines generated from 4 healthy donors.
) and CCR4+ (■) CD8+ T cells toward TARC gradients, using a transwell migration assay. The panel indicates that migration toward TARC is significantly increased when CD8+ T cells overexpress CCR4. The data are the mean ± SD for CD8+ T-cell lines generated from 4 healthy donors.
T lymphocytes expressing transgenic CCR4 do not acquire inhibitory properties.
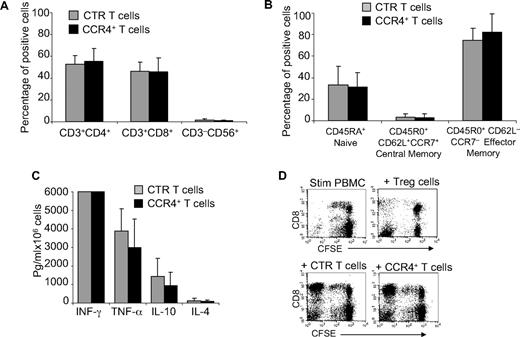
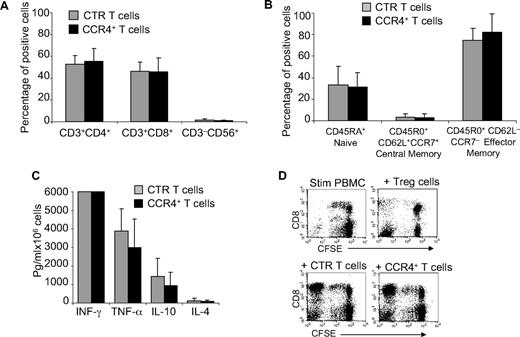
As TARC/CCR4 interaction seems to play an important role in attracting Th2 and Tregs,10,13 we next determined whether forced expression of CCR4 conferred inhibitory properties to the modified cells. We first compared the expression of T-cell markers (CD3, CD4, CD8, CD56, CD45RA, CD45RO, CD62L, and CCR7) by control and CCR4+-activated T cells and found that CCR4+ cells were comparable phenotypically to control T cells (Figure 3A), including the components of naive and effector-memory cells described previously23 (Figure 3B). We then examined the cytokines released by these cells after activation with OKT3 monoclonal antibody. As shown in Figure 3C, CCR4+ T cells continued to secrete interferon-γ (IFN-γ, > 6000 pg/mL per 106 cells) and tumor necrosis factor-α (TNF-α, 2900 ± 1550 pg/mL per 106 cells) similar to control T cells (> 6000 pg/mL per 106 cells and 3890 ± 1200 pg/mL per 106 cells, respectively). Moreover, production of the cytokines IL-4 and IL-10, which are associated with Th2 or Treg cells, was not significantly increased in CCR4+ cells (101 ± 76 pg/mL per 106 cells and 960 ± 688 pg/mL per 106, respectively) compared with control T cells (130 ± 109 pg/mL per 106 cells and 1450 ± 955 pg/mL per 106, respectively). To discover whether CCR4+ T cells acquired suppressive function, PBMCs were labeled with CFSE and then stimulated with irradiated allogeneic antigen-presenting cells (APCs) and OKT3, with or without the addition of control or CCR4+ T cells. As shown in Figure 3D, the proliferation of CD8+ T cells was unaffected by either control or CCR4+ T cells, suggesting that CCR4+ cells do not acquire inhibitory properties. In contrast, the proliferation of CD8+ T cells was significantly inhibited when CD4+CD25bright Tregs were added to the culture.
Immunophenotype and function of T cells overexpressiong CCR4 are retained. (A) The phenotypic composition of NT ( ) and CCR4+ (■) T cells. The data are mean ± SD of 5 healthy donors. T-cell markers are shown on the x-axis. No significant differences were observed if cells overexpressed CCR4. (B) Expression of naive, central memory, and effector memory surface markers on CTR (
) and CCR4+ (■) T cells. The data are mean ± SD of 5 healthy donors. T-cell markers are shown on the x-axis. No significant differences were observed if cells overexpressed CCR4. (B) Expression of naive, central memory, and effector memory surface markers on CTR ( ) and CCR4+ (■) T cells is not significantly different. The data are mean ± SD of 4 donors. (C) The production of Th1 (IFN-γ and TNF-α) and Th2 (IL-10 and IL-4) cytokines by CTR (
) and CCR4+ (■) T cells is not significantly different. The data are mean ± SD of 4 donors. (C) The production of Th1 (IFN-γ and TNF-α) and Th2 (IL-10 and IL-4) cytokines by CTR ( ) and CCR4+ (■) T cells 24 hours after stimulation with OKT3. No significant differences in cytokine production were detected, suggesting that the transgenic expression of CCR4 does not induce the acquisition of a Th2 profile. (D) CCR4+ T cells do not acquire inhibitory function. The inhibitory activity of T cells was evaluated using a CFSE-based suppression assay in which PBMCs labeled with CFSE are stimulated with irradiated allogeneic PBMCs and OKT3 in the absence (top left graph) or in the presence of freshly isolated CD4+CD25bright cells (top right graph), CTR (bottom left graph), or CCR4+ T cells (bottom right graph). The panel indicates a significant number of divisions (CFSE partitioning) of T cells in the absence or presence of CTR or CCR4+ T cells (evident in the left quadrants). In contrast, the divisions are significantly reduced in the presence of Treg cells. Shown is one of 3 donors studied, illustrative of results from all.
