Abstract
Curcumin is a natural product currently in human clinical trials for a variety of neoplastic, preneoplastic, and inflammatory conditions. We previously observed that, in cultured cells, curcumin exhibits properties of an iron chelator. To test whether the chelator activity of curcumin is sufficient to induce iron deficiency in vivo, mice were placed on diets containing graded concentrations of both iron and curcumin for 26 weeks. Mice receiving the lowest level of dietary iron exhibited borderline iron deficiency, with reductions in spleen and liver iron, but little effect on hemoglobin, hematocrit, transferrin saturation, or plasma iron. Against this backdrop of subclinical iron deficiency, curcumin exerted profound 2 effects on systemic iron, inducing a dose-dependent decline in hematocrit, hemoglobin, serum iron, and transferrin saturation, the appearance of microcytic anisocytotic red blood cells, and decreases in spleen and liver iron content. Curcumin repressed synthesis of hepcidin, a peptide that plays a central role in regulation of systemic iron balance. These results demonstrate that curcumin has the potential to affect systemic iron metabolism, particularly in a setting of subclinical iron deficiency. This may affect the use of curcumin in patients with marginal iron stores or those exhibiting the anemia of cancer and chronic disease.
Introduction
The polyphenol curcumin is the active ingredient in the herbal remedy and dietary spice turmeric. This vibrant yellow spice, derived from the rhizome of the plant Curcuma longa, has a long history of use in traditional medicines of China and India, where it is used to treat inflammatory diseases, abdominal disorders, and a variety of other ailments.1 Recent work has suggested that curcumin may have activity as a cancer chemopreventive and chemotherapeutic agent. For example, curcumin inhibits diethylnitrosamine-induced liver tumors2 and has a dose-dependent chemopreventive effect in rodent models of colon, duodenal, stomach, esophageal, and oral cancer,3-7 among others.8,9
Clinical trials of curcumin in humans have been promising. Phase 1 studies demonstrated virtually no toxicity in humans consuming up to 8 g curcumin per day for 3 months10 or a single dose of up to 12 g.11 Based on the encouraging preclinical and phase 1 clinical data, several additional human trials have been initiated and are currently enrolling patients. This includes trials testing the activity of curcumin in patients with colon cancer, pancreatic cancer, multiple myeloma, and myelodisplasia (www.clinicaltrials.gov).
Several mechanisms of action of curcumin have been demonstrated. Curcumin inhibits activation of nuclear factor-κB through blockade of I-κB kinase, and inhibits activation of cyclooxygenase 2 (COX2).12-15 Curcumin also alters activator protein 1 (AP1) complexes16 and inhibits Akt.17 Curcumin induces cytoprotective enzymes, including glutamate-cysteine ligase,18 isoforms of glutathione-S-transferase,19 as well as heme oxygenase,20 and NAD(P)H:quinone oxidoreductase 1.21 In addition to these effects on transcription and cell signaling, curcumin possesses chemical features that may further modulate its chemopreventive activity. For example, curcumin exhibits both pro- and antioxidant activity and is a free radical scavenger.22
We recently observed that liver cells treated with curcumin exhibit hallmarks of iron depletion, including decreases in the H and L subunits of the iron storage protein ferritin, increases in the iron transport protein transferrin receptor 1, and activation of iron-regulatory protein.23 A decrease in ferritin in the liver of FVB mice treated with curcumin for 12 weeks was also observed.23
Iron depletion can be induced by iron chelators. These small molecules have activity in the prevention and treatment of cancer in animal models and in patients.24,25 Curcumin has chemical properties consistent with iron chelator activity.26 The unexpected observation that cells treated with curcumin exhibited a decrease in ferritin raised the possibility that the chelator activity of curcumin might be sufficient to induce systemic iron depletion, potentially triggering or exacerbating subclinical or clinical iron deficiency. To directly test this possibility, we used dietary manipulation to model subclinical iron deficiency in mice and assessed the effects of curcumin on iron homeostasis in this setting. We demonstrate that curcumin can induce iron depletion in vivo, particularly in mice with low levels of body iron.
Methods
Animals and treatment
Mice were treated according to the guidelines established by the Animal Care and Use Committee of Wake Forest University and followed approved protocols. Male C3H/HeNCrl mice, 5 weeks of age, weighing an average of 17 g, were purchased from Charles River Breeding Laboratories (Portage, MI). Mice were maintained in a room controlled at 25°C with a relative humidity of 60% and 12-hour light/dark cycle. After arrival, mice were allowed to acclimate for 5 days on a basal AIN93M diet before being switched to defined diets. Ten mice were used for controls; all other groups consisted of 5 mice.
Diets were prepared by Dyets (Bethlehem, PA) and were based on AIN93M basal diet modified to contain various amounts of iron and curcumin as follows: 5, 12, 50, or 1000 mg iron/kg diet plus curcumin at 0% (control), 0.2%, 0.5%, and 2.0% (wt/wt). Dietary iron was purchased as ferric citrate (used for 5-, 12-, and 50-mg iron diets) and USP grade carbonyl iron powder (Reade Advanced Materials, Riverside, RI; used for 1000-mg iron diet). This resulted in slight variations in citrate content of the diets. Citrate content of the 5-, 12-, 50-, and 1000-mg iron diets was 836, 866, 1032, and 814 mg/kg, respectively. Curcumin was purchased as Turmeric type 97 (stock no. 12-970-05) from Kalsec (Kalamazoo, MI). The analysis of type 97 curcumin is 94.93% curcumin, with the residual fraction including oils and resins naturally occurring in turmeric, such as the sesquiterpene ketones and alcohols α-turmeron, β-turmeron, curlon, zingiberen, ar-turmeron, turmenorol A, and turmeronol B.27,28 These contaminants are not known to possess iron chelator activity.
