Abstract
Staphylococcus aureus secretes several virulence factors modulating immune responses. Staphylococcal superantigen-like (SSL) proteins are a family of 14 exotoxins with homology to superantigens, but with generally unknown function. Recently, we showed that SSL5 binds to P-selectin glycoprotein ligand 1 dependently of sialyl Lewis X and inhibits P-selectin–dependent neutrophil rolling. Here, we show that SSL5 potently and specifically inhibits leukocyte activation by anaphylatoxins and all classes of chemokines. SSL5 inhibited calcium mobilization, actin polymerization, and chemotaxis induced by chemokines and anaphylatoxins but not by other chemoattractants. Antibody competition experiments showed that SSL5 targets several chemokine and anaphylatoxin receptors. In addition, transfection studies showed that SSL5 binds glycosylated N-termini of all G protein–coupled receptors (GPCRs) but only inhibits stimuli of protein nature that require the receptor N-terminus for activation. Furthermore, SSL5 increased binding of chemokines to cells independent of chemokine receptors through their common glycosaminoglycan-binding site. Importance of glycans was shown for both GPCR and chemokine binding. Thus, SSL5 is an important immunomodulatory protein of S aureus that targets several crucial, initial stages of leukocyte extravasation. It is therefore a potential new antiinflammatory compound for diseases associated with chemoattractants and their receptors and disorders characterized by excessive recruitment of leukocytes.
Introduction
Staphylococcus aureus is a common human pathogen that causes severe community-acquired and nosocomial infections. This Gram-positive bacterium produces a wide variety of virulence proteins that aid in its infection, colonization, and disease progress. These surface-associated and secreted proteins often target the immune system.1
Chemotaxis inhibitory protein of S aureus (CHIPS) is such an excreted virulence factor of S aureus. CHIPS binds the formyl peptide receptor (FPR) and the C5a receptor (C5aR) and thereby inhibits cell activation by formylated peptides and C5a, respectively.2,3 Consequently, it attenuates the initial activation and migration of neutrophils to the site of infection. Structurally, CHIPS shows homology to the C-terminal domain of staphylococcal superantigen-like (SSL) proteins.4
SSLs are a family of 14 exoproteins. Eleven of them are encoded on staphylococcal pathogenicity island 2 present in all S aureus strains, and the remaining 3 are found on the immune evasion cluster 2.5 They were identified through sequence and structural homology with superantigens, but they do not show superantigenic activity.6 Latest evidence suggests that SSLs play a role in immune evasion by other means. SSL7 was described to bind C5 and immunoglobulin A (IgA) blocking the complement system and FcαRI binding, respectively.7 Recently, we described that SSL5 binds to P-selectin glycoprotein ligand 1 (PSGL-1).8 Thereby, SSL5 inhibits neutrophil rolling on endothelial cells by disrupting the interaction of PSGL-1 with its natural ligand P-selectin. Sugar moieties were shown to be crucial in this effect. Recently, the crystal structure of SSL5 in complex with sialyl Lewis X (sLex) was determined.9 SSL11 was also shown to interact with sLex,10 and both proteins bind sLex through their C-terminal domain. Therefore, at least a subset of SSLs affects the immune system by targeting glycoproteins.
The immune response rapidly reacts to invading pathogens. The response is initiated by extravasation of leukocytes from the blood stream to the site of infection. It is regulated by various chemotactic factors that are produced endogenously by endothelial cells and leukocytes as well as exogenously by the invading pathogen. These comprise the chemoattractants, which include the anaphylatoxins C3a and C5a, formylated peptides, platelet-activating factor (PAF) and leukotriene B4 (LTB4), and chemokines. Chemokines are small proteins of 8 to 10 kDa that are divided in 4 groups (CXC, CC, CX3C, and C) according to the number and relative position of their cysteine residues.11 All chemoattractants act on target cells through specific receptors belonging to the superfamily of 7-transmembrane, G protein–coupled receptors (GPCRs). Although chemoattractants act as proinflammatory signals, aberrant expression has been implicated in several chronic and acute diseases such as stroke, reperfusion/ischemia, transplant rejection, rheumatoid arthritis, and tumor progression.12 Therefore, a molecule targeting leukocyte activation by chemoattractants is of great interest as an antiinflammatory compound.