) and CCR4+ (■) T cells 24 hours after stimulation with OKT3. No significant differences in cytokine production were detected, suggesting that the transgenic expression of CCR4 does not induce the acquisition of a Th2 profile. (D) CCR4+ T cells do not acquire inhibitory function. The inhibitory activity of T cells was evaluated using a CFSE-based suppression assay in which PBMCs labeled with CFSE are stimulated with irradiated allogeneic PBMCs and OKT3 in the absence (top left graph) or in the presence of freshly isolated CD4+CD25bright cells (top right graph), CTR (bottom left graph), or CCR4+ T cells (bottom right graph). The panel indicates a significant number of divisions (CFSE partitioning) of T cells in the absence or presence of CTR or CCR4+ T cells (evident in the left quadrants). In contrast, the divisions are significantly reduced in the presence of Treg cells. Shown is one of 3 donors studied, illustrative of results from all.
Immunophenotype and function of T cells overexpressiong CCR4 are retained. (A) The phenotypic composition of NT ( ) and CCR4+ (■) T cells. The data are mean ± SD of 5 healthy donors. T-cell markers are shown on the x-axis. No significant differences were observed if cells overexpressed CCR4. (B) Expression of naive, central memory, and effector memory surface markers on CTR (
) and CCR4+ (■) T cells. The data are mean ± SD of 5 healthy donors. T-cell markers are shown on the x-axis. No significant differences were observed if cells overexpressed CCR4. (B) Expression of naive, central memory, and effector memory surface markers on CTR ( ) and CCR4+ (■) T cells is not significantly different. The data are mean ± SD of 4 donors. (C) The production of Th1 (IFN-γ and TNF-α) and Th2 (IL-10 and IL-4) cytokines by CTR (
) and CCR4+ (■) T cells is not significantly different. The data are mean ± SD of 4 donors. (C) The production of Th1 (IFN-γ and TNF-α) and Th2 (IL-10 and IL-4) cytokines by CTR ( ) and CCR4+ (■) T cells 24 hours after stimulation with OKT3. No significant differences in cytokine production were detected, suggesting that the transgenic expression of CCR4 does not induce the acquisition of a Th2 profile. (D) CCR4+ T cells do not acquire inhibitory function. The inhibitory activity of T cells was evaluated using a CFSE-based suppression assay in which PBMCs labeled with CFSE are stimulated with irradiated allogeneic PBMCs and OKT3 in the absence (top left graph) or in the presence of freshly isolated CD4+CD25bright cells (top right graph), CTR (bottom left graph), or CCR4+ T cells (bottom right graph). The panel indicates a significant number of divisions (CFSE partitioning) of T cells in the absence or presence of CTR or CCR4+ T cells (evident in the left quadrants). In contrast, the divisions are significantly reduced in the presence of Treg cells. Shown is one of 3 donors studied, illustrative of results from all.
) and CCR4+ (■) T cells 24 hours after stimulation with OKT3. No significant differences in cytokine production were detected, suggesting that the transgenic expression of CCR4 does not induce the acquisition of a Th2 profile. (D) CCR4+ T cells do not acquire inhibitory function. The inhibitory activity of T cells was evaluated using a CFSE-based suppression assay in which PBMCs labeled with CFSE are stimulated with irradiated allogeneic PBMCs and OKT3 in the absence (top left graph) or in the presence of freshly isolated CD4+CD25bright cells (top right graph), CTR (bottom left graph), or CCR4+ T cells (bottom right graph). The panel indicates a significant number of divisions (CFSE partitioning) of T cells in the absence or presence of CTR or CCR4+ T cells (evident in the left quadrants). In contrast, the divisions are significantly reduced in the presence of Treg cells. Shown is one of 3 donors studied, illustrative of results from all.
Forced expression of CCR4 can be obtained in T lymphocytes isolated from HL patients.
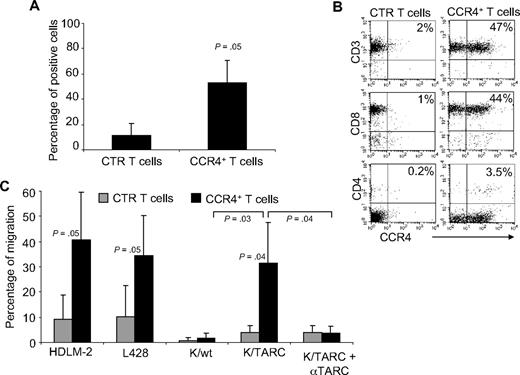
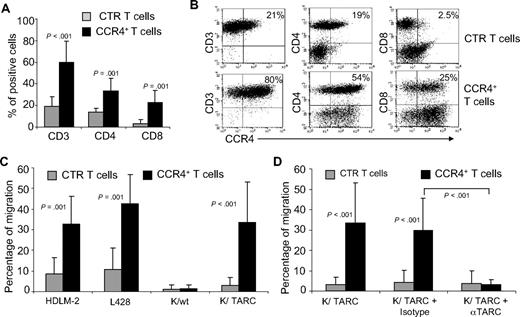
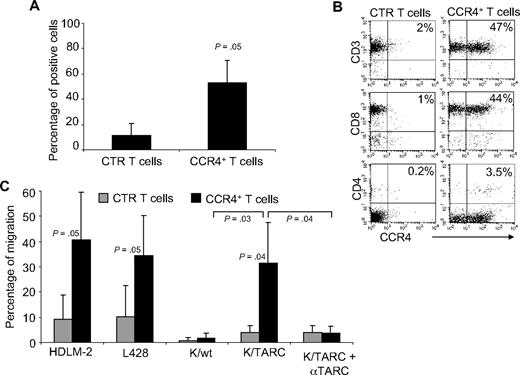
To ensure this approach was feasible in patients affected by HL, we evaluated whether CCR4 could be expressed in primary T cells from the peripheral blood of HL patients with active disease. After transduction, CCR4 was expressed by 53% plus or minus 18% of T cells, whereas only 11% plus or minus 9% of control T cells expressed this chemokine receptor (Figure 4A). Similar to observations made with T cells from healthy donors, expression of CCR4 could be detected on both CD4+ and CD8+ T cells after transduction (Figure 4B). CCR4 was also functional in these patient-derived T lymphocytes. As shown in Figure 4C, migration in TARC gradients toward HDLM-2 and L428 was significantly improved for CCR4+ T cells (41% ± 18% and 34% ± 16%) compared with control T cells (9% ± 19% for HDLM-2 and 10% ± 12% for L428; P = .05). In addition, improved migration was observed toward Karpas/TARC (32% ± 16%) compared with Karpas/wt (2% ± 2%; P = .03), whereas no migration was observed for control T cells to Karpas/wt (1% ± 1%) or Karpas/TARC (4% ± 2%). As anticipated, migration was TARC specific as it was significantly inhibited in the presence of TARC-blocking antibodies but not by an isotype control (percentage migration, 4% ± 3% vs 32% ± 16%; P = .04).