Mice were killed after 6 months. Blood was collected using a heparinized needle, and tissues were snap-frozen in liquid nitrogen or fixed in neutral-buffered formalin for subsequent analysis. Weights of the mice at death averaged 37.8 plus or minus 4.1 g (mean ± SD); curcumin had no effect on weights in mice on 12- and 50-mg/kg iron diets. In mice receiving 5 mg/kg dietary iron, mean weight of mice on 0.5% curcumin was 36 plus or minus 4.4 g and weight of mice on 2.0% curcumin was 31.4 plus or minus 3.6 g (mean ± SD). In mice receiving 1000 mg/kg dietary iron, mean weight of mice on 0.5% curcumin was 31.7 plus or minus 4.5 g and weight of mice on 2% curcumin was 34.7 plus or minus 2.0 g.
Tissue culture
HepG2 cells were procured from the ATCC (Manassas, VA) and propagated in Dulbecco modified Eagle medium (DMEM/High Modified; HyClone Laboratories, Logan, UT) containing 10% fetal bovine serum (Gemini, Irvine, CA). Cells were cultured in a humidified chamber maintained at 37°C and 5% CO2. For curcumin treatments, control cells received basal media, whereas treated cells received basal media containing curcumin (Sigma-Aldrich, St Louis, MO) at final concentrations of 6.25, 12.5, and 25 μM.
Hemoglobin
Samples for hemoglobin assay were prepared from fresh blood diluted 1:100 in deionized water, and measurement was performed using the QuantiChrom Hemoglobin Assay Kit (BioAssay Systems, Hayward, CA).
Hematocrit measurement
Samples were collected from fresh blood using heparinized 40-mm microhematocrit tubes (StatSpin, Norwood, MA). Sealed hematocrit tubes were then centrifuged for 20 minutes at 4000 rpm at 4°C, and the percentage cell volume was determined using the Critocaps Micro-Hematocrit Capillary Tube Reader (Oxford Labware, St Louis, MO). Mean cell hemoglobin concentration was computed by dividing hemoblogin (g/dL) by hematocrit.
Blood smears
Blood smears were prepared from fresh blood on clean glass slides and air dried at room temperature. Wright stain reagent (WS16; Sigma-Aldrich) was used to stain blood smears according to the manufacturer's protocol. Slides were imaged using a Nikon Eclipse 50i microscope (Nikon, Melville, NY) and QImaging MicroPublisher 3.3 RTV camera (QImaging, Surrey, BC) and the image processing program ImageJ. Mean corpuscular volume was estimated semiquantitatively by comparing mean diameters of red blood cells (RBCs) to lymphocytes in peripheral blood smears. Images of smears from 2 mice from each group were captured on ImageJ and measurements made on 100 individual RBCs and approximately 20 lymphocytes from the same fields. Mean diameters of lymphocytes were not different (7.3 ± 0.9 μm in controls vs 7.8 ± 0.8 μm in curcumin-treated mice, P = .9). Mean diameter of RBCs from control mice was 5.7 plus or minus 0.5 μm, whereas mean diameter of cells from mice that had been treated with 2% curcumin was 5.2 plus or minus 0.3 μm, a statistically significant reduction (P < .001). The extent of anisocytosis was estimated semiquantitatively by an experienced hematologist (M.A.K.) using a scale of 0 to 4, as described.29
Iron/TIBC assay
Iron and unsaturated iron-binding capacity (UIBC) were measured using the Iron/TIBC Reagent Set (TECO Diagnostics, Anaheim, CA) according to the manufacturer's protocol. Total iron-binding capacity (TIBC) was calculated by the formula: iron level + UIBC = TIBC (μg/dL). Percentage iron saturations were calculated as: iron level/TIBC × 100% = % saturation.
Nonheme iron measurement
Nonheme iron was measured in 96-well plates as previously described.30
Preparation of tissues for pathology
Tissues collected at necropsy for routine histologic examination included liver, spleen, lung, heart, kidney, bone, and intestine. Tissues were immediately fixed in 10% neutral-buffered formalin for 24 hours at room temperature and stored in 70% ethanol until processing. Following fixation, femurs were fixed as above and decalcified using Decalcifier II (Surgipath Medical Industries, Richmond, IL) according to the manufacturer's protocol and stored in 70% ethanol until processing. Tissues were embedded in paraffin, processed routinely for histology, sectioned at 4 to 6 μm, and stained with hematoxylin and eosin and Perl stain. Slides were imaged using a Nikon Eclipse 50i microscope (Nikon) and QImaging MicroPublisher 3.3 RTV camera (QImaging) and the image processing program ImageJ.
Tissue homogenate preparation
Livers were ground using a liquid nitrogen–chilled mortar and pestle after the addition of tissue lysis buffer (25 mM Tris, pH 7.4, 1% Triton X-100, 1% sodium dodecyl sulfate, 1% sodium deoxycholate, 150 mM NaCl, 2 μg/mL aprotinin, 1 mM phenylmethylsulfonyl fluoride). Tissues were further homogenized on ice using a polytron style homogenizer and subjected to freeze/thawing. The homogenate was clarified by centrifugation at 18 000g for 15 minutes at 4°C. Supernatants were removed and protein concentration was determined by Bio-Rad protein assay (Hercules, CA).