Here, we describe that SSL5 potently and specifically inhibits leukocyte activation by chemokines and anaphylatoxins. It does so through binding to the N-terminus of all tested GPCRs in a glycan-dependent manner. Ligand specificity of SSL5 is exerted by the nature of the ligand. SSL5 only inhibits stimuli of protein nature that act at least through the N-terminal region of the target GPCR, in contrast to lipid and peptide ligands that activate GPCRs independent of the N-terminus. In case of chemokines, SSL5 has a second mechanism of action. SSL5 sequesters chemokines from the chemokine receptors through glycoproteins and acts as a scavenger.
Methods
Reagents and antibodies
Reagents and antibodies used in this study are reported in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).13
Recombinant proteins
SSL5 and SSL11 of S aureus strain NCTC8325 were cloned, expressed and purified as described.8 Generation of N-terminal peptides of CXCR1, CXCR4, and C5aR are described in Document S1.
Cells
Human leukocytes and human umbilical vein endothelial cells were isolated as described.8,14 Informed consent was obtained from all subjects in accordance with the Declaration of Helsinki. Approval was obtained from the medical ethics committee of the University Medical Center Utrecht (The Netherlands). The human promonocytic U937 cells transfected with or without the C5aR (U937-C5aR) or CXCR2 (U937-CXCR2) were a generous gift from Dr E. Prossnitz (University of New Mexico, Albuquerque, NM).15,16 Human embryonic kidney (HEK) cells and Jurkat T cells were from ATCC (Rockville, MD).
Cloning and transfection of N-termini of human GPCRs into HEK cells
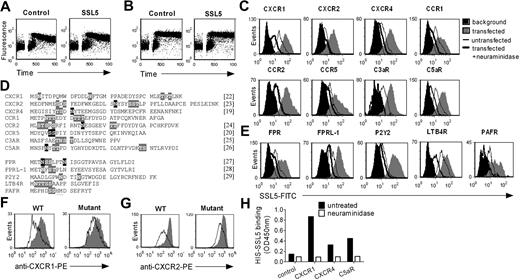
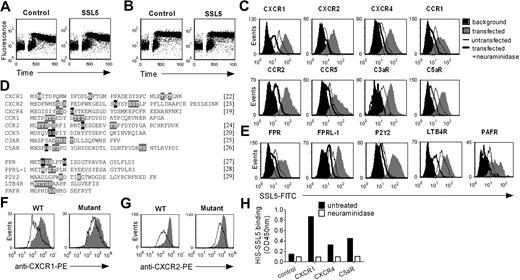
The N-termini of CXCR2, CXCR4, CCR1, CCR2, CCR5, C3aR, FPR, FPRL1, P2Y2, LTB4 receptor (LTB4R), and PAF receptor (PAFR) were cloned in the pDISPLAY vector (Invitrogen, Carlsbad, CA) downstream of a hemagglutinin (HA) tag as described for CXCR1 and C5aR.2 Mutations in N-CXCR1 (N3Q, N16Q, T34A, T36A) and N-CXCR2 (S10A, N22Q, S26A, S27A) leading to receptors devoid of glycosylation sites were introduced with the use of primers. Primer sets are listed in Document S1. HEK cells were transiently transfected with the vectors using Lipofectamine. Two days after transfection, cells were used for binding assays. Cells with successful receptor expression were identified by staining with mouse anti–HA monoclonal antibody (mAb) and goat anti–mouse IgG-APC. Anti–HA-negative cells from the same transfection were examined as untransfected cells. Viable cells were identified through negative staining with propidium iodide.2 Transfection efficiency was between 3% and 10%, and equal intensities of anti-HA mAb binding for all transfected receptors signified comparable receptor expressions.
Calcium mobilization
Neutrophils were labeled with 2 μM Fluo-3-AM, a fluorescent probe for free intracellular calcium. The cells were treated with or without SSL5 for 30 seconds, and calcium mobilization was measured with a flow cytometer (FACSCalibur; Becton Dickinson, Franklin Lanes, NJ) for 10 seconds before and 40 seconds after stimulation with chemoattractants, ionomycin or ATP. Monocyte calcium mobilization was monitored by gating on side scatter and anti–CD14-PE staining. Where indicated, cells were first treated with 0.2 U/mL neuraminidase at 37°C for 45 minutes. Efficacy of treatment was assessed by staining with anti-CD15s. In some experiments, SSL5 was first preincubated with increasing concentrations of sLex for 30 minutes. For all stimuli, percentage of activation was determined at 5 seconds after stimulation and was calculated relative to the fluorescence of cells that exhibited maximal stimulation, after subtracting the background fluorescence.