Expression and improved migration of CCR4+ T cells established from persons affected by HL. (A) The expression of CCR4 by CTR ( ) and transduced (■) T cells established from 4 persons with HL. The data are mean ± SD. (B) A representative phenotypic analysis. (C) The migration of CTR (
) and transduced (■) T cells established from 4 persons with HL. The data are mean ± SD. (B) A representative phenotypic analysis. (C) The migration of CTR ( ) and CCR4+ (■) T cells toward TARC gradients, using a transwell migration assay. T-cell migration was evaluated using culture supernatants collected from HDLM-2 and L428, which physiologically produce high amounts of TARC, and against Karpas genetically modified to produce TARC (K/TARC). K/wt represents migration toward Karpas wild-type, which produces a negligible amount of TARC, as a negative control. Migration is significantly increased for CCR4+ T cells compared with CTR T cells. In addition, the figure shows that the improved migration of CCR4+ T cells (■) is TARC mediated as it is inhibited by addition of anti-TARC antibodies but not by addition of an isotype control.
) and CCR4+ (■) T cells toward TARC gradients, using a transwell migration assay. T-cell migration was evaluated using culture supernatants collected from HDLM-2 and L428, which physiologically produce high amounts of TARC, and against Karpas genetically modified to produce TARC (K/TARC). K/wt represents migration toward Karpas wild-type, which produces a negligible amount of TARC, as a negative control. Migration is significantly increased for CCR4+ T cells compared with CTR T cells. In addition, the figure shows that the improved migration of CCR4+ T cells (■) is TARC mediated as it is inhibited by addition of anti-TARC antibodies but not by addition of an isotype control.
Expression and improved migration of CCR4+ T cells established from persons affected by HL. (A) The expression of CCR4 by CTR ( ) and transduced (■) T cells established from 4 persons with HL. The data are mean ± SD. (B) A representative phenotypic analysis. (C) The migration of CTR (
) and transduced (■) T cells established from 4 persons with HL. The data are mean ± SD. (B) A representative phenotypic analysis. (C) The migration of CTR ( ) and CCR4+ (■) T cells toward TARC gradients, using a transwell migration assay. T-cell migration was evaluated using culture supernatants collected from HDLM-2 and L428, which physiologically produce high amounts of TARC, and against Karpas genetically modified to produce TARC (K/TARC). K/wt represents migration toward Karpas wild-type, which produces a negligible amount of TARC, as a negative control. Migration is significantly increased for CCR4+ T cells compared with CTR T cells. In addition, the figure shows that the improved migration of CCR4+ T cells (■) is TARC mediated as it is inhibited by addition of anti-TARC antibodies but not by addition of an isotype control.
) and CCR4+ (■) T cells toward TARC gradients, using a transwell migration assay. T-cell migration was evaluated using culture supernatants collected from HDLM-2 and L428, which physiologically produce high amounts of TARC, and against Karpas genetically modified to produce TARC (K/TARC). K/wt represents migration toward Karpas wild-type, which produces a negligible amount of TARC, as a negative control. Migration is significantly increased for CCR4+ T cells compared with CTR T cells. In addition, the figure shows that the improved migration of CCR4+ T cells (■) is TARC mediated as it is inhibited by addition of anti-TARC antibodies but not by addition of an isotype control.
T lymphocytes expressing transgenic CCR4 have improved migration toward TARC-secreting tumors in vivo.
We next determined whether forced expression of CCR4 by activated T cells produced improved migration in vivo. Sublethally irradiated SCID mice were engrafted subcutaneously with 5 × 106 Karpas/wt on the left flank and 5 × 106 Karpas/TARC on the right flank to evaluate TARC-mediated T-cell migration within the same animal. Thus, each mouse acted as a “self-control” for unmodified and CCR4+ T cells. We ensured that the 2 cell lines Karpas/wt and Karpas/TARC had comparable in vivo growth (data not shown) and that TARC could be detected in the plasma of mice engrafted with tumor cells (696 ± 141 pg/mL when the engrafted tumor was 1 cm3). After tumor engraftment, mice were infused intravenously with either 10 × 106 control or CCR4+ T lymphocytes. To monitor the migration over time of infused T cells using the IVIS Xenogen imager, control and CCR4+ T cells were further modified to express eGFP-FFLuc as previously described (Figure 5A).16,18,21 As shown in Figure 5B and C, we observed that CCR4+ T cells preferentially accumulated at tumors producing TARC by days 9 to 12 (Karpas/TARC = 4.4 × 105 ± 2.7 × 105 p/sec/cm2/sr vs Karpas/wt = 5 × 104 ± 2.1 × 104 p/sec/cm2/sr; P = .04). In contrast, the signals detected at the tumor sites for control T cells were low and showed no significant differences between Karpas/TARC (2.7 × 104 ± 5.6 × 103 p/sec/cm2/ser) or Karpas/wt (2.6 × 104 ± 1 × 104 p/sec/cm2/sr; P = .8). Figure 5D shows that the increase of the bioluminescence signal corresponded to an increase in T cells localized at the TARC-secreting tumor site.