Antibodies
Rabbit antimouse ferritin H and L antibodies were prepared by Biosource (Invitrogen, Carlsbad, CA). Rat antimouse transferrin receptor (TfR) antibody was purchased from Alpha Diagnostic International (San Antonio, TX). Mouse antirabbit glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was purchased from Fitzgerald Industries (Concord, MA). Antiferroportin antibody was purchased from Alpha Diagnostic International. The secondary antibody goat antirabbit IgG horseradish peroxidase (HRP) was purchased from Bio-Rad, and the secondary antibody goat antimouse IgG HRP was purchased from Calbiochem (San Diego, CA).
Western blots
Western blots were performed essentially as described.31 Proteins were resolved on 12% sodium dodecyl sulfate polyacrylamide gels and transferred to nitrocellulose membranes (Whatman, Clifton, NJ) before incubation with primary and secondary antibodies. Equivalent protein loading was demonstrated by staining membranes in 1% Ponceau S. HRP-conjugated secondary antibodies were visualized using the SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical, Rockford, IL). Films were quantified using UN-SCAN-IT Automatic digitizing system, version 5.1. Fold changes were calculated after normalization to GAPDH.
RNA-binding protein gel-shift assay
Tissue extracts were prepared and band-shift assays were conducted as described.32 Liver-tissue extracts (20 μg) were incubated with a [α-32P]UTP-radiolabeled human ferritin H iron-responsive element (IRE) probe prepared from in vitro transcription of BamHI-linearized pST18 plasmid (a generous gift of Dr P. Ponka, McGill University, Montreal, QC). RNA-protein complexes were analyzed on a 6% nondenaturing polyacrylamide gel. Gels were dried and subjected to autoradiography and Phosphorimaging. Quantification of IRE/iron-regulatory proteins (IRP) complexes was based on Phosphorimaging analysis (ImageQuaNT version 5.2; Molecular Dynamics, Sunnyvale, CA).
Real-time RT-PCR
Total RNA was isolated from mice liver tissue or HepG2 cells using TRIzol reagent at the ratio of 1 mg tissue/10 μL or 106 cells/mL TRIzol reagent. Real-time reverse-transcriptase polymerase chain reaction (RT-PCR) was performed essentially as described.33 The following primers were used: hepcidin forward primer: 5′-CTG AGC AGC ACC ACC TAT CTC-3′, reverse primer: 5′-TGG CTC TAG GCT ATG TTT TGC-3′. Samples were normalized using β-actin, forward primer: 5′-AGA GGG AAA TCG TGC GTG AC-3′, and reverse primer: 5′-CAA TAG TGA TGA CCT GGC CGT-3′.
Statistical analysis
Descriptive data (means and SD) were presented for each level of the factor of interest. Comparisons between groups were made by fitting a series of analysis of variance (ANOVA) models. For different outcomes, the independent variables were either curcumin, iron levels, or both, depending on the measure being compared (ie, a series of 1-way or 2-way ANOVA models were fit). For each model, we examined the overall effect of the independent variable(s) (curcumin or iron group) and then performed pairwise comparisons between levels of each group using specific contrasts within the ANOVA models. All models were fit using SAS, version 9.1.3.
Results
Modulation of iron status in C3H mice
Levels of iron and the response to alterations in dietary iron availability vary dramatically in a strain-specific manner among mouse species.34 To determine whether curcumin affects systemic iron, we first assessed the response of C3H mice to alterations in levels of dietary iron. We chose this strain because it has been used extensively in the study of chemopreventive agents, including curcumin.2,35-39 Mice were fed defined diets containing 5, 12, 50, and 1000 mg iron/kg diet beginning at 5 weeks of age. Basal AIN93M diet contains 48.4 mg iron/kg diet. We therefore operationally defined 50 mg/kg as a “normal” iron diet. Levels of iron in our diets thus ranged from 10-fold lower to 20-fold higher than normal iron levels. Mice were killed after 6 months. There was no effect of these dietary iron concentrations on weight gain over this period (not shown).
As seen in Table 1, hematologic parameters of iron, including hematocrit, hemoglobin, transferrin saturation, and serum iron, were similar in all mice after 6 months, despite the wide variation in dietary iron content. In contrast, direct measurements of iron in the spleen revealed that dietary iron substantially affected the content of spleen nonheme iron (Table 1).
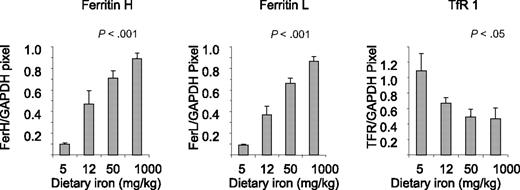
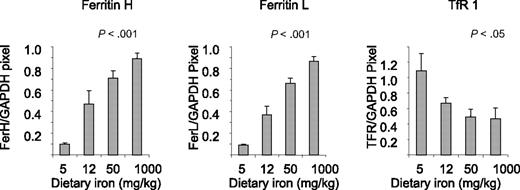
To assess effects of dietary iron on compartments of iron storage, we measured nonheme iron in the liver (Table 1). Dietary iron dramatically modulated liver iron, with iron content ranging from an average of 38 μg/g liver in mice fed 5 mg/kg diet to 552-μg/g in mice fed 1000-mg iron/kg diet. To assess whether these changes in iron content were associated with corresponding alterations in proteins of iron metabolism in the liver, we measured ferritin, a protein of iron storage,40 and TfR1, a protein that mediates iron uptake.41 These proteins are regulated by iron in an inverse way: under conditions of iron repletion, ferritin rises and TfR1 declines; conversely, in iron-depleted states, ferritin declines and TfR1 rises.40 As seen in Figure 1, dietary iron dramatically affected levels of both subunits of ferritin, ferritin H and ferritin L: in mice fed the lowest levels of iron, ferritin was nearly undetectable; at the 1000-mg iron/kg diet, ferritin rose approximately 9-fold. TfR1 was inversely affected, rising in iron-depleted mice and declining under conditions of iron repletion (Figure 1).