Actin polymerization
Neutrophils were incubated with or without 30 μg/mL SSL5 for 1 minute at room temperature and stimulated with 3 nM fMLP, 10 nM CXCL8, or 1 nM C5a. Actin polymerization was assessed over time with fluorescent phallacidin, which binds specifically to the active state of actin, as described.17 Polymerization was calculated as the relative increase in fluorescence compared with nonstimulated cells (at 0 seconds).
Chemotaxis
Neutrophils were labeled with 2 μM calcein-AM. For chemotaxis, a 96-well transmembrane system with an 8-μm pore size polycarbonate membrane (ChemoTX; NeuroProbe, Gaithersburg, MD) was used. Chemoattractants were added to the lower compartment. For spontaneous migration, solely buffer was used, and for total migration labeled cells were used. The membrane holder was assembled, and cells were added to the upper compartment. After 30 minutes at 37°C (5% CO2), the membrane was washed, and migrated cells were quantified using a plate reader fluorometer (FlexStation; Molecular Devices, Sunnyvale, CA). Percentage chemotaxis was calculated relative to the fluorescence value of cells added directly to the lower compartment.
Binding assays
To determine binding of SSL5 to cells, SSL5 was first labeled with FITC.8 Neutrophils or HEK transfectants were incubated with 10 μg/mL SSL5-FITC in RPMI/HSA for 30 minutes on ice. After washing, fluorescence was measured with a flow cytometer. In some experiments, SSL5-FITC was first preincubated with sLex for 30 minutes. For competition experiments, neutrophils, monocytes, Jurkat cells, or U937 cells were first incubated with SSL5 for 15 minutes on ice. Subsequently, 1 μg/mL mAbs against several GPCRs were added for 30 minutes on ice. The cells were washed and stained with goat anti–mouse Ig-FITC for another 30 minutes. For competition experiments with HEK transfectants, cells were incubated with 10 μg/mL SSL5 for 15 minutes before staining with 0.5 μg/mL PE-labeled antibody against the transfected receptor for 30 minutes. For detection of chemokine binding to cells, biotinylated chemokine fluorokine kits were used according to the manufacturer's protocol (R&D Systems, Minneapolis, MN). After incubation of cells with or without SSL5, 0.7 μg/mL biotinylated chemokine or control biotinylated protein (soybean trypsin inhibitor) was added for 1 hour and subsequently stained with avidin-FITC for 30 minutes. Where indicated, SSL5 was first preincubated with 300 μg/mL sLex for 30 minutes at room temperature, and biotinylated chemokines were first incubated with 100 μg/mL heparan sulfate for 30 minutes at room temperature or polyclonal, blocking anti–chemokine antibodies according to the manufacturer's protocol. For binding of C5a to U937 cells, C5a was first labeled with FITC as described for SSL5.8 C5a-FITC (1 μg/mL) was added to cells treated with SSL5 for 15 minutes.
Enzyme-linked immunosorbent assay
Growth hormone (GH)–tagged N-terminal peptides of CXCR1, CXCR4, and C5aR and control GH protein were coated at 10 μg/mL in a microtiter plate. Wells were blocked with 4% skimmed milk in PBS/0.05%Tween. Where indicated, the proteins were treated with neuraminidase. Thereafter, 10 μg/mL HIS-SSL5 was allowed to bind to proteins for 1 hour at 37°C. Bound HIS-SSL5 was detected with successive incubations with anti-Xpress and goat anti–mouse-HRP and visualized as described.18
Supplemental data
The supplemental data include supplemental experimental procedures and supplemental figures.