Improved in vivo migration of CCR4+ T cells. CTR and CCR4+ T cells were transduced to express eGFP-FFLuc to monitor their migration in vivo in SCID mice, using the IVIS imager system. (A) The expression of eGFP-FFLuc on CTR (top plot) and CCR4+ (bottom plot) T cells evaluated by GFP. (B) The bioluminescence signal from CTR and CCR4+ T cells in 3 representative SCID mice/group engrafted with TARC− tumor (K/wt) on the left side and the TARC+ tumor (K/TARC) on the right side. Whereas no significant expansion of the bioluminescent signal was observed to either site of tumor in mice receiving CTR T cells (top picture in each pair), an increase in bioluminescence was observed in mice receiving CCR4+ T cells (bottom picture in each pair) only at the site of the tumor producing TARC. (C) The fold change of bioluminescence signal between K/wt and K/TARC sites for CTR (○) and CCR4+ (■) T cells. Data are mean ± SD of 6 mice. (D) The immunophenotype of K/wt (left plot) and K/TARC (right plot) tumors isolated from one representative mouse that received CCR4+ T cells, killed on day 9. After removal, the tumors were homogenized and cells stained to distinguish T cells (using an antihuman CD3 antibody) from Karpas tumor cells (using an antihuman CD30 antibody). As shown in the panel, the proportion of cells detectable at the site of tumor-secreting TARC (5.6%) was more than 10-fold higher compared with K/wt (0.5%). This correlated with the increase in bioluminescence signal at the site of K/TARC tumor (2.1 × 106 p/sec/cm2/sr) compared with the site of K/wt tumor (1.4 × 105 p/sec/cm2/sr).
Improved in vivo migration of CCR4+ T cells. CTR and CCR4+ T cells were transduced to express eGFP-FFLuc to monitor their migration in vivo in SCID mice, using the IVIS imager system. (A) The expression of eGFP-FFLuc on CTR (top plot) and CCR4+ (bottom plot) T cells evaluated by GFP. (B) The bioluminescence signal from CTR and CCR4+ T cells in 3 representative SCID mice/group engrafted with TARC− tumor (K/wt) on the left side and the TARC+ tumor (K/TARC) on the right side. Whereas no significant expansion of the bioluminescent signal was observed to either site of tumor in mice receiving CTR T cells (top picture in each pair), an increase in bioluminescence was observed in mice receiving CCR4+ T cells (bottom picture in each pair) only at the site of the tumor producing TARC. (C) The fold change of bioluminescence signal between K/wt and K/TARC sites for CTR (○) and CCR4+ (■) T cells. Data are mean ± SD of 6 mice. (D) The immunophenotype of K/wt (left plot) and K/TARC (right plot) tumors isolated from one representative mouse that received CCR4+ T cells, killed on day 9. After removal, the tumors were homogenized and cells stained to distinguish T cells (using an antihuman CD3 antibody) from Karpas tumor cells (using an antihuman CD30 antibody). As shown in the panel, the proportion of cells detectable at the site of tumor-secreting TARC (5.6%) was more than 10-fold higher compared with K/wt (0.5%). This correlated with the increase in bioluminescence signal at the site of K/TARC tumor (2.1 × 106 p/sec/cm2/sr) compared with the site of K/wt tumor (1.4 × 105 p/sec/cm2/sr).
Functional coexpression of CCR4 and CAR-CD30 in T lymphocytes can be achieved using a bicistronic vector.
After establishing that the forced expression of CCR4 by activated T cells enhances their migration toward TARC-secreting tumors without altering their Th1/T effector profile, we determined whether the enhanced migration of these cells to HL tumors could be coupled with antilymphoma specificity by providing them with a CAR targeting the CD30 molecule (CAR-CD30).5,16
Transduction efficiency.
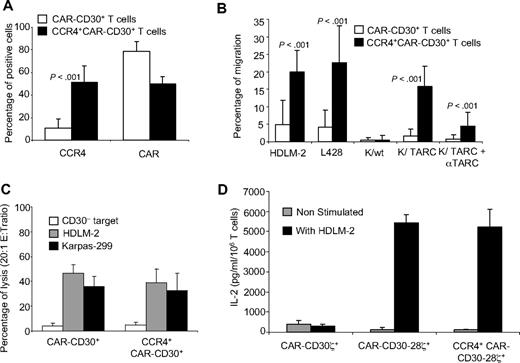
Activated T cells established from 10 healthy donors were transduced with a CCR4(I)CAR-CD30 bicistronic vector. As a control, we used the same T cells transduced with a CAR-CD30 monocistronic vector. As shown in Figure 6A, T cells transduced with either the CAR-CD30 or CCR4(I)CAR-CD30 vector expressed significant levels of CAR-CD30, although the expression of this molecule was higher in cells transduced with the vector encoding CAR-CD30 alone (79% ± 8% vs 50% ± 6%; P < .001). In contrast, only T cells transduced with CCR4(I)CAR-CD30 expressed significant levels of CCR4, and the expression of this molecule remained low in cells transduced with CAR-CD30 alone (52% ± 14% vs 11% ± 8%; P < .001).