Dietary iron modulates ferritin L, ferritin H, and TfR1 in the liver. Liver homogenates were analyzed for expression of ferritin H, ferritin L, and TfR1 by Western blotting as described in “Western blots.” Shown are means and SD; n = 5. Protein levels showed a statistically significant change in response to iron dose (P < .001 for ferritin H and L and < .05 for TfR1).
Dietary iron modulates ferritin L, ferritin H, and TfR1 in the liver. Liver homogenates were analyzed for expression of ferritin H, ferritin L, and TfR1 by Western blotting as described in “Western blots.” Shown are means and SD; n = 5. Protein levels showed a statistically significant change in response to iron dose (P < .001 for ferritin H and L and < .05 for TfR1).
Effect of curcumin on hematologic parameters of iron metabolism
Having established conditions of subclinical iron deficiency as well as iron excess, we examined the influence of dietary curcumin on these parameters of iron metabolism. Curcumin was added to all diets at final concentrations of 0%, 0.2%, 0.5%, and 2.0%, with the highest dose corresponding approximately to the upper range of well-tolerated doses of curcumin in human clinical trials (8-12 g/day)10,11 Routine histologic examination revealed no significant lesions in any tissues examined. In particular, there was no evidence of toxicity of curcumin to the intestine, the site of iron absorption (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
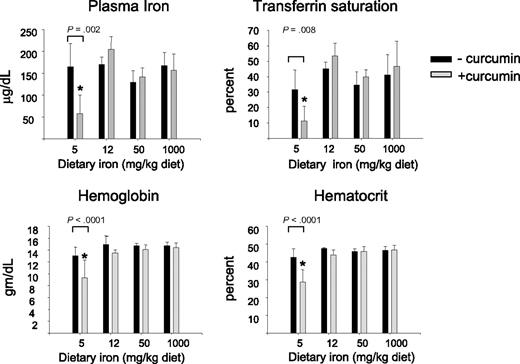
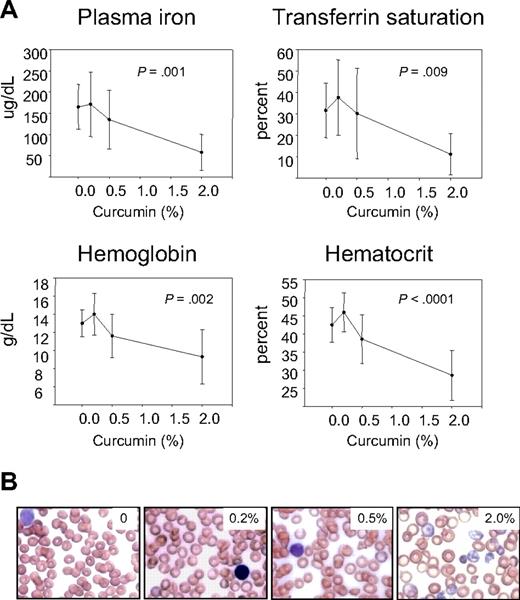
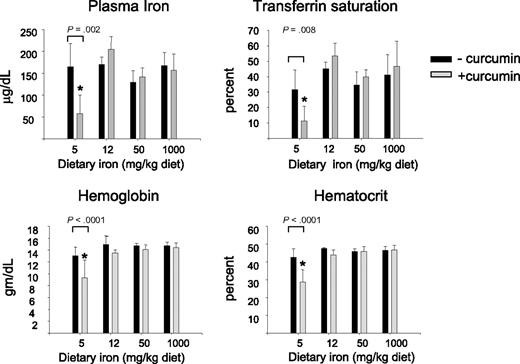
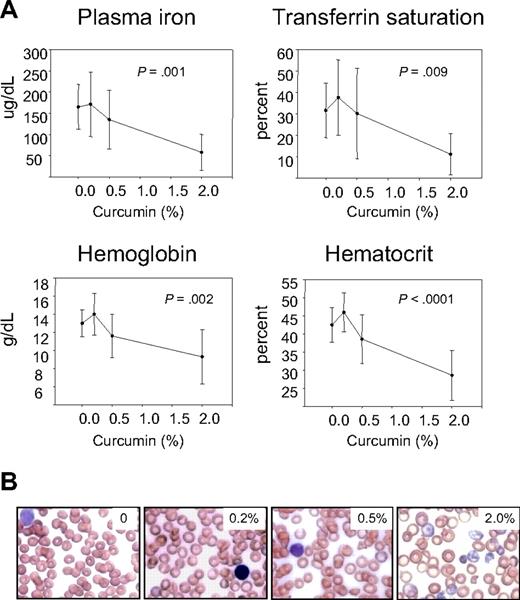
We first examined hematologic parameters of iron metabolism. As seen in Figure 2, there was no effect of curcumin on hematocrit, hemoglobin, serum iron, or transferrin saturation in mice fed iron diets containing 12- to 1000-mg iron/kg diet. However, there was a dramatic effect of curcumin on these hematologic parameters in mice consuming a low-iron diet (5 mg iron/kg diet), with curcumin causing a reduction in all parameters measured. The decline in hematocrit, hemoglobin, serum iron, and transferrin saturation was dependent on the curcumin dose (Figure 3A). Because these changes suggested that curcumin induced an iron deficiency anemia in mice on a low-iron diet, we also examined blood smears. RBCs from mice receiving 5 to 1000 mg dietary iron appeared similar to controls at all curcumin doses (not shown). Consistent with biochemical measures of iron in the blood, RBCs in peripheral blood isolated from mice fed the lowest level of dietary iron in combination with curcumin exhibited microcytosis that increased in proportion to curcumin intake (Figure 3B). Mean RBC size was reduced approximately 11%. Cells from mice treated with curcumin also appeared hypochromic, although the reduction in hemoglobin/packed cell volume (mean cell hemoglobin