Results
SSL5 inhibits neutrophil activation by CXCL8, C3a, and C5a
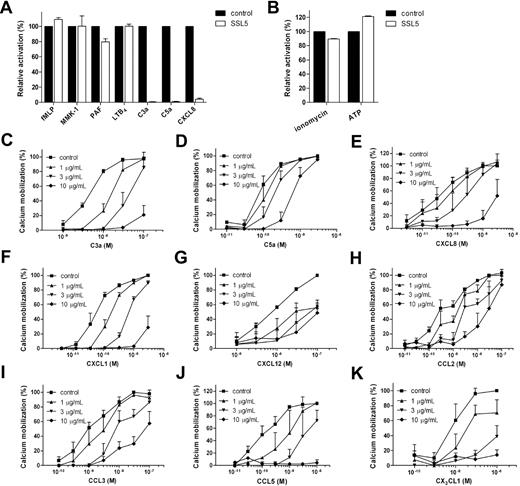
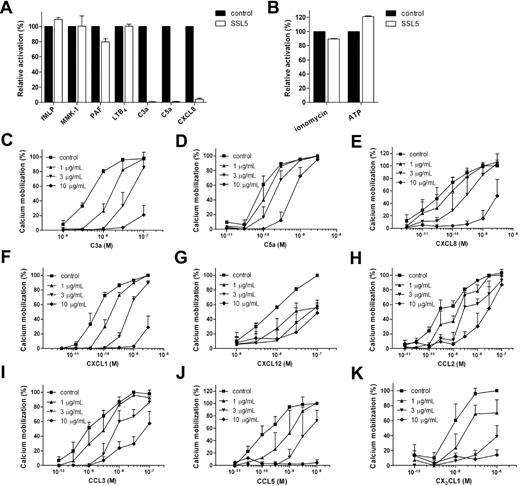
To investigate the effect of SSL5 on chemoattractant-induced neutrophil activation, a calcium mobilization assay was performed. For this purpose, neutrophils were treated with SSL5 and subsequently stimulated with suboptimal concentrations of the chemoattractants fMLP, MMK-1, PAF, LTB4, C3a, C5a, and CXCL8. These bacteria-, protein- or lipid-derived stimuli act through various GPCRs. SSL5 inhibited neutrophil stimulation induced by C3a, C5a, and CXCL8 but did not affect cell activation induced by the other chemoattractants (Figure 1A). Subsequently, SSL5 was tested for its effect on neutrophil activation by the nucleotide ATP and ionophore ionomycin (Figure 1B). SSL5 did not show any effect on these 2 stimuli nor did it induce a calcium mobilization when tested as a stimulus (data not shown). Toxicity on neutrophils, monocytes, lymphocytes, and endothelial cells was examined by evaluating propidium iodide staining by flow cytometry and trypan blue staining by microscopy. After treatment of cells for 1 hour with 10 μg/mL SSL5, the percentage of viable cells treated with SSL5 always exceeded 98% (data not shown). SSL5 is thus not toxic, and it is specific for a subset of GPCRs.
SSL5 inhibits leukocyte activation by chemokines and anaphylatoxins. (A,B) Neutrophils were incubated with (□) or without (■) 10 μg/mL SSL5 for 30 seconds and stimulated with (A) 1 nM fMLP, 10 nM MMK-1, 0.1 nM PAF, 0.1 nM LTB4, 3 nM C3a, 0.1 nM C5a, or 0.5 nM IL-8 and with (B) 30 nM ionomycin or 1 μM ATP. Cell activation was measured by calcium mobilization (C-K). Neutrophils (C-F) or monocytes (G-K) were preincubated with 0 (■), 1 (▴), 3 (▾), or 10 (♦) μg/mL SSL5 for 30 seconds. Calcium mobilization was measured on stimulation with C3a (C), C5a (D), CXCL8 (E), CXCL1 (F), CXCL12 (G), CCL2 (H), CCL3 (I), CCL5 (J), or CX3CL1 (K). Results represent mean plus or minus SEM of 3 independent experiments and are expressed relative to buffer-treated cells.
SSL5 inhibits leukocyte activation by chemokines and anaphylatoxins. (A,B) Neutrophils were incubated with (□) or without (■) 10 μg/mL SSL5 for 30 seconds and stimulated with (A) 1 nM fMLP, 10 nM MMK-1, 0.1 nM PAF, 0.1 nM LTB4, 3 nM C3a, 0.1 nM C5a, or 0.5 nM IL-8 and with (B) 30 nM ionomycin or 1 μM ATP. Cell activation was measured by calcium mobilization (C-K). Neutrophils (C-F) or monocytes (G-K) were preincubated with 0 (■), 1 (▴), 3 (▾), or 10 (♦) μg/mL SSL5 for 30 seconds. Calcium mobilization was measured on stimulation with C3a (C), C5a (D), CXCL8 (E), CXCL1 (F), CXCL12 (G), CCL2 (H), CCL3 (I), CCL5 (J), or CX3CL1 (K). Results represent mean plus or minus SEM of 3 independent experiments and are expressed relative to buffer-treated cells.