Improved migration of activated T lymphocytes genetically modified to overexpress CCR4 and a CAR targeting the CD30 antigen expressed by HL. A bicistronic vector encoding CCR4 and CAR-CD30 was constructed and used to transduce T cells from 8 healthy donors. (A) The expression of CCR4 and CAR-CD30 by T cells transduced with a retroviral vector encoding CAR-CD30 (□) or with a bicistronic retroviral vector encoding CCR4 and CAR-CD30 (■). Surface expression of the CCR4 and CAR was evaluated by FACS analysis. (B) The migration of CAR-CD30+ (□) and CCR4+CAR-CD30+ (■) T cells toward TARC gradients, using a transwell migration assay. T-cell migration was evaluated using culture supernatants collected from HDLM-2 and L428, which physiologically produce high amounts of TARC, and against Karpas genetically modified to produce TARC (K/TARC). Karpas wild type (K/wt) was used as a control. The panel indicates that migration toward TARC is significantly improved for T cells genetically modified to overexpress CCR4 using the bicistronic vector CCR4(I)CAR-CD30. This improved migration was TARC mediated as it was inhibited by addition of anti-TARC antibodies but not by addition of an isotype control. (C) Killing of CD30+ (HDLM-2,  ; Karpas, ■) and CD30− (□) tumor cells by CAR-CD30+ and CCR4+CD30-CAR+ T cells. (D) The measurement of IL-2 cytokine released in the supernatant of T cells cocultured with or without HDLM-2 and assessed using a specific ELISA assay. T cells were transduced with either CAR-CD30 or CCR4(I)CAR-CD30, where CAR molecules also incorporate the CD28 endodomain. As a control, T cells were also transduced with the same CAR targeting CD30 but lacking the CD28 endodomain (CD30CARζ). As anticipated, we observed enhanced production of IL-2 by T cells transduced with the CAR containing the CD28 endodomain (CAR-CD30), regardless of coexpression of CCR4, but not by T cells transduced with CD30CARζ lacking CD28. The figure indicates that T cells transduced with CCR4(I)CAR-CD30 vector produce IL-2 in amounts comparable with that of T cells transduced with the vector encoding CAR-CD30 alone, confirming that the CD28 pathway is not impaired by the coexpression of CCR4. Data are mean ± SD of 10 donors.
; Karpas, ■) and CD30− (□) tumor cells by CAR-CD30+ and CCR4+CD30-CAR+ T cells. (D) The measurement of IL-2 cytokine released in the supernatant of T cells cocultured with or without HDLM-2 and assessed using a specific ELISA assay. T cells were transduced with either CAR-CD30 or CCR4(I)CAR-CD30, where CAR molecules also incorporate the CD28 endodomain. As a control, T cells were also transduced with the same CAR targeting CD30 but lacking the CD28 endodomain (CD30CARζ). As anticipated, we observed enhanced production of IL-2 by T cells transduced with the CAR containing the CD28 endodomain (CAR-CD30), regardless of coexpression of CCR4, but not by T cells transduced with CD30CARζ lacking CD28. The figure indicates that T cells transduced with CCR4(I)CAR-CD30 vector produce IL-2 in amounts comparable with that of T cells transduced with the vector encoding CAR-CD30 alone, confirming that the CD28 pathway is not impaired by the coexpression of CCR4. Data are mean ± SD of 10 donors.
Improved migration of activated T lymphocytes genetically modified to overexpress CCR4 and a CAR targeting the CD30 antigen expressed by HL. A bicistronic vector encoding CCR4 and CAR-CD30 was constructed and used to transduce T cells from 8 healthy donors. (A) The expression of CCR4 and CAR-CD30 by T cells transduced with a retroviral vector encoding CAR-CD30 (□) or with a bicistronic retroviral vector encoding CCR4 and CAR-CD30 (■). Surface expression of the CCR4 and CAR was evaluated by FACS analysis. (B) The migration of CAR-CD30+ (□) and CCR4+CAR-CD30+ (■) T cells toward TARC gradients, using a transwell migration assay. T-cell migration was evaluated using culture supernatants collected from HDLM-2 and L428, which physiologically produce high amounts of TARC, and against Karpas genetically modified to produce TARC (K/TARC). Karpas wild type (K/wt) was used as a control. The panel indicates that migration toward TARC is significantly improved for T cells genetically modified to overexpress CCR4 using the bicistronic vector CCR4(I)CAR-CD30. This improved migration was TARC mediated as it was inhibited by addition of anti-TARC antibodies but not by addition of an isotype control. (C) Killing of CD30+ (HDLM-2,  ; Karpas, ■) and CD30− (□) tumor cells by CAR-CD30+ and CCR4+CD30-CAR+ T cells. (D) The measurement of IL-2 cytokine released in the supernatant of T cells cocultured with or without HDLM-2 and assessed using a specific ELISA assay. T cells were transduced with either CAR-CD30 or CCR4(I)CAR-CD30, where CAR molecules also incorporate the CD28 endodomain. As a control, T cells were also transduced with the same CAR targeting CD30 but lacking the CD28 endodomain (CD30CARζ). As anticipated, we observed enhanced production of IL-2 by T cells transduced with the CAR containing the CD28 endodomain (CAR-CD30), regardless of coexpression of CCR4, but not by T cells transduced with CD30CARζ lacking CD28. The figure indicates that T cells transduced with CCR4(I)CAR-CD30 vector produce IL-2 in amounts comparable with that of T cells transduced with the vector encoding CAR-CD30 alone, confirming that the CD28 pathway is not impaired by the coexpression of CCR4. Data are mean ± SD of 10 donors.
; Karpas, ■) and CD30− (□) tumor cells by CAR-CD30+ and CCR4+CD30-CAR+ T cells. (D) The measurement of IL-2 cytokine released in the supernatant of T cells cocultured with or without HDLM-2 and assessed using a specific ELISA assay. T cells were transduced with either CAR-CD30 or CCR4(I)CAR-CD30, where CAR molecules also incorporate the CD28 endodomain. As a control, T cells were also transduced with the same CAR targeting CD30 but lacking the CD28 endodomain (CD30CARζ). As anticipated, we observed enhanced production of IL-2 by T cells transduced with the CAR containing the CD28 endodomain (CAR-CD30), regardless of coexpression of CCR4, but not by T cells transduced with CD30CARζ lacking CD28. The figure indicates that T cells transduced with CCR4(I)CAR-CD30 vector produce IL-2 in amounts comparable with that of T cells transduced with the vector encoding CAR-CD30 alone, confirming that the CD28 pathway is not impaired by the coexpression of CCR4. Data are mean ± SD of 10 donors.