concentration) did not reach statistical significance. A tendency to anisocytosis was also noted at the highest level of dietary curcumin: on a scale of 0 to 4,29 peripheral blood smears from mice receiving the highest level of dietary curcumin received an average score of 2.8+ (moderate to prominent anisocytosis), whereas controls scored 1.4+ (absent or mild anisocytosis). Occasional polychromasia, presence of Howell-Jolly bodies, and irregularly shaped cells (poikilocytes) were also noted at the highest level of dietary curcumin (Figure 3B).
Curcumin affects hematologic parameters of iron in mice fed a low-iron diet. Serum iron, transferrin saturation, hemoglobin, and hematocrit were measured in mice fed different concentrations of dietary iron containing either 0% or 2% curcumin. Shown are means and SD; n = 5. Curcumin caused a statistically significant reduction in all blood parameters in mice fed 5 mg iron (P ≤ .008).
Curcumin affects hematologic parameters of iron in mice fed a low-iron diet. Serum iron, transferrin saturation, hemoglobin, and hematocrit were measured in mice fed different concentrations of dietary iron containing either 0% or 2% curcumin. Shown are means and SD; n = 5. Curcumin caused a statistically significant reduction in all blood parameters in mice fed 5 mg iron (P ≤ .008).
Curcumin-mediated reduction in hematologic parameters of iron metabolism in mice fed a low-iron diet depends on curcumin dose. (A) Plasma iron, transferrin saturation, hemoblogin, and hematocrit were measured in mice receiving 5 mg/kg dietary iron supplemented with curcumin at 0%, 0.2%, 0.5%, or 2.0%. Shown are means and SD; n = 5. The dose-dependent reduction in all parameters was statistically significant (P ≤ .009). (B) Representative peripheral blood smears from mice receiving 5 mg/kg dietary iron and 0%, 0.2%, 0.5%, or 2.0% curcumin.
Curcumin-mediated reduction in hematologic parameters of iron metabolism in mice fed a low-iron diet depends on curcumin dose. (A) Plasma iron, transferrin saturation, hemoblogin, and hematocrit were measured in mice receiving 5 mg/kg dietary iron supplemented with curcumin at 0%, 0.2%, 0.5%, or 2.0%. Shown are means and SD; n = 5. The dose-dependent reduction in all parameters was statistically significant (P ≤ .009). (B) Representative peripheral blood smears from mice receiving 5 mg/kg dietary iron and 0%, 0.2%, 0.5%, or 2.0% curcumin.
Effects of curcumin on iron in the spleen, bone marrow, and liver
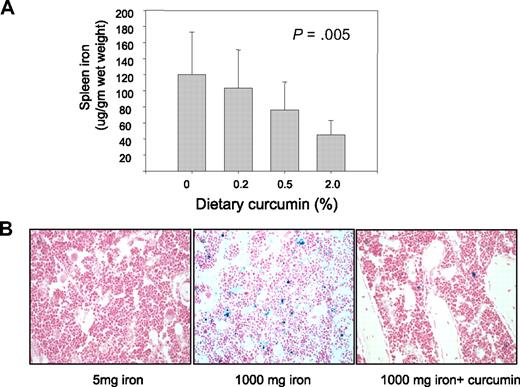
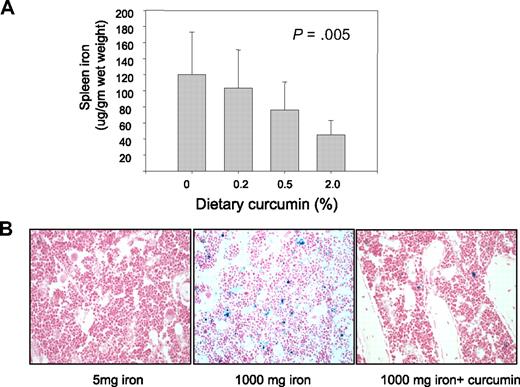
Measurements of iron in the spleen gave results similar to those observed in the peripheral blood: there was no effect of curcumin on the level of iron in the spleen of mice fed higher levels of iron (not shown), but curcumin led to a dose-dependent decline in iron in the spleen of mice fed a low-iron diet (Figure 4A).