The chemokine CXCL8 and complement fragments C3a and C5a are potent neutrophil stimuli. Therefore, the effectiveness of inhibition of these 3 stimuli by SSL5 was further investigated. Figure 1C-E shows that SSL5 inhibited C3a-, C5a-, and CXCL8-induced calcium mobilization in neutrophils in a dose-dependent manner. Although 3 μg/mL of SSL5 inhibited 50% of maximal neutrophil stimulation by C3a, 10 μg/mL was needed for neutrophil activation by CXCL8 and C5a.
SSL5 inhibits chemokine-induced cell activation
Because SSL5 inhibited neutrophil activation by the CXC chemokine CXCL8, the effect of SSL5 on other CXC chemokines that interact with different chemokine receptors was investigated. SSL5 not only inhibited calcium mobilization in neutrophils induced by CXCL8 that interacts with CXCR1 and CXCR2 but also by CXCL1 that acts solely on CXCR2 (Figure 1F). SSL5 also inhibited responses in monocytes induced by the CXCR4 ligand CXCL12 (Figure 1G). In addition, monocytes were stimulated with CC chemokines CCL2 (CCR2), CCL3 (CCR1, CCR5), and CCL5 (CCR1, CCR3, CCR5), and the CX3C chemokine CX3CL1 (CX3CR1). SSL5 potently inhibited calcium mobilization induced by these chemokines as well (Figure 1H-K). SSL5 thus strongly inhibited all tested chemokines from different chemokine classes that act on different chemokine receptors. In all cases, a dose-response effect was observed, and the chemokine concentration inducing 50% stimulation was completely abrogated by 3 μg/mL SSL5.
SSL5 inhibits actin polymerization induced by chemokines and anaphylatoxins
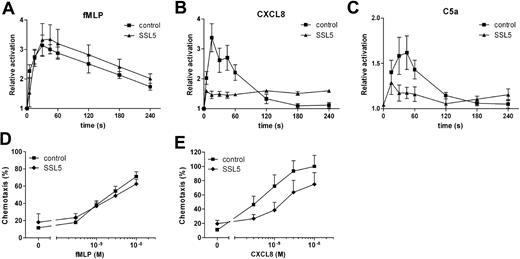
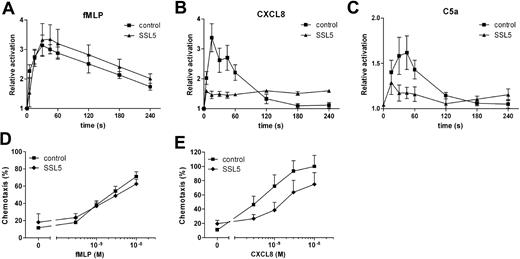
Stimulation of leukocytes does not only lead to transient calcium mobilization but also induces rapid actin polymerization. Therefore, SSL5 was tested for its ability to block stimulus-induced actin polymerization in neutrophils. For this purpose, neutrophils were treated with SSL5 and subsequently stimulated with fMLP, CXCL8, or C5a. Actin polymerization was measured over time. Figure 2A-C shows that SSL5 strongly inhibited actin polymerization induced by CXCL8 and C5a but not by fMLP, as was shown for calcium mobilization assays.
SSL5 inhibits actin polymerization and chemotaxis by chemokines and anaphylatoxins. (A-C) Neutrophils were incubated for 1 minute with (▴) or without (■) 30 μg/mL SSL5 and stimulated with 3 nM fMLP (A), 10 nM CXCL8 (B), or 1 nM C5a (C) for 5 minutes. At several time points samples were taken, and actin polymerization was measured. Results are expressed as the relative increase in fluorescence compared with unstimulated cells. (D,E) Neutrophils were incubated with (♦) or without (■) 10 μg/mL SSL5 and allowed to migrate toward fMLP (D) or CXCL8 (E). Chemotaxis was measured after 30 minutes. Results represent mean plus or minus SEM of 3 independent experiments.
SSL5 inhibits actin polymerization and chemotaxis by chemokines and anaphylatoxins. (A-C) Neutrophils were incubated for 1 minute with (▴) or without (■) 30 μg/mL SSL5 and stimulated with 3 nM fMLP (A), 10 nM CXCL8 (B), or 1 nM C5a (C) for 5 minutes. At several time points samples were taken, and actin polymerization was measured. Results are expressed as the relative increase in fluorescence compared with unstimulated cells. (D,E) Neutrophils were incubated with (♦) or without (■) 10 μg/mL SSL5 and allowed to migrate toward fMLP (D) or CXCL8 (E). Chemotaxis was measured after 30 minutes. Results represent mean plus or minus SEM of 3 independent experiments.