Migration.
We then tested whether CCR4 expression obtained using the bicistronic vector improved migration in vitro, using the transwell migration assay. The migration of CCR4+CAR-CD30+ T cells toward HDLM-2 and L-428 supernatants was significantly increased (percentage migration, 17% ± 6% and 19% ± 9%, respectively) compared with T cells transduced with the monocistronic vector encoding CAR-CD30 (percentage migration, 4% ± 6% and 5% ± 4%, respectively; P < .001; Figure 6B). In addition, migration toward Karpas/TARC supernatant was significantly improved (percentage migration, 13% ± 5%) compared with migration toward Karpas/wt supernatant (percentage migration, 0.4% ± 1%; P < .001; Figure 6B). In contrast, migration of T cells transduced with CAR-CD30 alone was minimal toward both Karpas/wt and Karpas/TARC supernatants (percentage migration, 0.6% ± 1% and 0.9% ± 1%, respectively; Figure 6B).
Effector function.
We next tested whether the expression of CAR-CD30 from the bicistronic vector made T lymphocytes cytotoxic to CD30+ tumor cells. T cells transduced with the bicistronic vector CCR4(I)CAR-CD30 retained their killing of CD30+ tumor cells (39% ± 15% for HDLM-2 and 33% ± 16% for Karpas-299 at 20:1 effector/target ratio) at levels comparable with that of T cells transduced with the monocistronic vector CAR-CD30 (47% ± 11% and 36% ± 8%, respectively; Figure 6C). Killing of CD30− targets was always less than 10% for both CCR4+CAR-CD30+ and CAR-CD30+ T lymphocytes. As expected, no cytotoxicity against CD30+ targets was produced by control T cells (1.4% ± 0.5% for HDLM-2 and 2.7% ± 1% for Karpas-299, respectively), and no significant killing of CD30− targets was produced by control T cells (data not shown).
Because CAR-CD30 also contains the costimulatory endodomain CD28, we measured the IL-2 released by CAR-CD30+ cells after exposure to CD30+ targets. IL-2 was detected in supernatants collected from T cells transduced with either CAR-CD30 or CCR4(I)CAR-CD30 (5427 ± 427 pg/mL per 106 cells and 5238 ± 873 pg/mL per 106 cells, respectively), confirming that the CD28 pathway is not impaired by the coexpression of CCR4. As expected, T lymphocytes transduced with a CAR targeting CD30 but lacking the coexpression of CD28 endodomain did not produce significant amounts of IL-2 in response to CD30+ tumor cells (Figure 6D).
Forced expression of CCR4 in CAR-CD30+ T cells improves migration and antitumor activity in vivo
Improved in vivo migration.
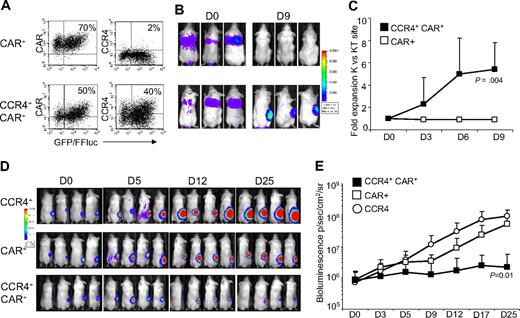
To evaluate the in vivo migration of T cells, SCID mice (8 mice/group) were engrafted subcutaneously with Karpas/wt and Karpas/TARC cells (5 × 106 cells/mice) on the left and right flank, respectively. Seven days later, mice were infused intravenously either with CAR-CD30+ or CCR4+CAR-CD30+ T cells (10 × 106 cells per mouse) expressing eGFP-FFLuc (Figure 7A). When mice were injected with CCR4+CAR-CD30+ cells, the bioluminescence signal, and thus T-cell migration, was greater at the site of the Karpas/TARC cells (3.2 × 105 ± 1.9 × 105 p/sec/cm2/sr) by days 6 to 9, compared with the Karpas/wt cell site (5.5 × 104 ± 3.2 × 104 p/sec/cm2/sr; P = .01; Figure 7B,C). In contrast, no significant signal accumulation was detected at the Karpas/wt site or Karpas/TARC site when mice were infused with T cells expressing only CAR-CD30 (Karpas/TARC cells = 3.7 × 104 ± 1.8 × 104 p/sec/cm2/sr vs Karpas/wt cells = 4.4 × 104 ± 2.8 × 104 p/sec/cm2/sr; P = .2).
Improved in vivo migration and antitumor activity of T cells transduced with a bicistronic vector encoding both CCR4 and CAR-CD30. For in vivo experiments, T cells transduced with CAR-CD30 or the bicistronic vector encoding CCR4 and CAR-CD30 were further transduced to express eGFP-FFLuc to monitor their migration in vivo in SCID mice using the IVIS system. (A) The expression of eGFP-FFLuc on CAR-CD30+ and CCR4+CAR-CD30+ cells in one representative donor. (B) The bioluminescence signal from T cells in 3 representative SCID mice engrafted with TARC− tumor (Karpas/wt) on the left side and the TARC+ tumor (Karpas/TACR) on the right side. Whereas no significant expansion of the bioluminescent signal was observed at either tumor site in mice receiving CAR-CD30+ T cells (top panels), a significant increase of bioluminescence was observed in mice receiving CCR4+CAR-CD30+ T cells (bottom panels) at the site of tumor-producing TARC. (C) The fold change of bioluminescence signal between K/wt and K/TARC site for CAR-CD30+ (□) and CCR4+CAR-CD30+ (■) T cells. Data are mean ± SD of 8 mice. Data confirm that CCR4 expressed by T cells using a bicistronic vector remain functional in vivo. To evaluate in vivo antitumor activity of T cells transduced with the bicistronic vector, the HL-derived cell line HDLM-2 was further transduced with FFLuc and inplanted subcutaneously into NOG-SCID mice (7 mice/group). Tumor growth was monitored by measuring bioluminescence signals with the IVIS system. Mice then received intravenous T cells transduced with retroviral vectors encoding either CCR4 or CAR-CD30, or the bicistronic vector CCR4(I)CAR-CD30. (D) The bioluminescence signal of tumor cells in 4 representative mice/group. In mice receiving CCR4+ or CAR-CD30+ T cells, the bioluminescence signal, and thus tumor, increased over time. In contrast, in mice that received CCR4+CAR-CD30+ T cells, the signal remained stable, indicating tumor control. (E) The data from all 7 mice/group.