Curcumin reduces spleen iron and stainable iron in the bone marrow. (A) Spleen nonheme iron was measured in mice receiving 5 mg/kg dietary iron supplemented with curcumin at 0%, 0.2%, 0.5%, or 2.0%. Shown are means and SD; n = 5. Spleen iron showed a statistically significant change in response to curcumin dose (P = .005 for trend). (B) Bone marrow was obtained from decalcified femurs of representative mice and stained with hematoxylin and eosin and Perl stain. Mice had received either 5 mg/kg iron plus 0 curcumin, 1000 mg/kg iron plus 0 curcumin, and 1000 mg/kg iron plus 0.2% curcumin.
Curcumin reduces spleen iron and stainable iron in the bone marrow. (A) Spleen nonheme iron was measured in mice receiving 5 mg/kg dietary iron supplemented with curcumin at 0%, 0.2%, 0.5%, or 2.0%. Shown are means and SD; n = 5. Spleen iron showed a statistically significant change in response to curcumin dose (P = .005 for trend). (B) Bone marrow was obtained from decalcified femurs of representative mice and stained with hematoxylin and eosin and Perl stain. Mice had received either 5 mg/kg iron plus 0 curcumin, 1000 mg/kg iron plus 0 curcumin, and 1000 mg/kg iron plus 0.2% curcumin.
We also assessed the effects of curcumin on stainable iron in the bone marrow (Figure 4B). Because no iron staining was evident in mice fed low levels of dietary iron, it was not possible to visualize further reductions using this method in mice fed low-iron diets. However, intense iron staining was seen in mice fed a high-iron diet, and this was decreased by curcumin (Figure 4B).
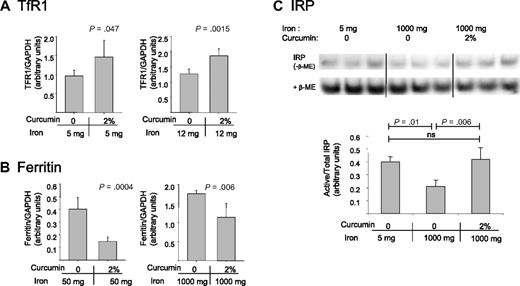
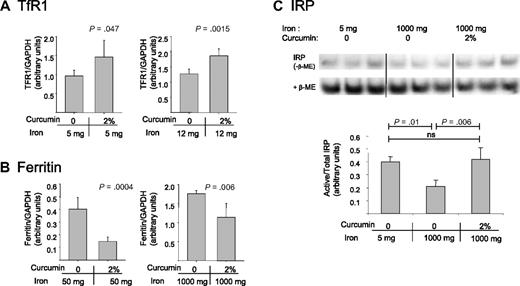
To assess whether curcumin also affected the liver, the major storage compartment for iron, we measured levels of proteins of iron metabolism in the liver. Because high dietary iron levels reduce basal levels of TfR1, curcumin's effects in mice fed high-iron diets were assessed using measurements of ferritin. Similarly, because ferritin is difficult to detect in mice fed low-iron diets, we assessed effects of curcumin in this group of animals by assessing effects of curcumin on TfR1. As shown in Figure 5A,B, curcumin induced changes in liver proteins consistent with iron depletion: TfR1 increased and ferritin decreased.
Curcumin modulates iron-regulatory proteins in the liver. (A) TfR1 was analyzed in mice fed low-iron diets (containing either 5 mg/kg or 12 mg/kg dietary iron) in the presence or absence of 2% curcumin. Liver homogenates were prepared and TfR1 levels were assessed by Western blotting. Shown are means and SD; n = 5. (B) Ferritin was analyzed in mice fed normal or high-iron diets (containing either 50 mg/kg or 1000 mg/kg dietary iron) in the presence of absence of 2% curcumin. Total ferritin (ferritin H + ferritin L) was assessed by Western blotting in liver homogenates. Shown are means and SD; n = 5. (C) The ratio of active to total IRP was analyzed using bandshift assays as described in “RNA-binding protein gel-shift assay.” Liver homogenates were prepared from mice receiving 5 mg/kg dietary iron plus 0 curcumin, 1000 mg/kg dietary iron plus 0 curcumin, or 1000 mg/kg dietary iron plus 2% curcumin. Shown are means and SD; n = 3.
Curcumin modulates iron-regulatory proteins in the liver. (A) TfR1 was analyzed in mice fed low-iron diets (containing either 5 mg/kg or 12 mg/kg dietary iron) in the presence or absence of 2% curcumin. Liver homogenates were prepared and TfR1 levels were assessed by Western blotting. Shown are means and SD; n = 5. (B) Ferritin was analyzed in mice fed normal or high-iron diets (containing either 50 mg/kg or 1000 mg/kg dietary iron) in the presence of absence of 2% curcumin. Total ferritin (ferritin H + ferritin L) was assessed by Western blotting in liver homogenates. Shown are means and SD; n = 5. (C) The ratio of active to total IRP was analyzed using bandshift assays as described in “RNA-binding protein gel-shift assay.” Liver homogenates were prepared from mice receiving 5 mg/kg dietary iron plus 0 curcumin, 1000 mg/kg dietary iron plus 0 curcumin, or 1000 mg/kg dietary iron plus 2% curcumin. Shown are means and SD; n = 3.