SSL5 inhibits neutrophil migration toward CXCL8
Chemoattractants are potent inducers of neutrophil migration. To examine the effect of SSL5 on neutrophil chemotaxis, neutrophils were incubated with SSL5 and allowed to migrate toward fMLP or CXCL8. As shown in Figure 2D,E, SSL5 had no effect on chemotaxis of neutrophils toward fMLP, whereas chemotaxis toward CXCL8 was inhibited. Although calcium mobilization induced by CXCL8 was greatly affected by SSL5, migration was only inhibited by 50%.
Inhibition of chemokine- and anaphylatoxin-induced cell activation is dependent on sLex at the cell surface
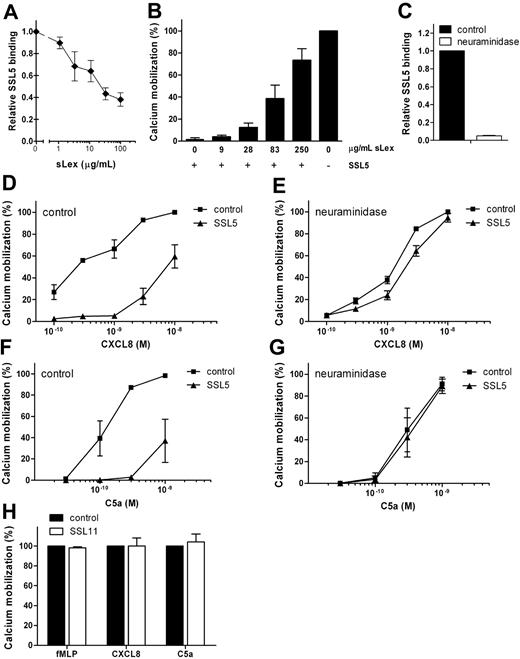
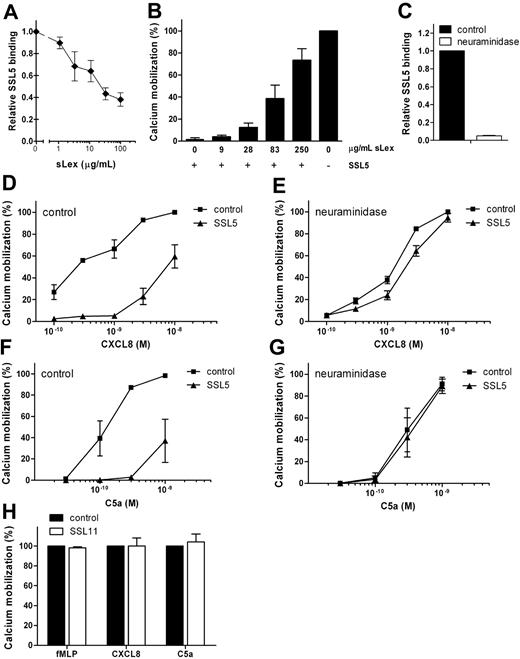
SSL5 was previously shown to bind PSGL-1 dependently on sLex sugar moieties.8 Therefore, we investigated the importance of sLex in the antagonistic effect of SSL5 by 2 means. SSL5 was first preincubated with soluble sLex. Loading SSL5 with increasing concentrations of sLex inhibited SSL5 binding to cells (Figure 3A) and consequently eliminated SSL5 activity on CXCL8-induced cell activation (Figure 3B). Glycan-dependent effects were also investigated on neutrophils treated with neuraminidase, which cleaves of sialic acid and thereby disturbs the sLex epitope. This completely abolished SSL5 (Figure 3C), P-selectin/Fc and anti-CD15s binding (data not shown), showing effective removal of sialic acids. Although neuraminidase treatment slightly affected neutrophil responses to CXCL8 and C5a, it clearly abolished their inhibition by SSL5 (Figure 3D-G). Thus, glycan-dependent binding of SSL5 to cells is needed for its antagonistic effect on chemokines and anaphylatoxins.