Improved in vivo migration and antitumor activity of T cells transduced with a bicistronic vector encoding both CCR4 and CAR-CD30. For in vivo experiments, T cells transduced with CAR-CD30 or the bicistronic vector encoding CCR4 and CAR-CD30 were further transduced to express eGFP-FFLuc to monitor their migration in vivo in SCID mice using the IVIS system. (A) The expression of eGFP-FFLuc on CAR-CD30+ and CCR4+CAR-CD30+ cells in one representative donor. (B) The bioluminescence signal from T cells in 3 representative SCID mice engrafted with TARC− tumor (Karpas/wt) on the left side and the TARC+ tumor (Karpas/TACR) on the right side. Whereas no significant expansion of the bioluminescent signal was observed at either tumor site in mice receiving CAR-CD30+ T cells (top panels), a significant increase of bioluminescence was observed in mice receiving CCR4+CAR-CD30+ T cells (bottom panels) at the site of tumor-producing TARC. (C) The fold change of bioluminescence signal between K/wt and K/TARC site for CAR-CD30+ (□) and CCR4+CAR-CD30+ (■) T cells. Data are mean ± SD of 8 mice. Data confirm that CCR4 expressed by T cells using a bicistronic vector remain functional in vivo. To evaluate in vivo antitumor activity of T cells transduced with the bicistronic vector, the HL-derived cell line HDLM-2 was further transduced with FFLuc and inplanted subcutaneously into NOG-SCID mice (7 mice/group). Tumor growth was monitored by measuring bioluminescence signals with the IVIS system. Mice then received intravenous T cells transduced with retroviral vectors encoding either CCR4 or CAR-CD30, or the bicistronic vector CCR4(I)CAR-CD30. (D) The bioluminescence signal of tumor cells in 4 representative mice/group. In mice receiving CCR4+ or CAR-CD30+ T cells, the bioluminescence signal, and thus tumor, increased over time. In contrast, in mice that received CCR4+CAR-CD30+ T cells, the signal remained stable, indicating tumor control. (E) The data from all 7 mice/group.
Improved antitumor activity.
We used NOG/SCID/γcnull mice that allow significant and consistent engraftment of the HL-derived cell line HDLM-222 to examine the antitumor activity of CCR4+CAR-CD30+ cells. These NOG/SCID/γcnull mice were engrafted subcutaneously with HDLM-2 tumor cells expressing FFLuc (3 × 106 cells/mouse) and 3 to 5 days later, the mice (7/group) were infused intravenously with either CCR4+ or CAR-CD30+ or CCR4+CAR-CD30+ T cells (10 × 106 cells per mouse). Tumor growth was monitored longitudinally using bioluminescence detected by the IVIS imager. As shown in Figure 7D and E, by day 25, bioluminescence signals from the tumor cells were progressively increasing in mice that received either CCR4+ or CAR-CD30+ T lymphocytes (tumor signals increased from 0.7 × 106 ± 0.8 × 106 p/sec/cm2/sr to 92 × 106 ± 55 × 106 p/sec/cm2/sr and from 0.9 × 106 ± 0.7 × 106 p/sec/cm2/sr to 52 × 106 ± 37 × 106 p/sec/cm2/sr, respectively). In contrast, tumor bioluminescence only slowly increased in mice treated with CCR4+CAR-CD30+ T lymphocytes (from 0.8 × 106 ± 0.7 × 106 p/sec/cm2/sr to 2 × 106 ± 3 × 106 p/sec/cm2/sr); and indeed, by day 30 after T-cell infusion, tumor regression was documented in 4 of 7 mice (57%).
Discussion
Coupling the effector function of tumor-specific T cells with enhanced migration toward a specific tumor chemokine gradient has the potential to improve the clinical benefits of adoptive T-cell therapy in cancer patients. Using CD30+ HL that expresses the chemokine TARC as our model, we demonstrated that T lymphocytes engineered to coexpress the chemokine receptor, CCR4, and the effector molecule, CAR-CD30, have enhanced migration to the tumor when infused intravenously in a mouse model of HL and provide greater antilymphoma activity than T lymphocytes expressing the same CAR-CD30 receptor but lacking CCR4 expression.