Effects of curcumin on iron-regulatory proteins in the liver
We also tested whether curcumin affected the activity of IRPs. IRPs serve as important intracellular regulators of proteins of iron management.42,43 Under iron-deficient conditions, these proteins are activated, leading to translational ferritin repression and TfR1 mRNA stabilization. Conversely, under iron-replete conditions, IRPs are inactivated, thereby increasing the translation of ferritin mRNA and degradation of TfR1 mRNA. To assess IRP activity, we used a band-shift assay to measure the binding of IRPs to their mRNA recognition element, the IRE. As shown in Figure 5C, activity of IRPs (binding to the IRE) was increased under conditions of iron depletion relative to conditions of iron repletion, as expected. When IRP activity was examined in the livers of mice that had received the combination of high dietary iron and curcumin, IRP activity was increased (Figure 5C). Indeed, IRP activity in curcumin-treated mice was comparable with IRP activity in mice receiving 200-fold lower levels of dietary iron (Figure 5C).
Effects of curcumin on hepcidin
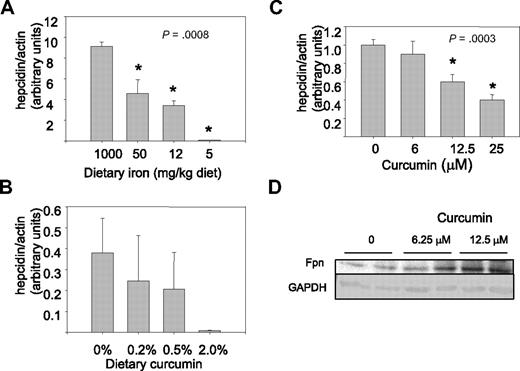
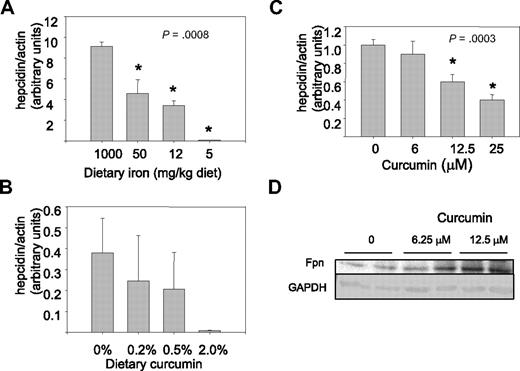
These results indicated that curcumin had a pronounced effect on iron metabolism. We therefore tested whether curcumin affected levels of hepcidin, a protein primarily produced by hepatocytes that plays a central role in regulation of systemic iron balance.41 Under conditions of iron excess, synthesis of hepcidin increases.44 Binding of hepcidin to ferroportin, an iron efflux pump in enterocytes, triggers the degradation of ferroportin and thus blocks further iron uptake. Conversely, under conditions of iron depletion, hepcidin synthesis is decreased, favoring increased systemic iron assimilation.45 Because antibodies to functional hepcidin protein are not generally available and hepcidin is controlled at the transcriptional level, we measured the effects of curcumin on hepcidin using real-time RT-PCR.46,47 As shown in Figure 6A, hepcidin levels in the liver were indeed modulated by dietary iron, with depletion of dietary iron reducing hepcidin expression. At the lowest dose of dietary iron, curcumin further reduced hepcidin levels (Figure 6B). As has been previously observed,48 levels of expression of hepcidin in mice fed a low-iron diet were highly variable. Although the trend of decreasing expression with increasing curcumin is evident, because of the variability in the data, differences in expression between mice receiving 0% and 2% curcumin were of borderline significance (P = .08). Therefore, we confirmed that curcumin could inhibit hepcidin synthesis using cultured HepG2 cells. As shown in Figure 6C, HepG2 cells exposed to curcumin for 8 hours exhibited a dose-dependent decline in hepcidin mRNA. To determine whether the decrease in hepcidin had functional consequences, we measured ferroportin, an iron efflux pump that is targeted for degradation by hepcidin.49 As anticipated, HepG2 cells treated with curcumin exhibited an increase in ferroportin compared with untreated cells (Figure 6D).
Hepcidin is decreased by dietary iron deficiency or by curcumin. (A) Hepcidin mRNA was measured by real-time RT-PCR in livers of mice receiving dietary iron ranging from 1000 to 5 mg/kg diet (n = 5). Data were normalized to β-actin mRNA. The decline in hepcidin was statistically significant. (B) Hepcidin was measured in mice receiving 5 mg/kg dietary iron supplemented with different concentrations of dietary curcumin (n = 5). The difference between the 0% and 2.0% dietary curcumin group is of borderline significance compared using a 2-sample t test for specific comparisons (P = .08); other differences were not statistically significant. (C) HepG2 hepatocytes were treated with increasing concentrations of curcumin for 8 hours and hepcidin mRNA measured by real-time RT-PCR. Data were normalized to β-actin mRNA. The decline in hepcidin was statistically significant (P < .001 for trend); 12.5 and 25 μM curcumin were statistically different from control (P ≤ .001); 6.25 μM curcumin was not different from control). (D) Duplicate cultures of HepG2 cells were treated with the indicated concentrations of curcumin for 48 hours, and ferroportin levels assessed by Western blot. GAPDH was used as a loading control.
Hepcidin is decreased by dietary iron deficiency or by curcumin. (A) Hepcidin mRNA was measured by real-time RT-PCR in livers of mice receiving dietary iron ranging from 1000 to 5 mg/kg diet (n = 5). Data were normalized to β-actin mRNA. The decline in hepcidin was statistically significant. (B) Hepcidin was measured in mice receiving 5 mg/kg dietary iron supplemented with different concentrations of dietary curcumin (n = 5). The difference between the 0% and 2.0% dietary curcumin group is of borderline significance compared using a 2-sample t test for specific comparisons (P = .08); other differences were not statistically significant. (C) HepG2 hepatocytes were treated with increasing concentrations of curcumin for 8 hours and hepcidin mRNA measured by real-time RT-PCR. Data were normalized to β-actin mRNA. The decline in hepcidin was statistically significant (P < .001 for trend); 12.5 and 25 μM curcumin were statistically different from control (P ≤ .001); 6.25 μM curcumin was not different from control). (D) Duplicate cultures of HepG2 cells were treated with the indicated concentrations of curcumin for 48 hours, and ferroportin levels assessed by Western blot. GAPDH was used as a loading control.
Discussion
The experiments reported here were undertaken as a systematic test of the ability of curcumin to modulate systemic iron homeostasis in vivo. Earlier studies of the chemistry of curcumin have revealed that curcumin exhibits iron-binding activity in cell-free systems: for example, cyclic voltammetry measurements have estimated a formation constant of 1022 for the Fe(III)–curcumin complex.26 However, we have calculated a pM of curcumin of 16.723 (where pM is the negative logarithim of the free iron concentration at 10 μM chelator and 1 μM iron), indicating moderate chelator activity relative to the highly potent chelators used for the treatment of iron overload, which have pM values that range from 19.5 to 26.6.24 Thus, curcumin's function as an iron chelator in vivo was uncertain. Previous work in our laboratory demonstrated that curcumin affects proteins of iron metabolism in cultured normal liver cells and reduces levels of ferritin in the liver, findings suggestive of functional iron chelator activity.23 Further, Srichairatanakool et al reported that curcumin increased the rate of nontransferrin-bound iron removal from thalassemic plasma when used in combination with deferiprone.50 The current and anticipated use of curcumin as a cancer chemopreventive and anticancer therapeutic prompted us to conduct a careful assessment of the extent to which curcumin modulates systemic iron in vivo.
Mice exhibit substantial strain-dependent variations in iron metabolism,34,51 so it was first necessary to determine the sensitivity of C3H mice to manipulations of iron. Our goal was to achieve modest, subclinical iron depletion. We felt this was a relevant scenario because, for most of the 1000+ years that curcumin has been used as a medicinal agent, the iron status of humans has been marginal. This is particularly true in India, where curcumin use is extensive and where diets are traditionally low in bio-available iron.52 We were able to scale levels of iron to achieve substantial depletion of iron levels in spleen, liver, and bone marrow, with limited effects on plasma hemoglobin. We also provided excess dietary iron to one group of animals. Over the 200-fold range of dietary iron provided in these diets, iron levels in the spleen and liver were substantially modified, varying over an approximately 10- and 16-fold range, respectively. Relative to a normal iron diet, the low-iron diet reduced spleen and liver iron approximately 6-fold (Table 1) with minor effects on hematologic iron parameters (Table 1). The ability to manipulate dietary iron to create states ranging from modest iron overload to subclinical iron deficiency provided an opportunity to test curcumin's ability to further modulate systemic iron.
We observed that curcumin was able to perturb all parameters of iron metabolism, particularly in mice fed a low-iron diet (5 mg iron/kg diet). In the absence of curcumin, hematopoiesis was not affected by alterations in dietary iron in these mice, although levels of storage iron in the liver and spleen were depleted relative to mice fed a norm-iron diet (Table 1). The addition of curcumin to the diets of these mice induced a phenotype of iron deficiency anemia, including a decline in serum iron, decreased hematocrit, decreased transferrin saturation, and appearance of hypochromic RBCs (Figures 2,3). Curcumin also decreased iron levels in the bone marrow and spleen (Figure 4). In the liver, curcumin-mediated changes in the liver were extensive: curcumin reduced liver iron, activated IRP, repressed ferritin, induced TfR1, and repressed synthesis of the systemic iron-regulatory hormone hepcidin (Figures 5,6). These results were not a consequence of intestinal toxicity of curcumin with resultant inhibition of iron absorption because histopathologic examination of intestinal sections showed no toxic effects of curcumin at any dose (Figure S1). Published studies have similarly demonstrated curcumin to be remarkably nontoxic in rodents and in humans,9 and to exert a protective rather than damaging effect on the rodent gastrointestinal tract.53,54
Collectively, these results demonstrate that curcumin has the potential to affect systemic iron metabolism. There are 2 important implications of these results. First, iron chelators have been shown to exert antitumor effects, both through the formation of redox-active iron complexes55 and by iron depletion.24 Thus, reduction in systemic iron resulting from the use of curcumin in the setting of a low-iron diet may contribute to the anticancer activity of curcumin. Second, curcumin may have the potential to contribute to the development of anemia in patients with marginal iron status. This may be an important consideration when curcumin is used to treat patients with marginal or depleted iron stores or those exhibiting the anemia of cancer and chronic disease.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants from the American Institute for Cancer Research (S.V.T.) and the National Institutes of Health (grant R37 DK 42412, F.M.T.; and grant KO1 DK065876, J.W.).
National Institutes of Health
Authorship
Contribution: Y.J. performed research and wrote the paper; J.W., X.D., W.W., and H.H. performed research; N.D.K., R.D., and M.A.K. analyzed data; and F.M.T. and S.V.T. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Suzy V. Torti, Department of Biochemistry, Wake Forest University School of Medicine, Winston Salem, NC 27157; e-mail: storti@wfubmc.edu.