The generation of T lymphocytes with specificity for tumor-associated antigens is the basis of most adoptive T-cell therapies. Using this approach, clinical responses have been obtained in a high proportion of EBV-positive HL patients receiving EBV-specific CTLs enriched for T-cell precursors targeting LMP-1 and LMP-2, the predominant EBV antigens expressed by Reed-Stenberg cells in EBV-positive HL.24 As CD30 is highly expressed by Reed-Stenberg cells,4 the infusion of T lymphocytes engineered to target CD30 by the expression of CAR-CD30 may be similarly effective in patients with EBV-negative HL. As previously reported, CAR-CD30-modified T cells do indeed show consistent cytotoxic activity against a variety of CD30+ tumor cells,5 and these benefits are seen without functional effects on other T cells because CD30 is only expressed by a small fraction of activated T cells, which may be nonessential to an effective immune response.16
Although the effectiveness of CAR-CD30 or other chimeric receptors can be enhanced by incorporation of costimulatory endodomains into the CAR molecules themselves17,18,25,26 or by expressing the receptors in antigen-specific CTLs,15,16,27,28 the antitumor effects of CTLs are also dependent on their ability to home to the sites of malignancy. Although gene marking2 and multimer staining studies1,24 showed that EBV-specific CTLs can accumulate in EBV-positive HL, these tumors, unlike EBV-negative HL, consistently express (IFN-γ)–inducible protein 10 (also known as CXCL10),8,9 the receptor for which (CXCR3) is expressed on EBV-specific CTLs.23 By contrast, the trafficking of CAR-expressing T lymphocytes to EBV-negative HL will probably be suboptimal because there is a disparity between the chemokines (such as TARC) produced by the tumors and the chemokine receptors expressed by CD8+ effector cells.
Trafficking of T cells to lymphoid organs and peripheral tissues is a multistage process,29 but soluble and tissue-bonded chemokines interacting with chemokine receptors expressed by T lymphocytes certainly play a pivotal role in determining migration under physiologic conditions and during inflammation.29 The production of chemokines by tumor cells can disrupt this process. Hence, the Reed-Stenberg cells of HL produce the chemokines TARC and MDC that selectively recruit CCR4-expressing cell subsets, including eosinophils, histiocytes, macrophages, plasma cells, and Th2 and Treg lymphocytes,8,9 which are all readily detected at tumor sites.8-10 By contrast, effector CD8+ cells express the chemokine receptors CCR2, CCR5, CXCR1, and CXCR3, allowing them to respond to the chemokines, including CCL2, CCL3, CCL4, CCL5 (Rantes), CXL10, and CXCL8,29 released during inflammation. These CD8+ effectors may still be able to traffic even to EBV-negative HL because molecular and histologic analyses of HL tissues indicate that some produce the monokine induced by IFN-γ (Mig) (CXCL9) and the chemokine CCL5 whose cognate receptors CXCR3 and CCR5 are indeed present on CD8+ cells. But although this will allow limited migration, Th2 and Treg cells are overwhelmingly present so that TARC and MDC are the more important determinants of migration. Further evidence for this importance comes from the observation that elevated serum levels of TARC/CCL17 correlate with unfavorable prognosis in patients with HL, so that this pattern of infiltrate has clinical correlates.30,31
Our data demonstrate that forced expression of CCR4 in CD8+ T lymphocytes provides them with the capacity to migrate toward TARC gradient, so that the functionality of this receptor is not restricted to the subset of T cells in which it is physiologically expressed. Conversely, expression of CCR4 in CD8+ T cells raises the concern that this molecule may also produce unwanted or altered functions, such as acquisition of inhibitory activity, diminished cytotoxic activity, or shifts in the polarity of released cytokines. Our data show that CCR4 expression in CD8+ T cells does not confer on them any discernible Th2 or Treg properties or otherwise modulate their function. Hence CCR4+CD8+ cells maintain their cytotoxic activity through CAR-CD30 against CD30+ tumors, produce IFN-γ, TNF-α, and IL-2 rather than IL-4 or IL-10, and do not acquire inhibitory function.
TARC and MDC are released by other tissues in which accumulation of CD8+ T lymphocytes may be toxic. For instance, CCR4-TARC/MDC interaction may also direct CD4+ cells to the skin, especially during inflammation.32,33 CD30 antigen, however, is not physiologically expressed in the skin, so that the CAR-CD30+ T cells should not produce cytotoxic, direct “off-target” effects even if they are present. Indeed, improved migration to the skin of CCR4+CAR-CD30+ T cells could be advantageous in extending this T-cell therapeutic approach form HL to cutaneous large cell anaplastic lymphomas that are frequently CD30+.34
In conclusion, coexpression of CCR4 by T lymphocytes can be used to improve the homing of CAR-CD30-modified T cells to CD30+ HL and thereby promote improved antilymphoma effects. A similar approach can be implemented for other types of cancer,7 provided that the targeted tumors produce specific chemokines for which T cells may not express the receptor, to maximize the antitumor activity of adoptively transferred antitumor T cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the Leukemia & Lymphoma Society Specialized Center of Research (grant no. 7018; H.E.H.) and Specialized Programs of Research Excellence in Lymphoma (H.E.H.). A.D.S. is supported by Fondazione Marco Semenza per la Ricerca sul Cancro (Milano, Italy) and by American Italian Cancer Foundation (New York, NY). G.D. is supported by the Doris Duke Charitable Foundation/Clinical Scientist development award and by a Leukemia & Lymphoma Society Translational Research grant. B.S. is supported by National Institutes of Health (grant R01CA131027) and the Leukemia & Lymphoma Society Translational Research.
National Institutes of Health
Authorship
Contribution: A.D.S., G.D., and B.S. designed the research, analyzed the data, and wrote the manuscript; A.D.S performed the majority of the experiments; B.D.A. and B.S. performed some in vitro and in vivo experiments; G.D. has supervised the generation of the retroviral vectors; A.E.F. provided assistance with some in vitro experiments; L.Z. and A.M. provided technical assistance for the in vitro and in vivo experiments, respectively; and C.M.R., H.E.H., and M.K.B. provided assistance in the design of the research and critically reviewed the manuscript. All authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Barbara Savoldo, Center for Cell and Gene Therapy, Baylor College of Medicine, 6621 Fannin St, MC 3-3320, Houston, TX 77030; e-mail: bsavoldo@bcm.tmc.edu.