SSL5 inhibition of chemokine- and anaphylatoxin-induced cell activation depends on sLex at the cell surface. (A) Binding of 10 μg/mL SSL5-FITC, preincubated with different concentrations of sLex, to neutrophils. Results are expressed relative to buffer-treated SSL5-FITC. (B) Calcium mobilization in neutrophils induced by 0.1 nM CXCL8 after incubation of cells with (+) or without (−) 10 μg/mL SSL5 that was preloaded with different concentrations of sLex for 15 minutes. (C) SSL5-FITC binding to untreated (■) or neuraminidase-treated (□) neutrophils. Results are expressed relative to control-treated cells. (D-G) Untreated or neuraminidase-treated neutrophils were incubated with (▴) or without (■) 10 μg/mL SSL5 and stimulated with CXCL8 (D,E) or C5a (F,G). Cell activation was measured by calcium mobilization. (H) Neutrophils were incubated with (□) or without (■) 10 μg/mL SSL11 for 30 seconds. Calcium mobilization was measured on stimulation with 1 nM fMLP, 0.5 nM CXCL8, or 0.1 nM C5a. Results represent mean plus or minus SEM of 3 independent experiments.
SSL5 inhibition of chemokine- and anaphylatoxin-induced cell activation depends on sLex at the cell surface. (A) Binding of 10 μg/mL SSL5-FITC, preincubated with different concentrations of sLex, to neutrophils. Results are expressed relative to buffer-treated SSL5-FITC. (B) Calcium mobilization in neutrophils induced by 0.1 nM CXCL8 after incubation of cells with (+) or without (−) 10 μg/mL SSL5 that was preloaded with different concentrations of sLex for 15 minutes. (C) SSL5-FITC binding to untreated (■) or neuraminidase-treated (□) neutrophils. Results are expressed relative to control-treated cells. (D-G) Untreated or neuraminidase-treated neutrophils were incubated with (▴) or without (■) 10 μg/mL SSL5 and stimulated with CXCL8 (D,E) or C5a (F,G). Cell activation was measured by calcium mobilization. (H) Neutrophils were incubated with (□) or without (■) 10 μg/mL SSL11 for 30 seconds. Calcium mobilization was measured on stimulation with 1 nM fMLP, 0.5 nM CXCL8, or 0.1 nM C5a. Results represent mean plus or minus SEM of 3 independent experiments.
In addition to SSL5, SSL11 is described to bind glycoproteins carrying the sLex epitope.10 Therefore, SSL11 was tested for its effect on neutrophil activation by chemokines and anaphylatoxins. Figure 3H shows that SSL11 did not inhibit neutrophil stimulation by fMLP, CXCL8, or C5a, whereas SSL11 clearly showed a glycan-dependent binding to neutrophils (data not shown). SSL5 therefore seems unique in its ability to inhibit neutrophil activation by chemokines and anaphylatoxins in a glycan-dependent manner.
SSL5 competes with antibodies directed against chemokine receptors
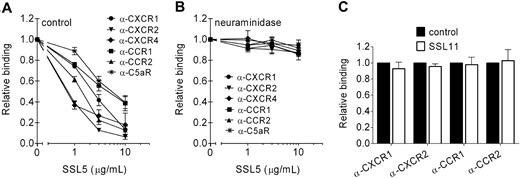
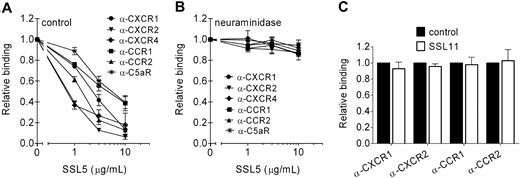
Neuraminidase treatment showed that cell binding of SSL5 is necessary for its inhibition of chemokine- and anaphylatoxin-induced GPCR activation. Therefore, the interaction of SSL5 with different GPCRs was further investigated. For this purpose, SSL5 was screened for its ability to block binding of anti-CXCR1, anti-CXCR2, and anti-C5aR mAbs to neutrophils; anti-CCR1 and anti-CCR2 mAbs to monocytes; and anti-CXCR4 to Jurkat cells. SSL5 competitively and dose dependently inhibited binding of all the tested antibodies (Figure 4A). Thus, SSL5 binds to the C5a and chemokine receptors.
SSL5 targets chemokine and anaphylatoxin receptors. (A,B) Binding of α-CXCR1 (●), α-CXCR2 (▾), and α-C5aR (*) mAbs to neutrophils, α-CXCR4 (♦) mAb to Jurkat cells, and α-CCR1 (■) and α-CCR2 (▴) mAbs to monocytes was measured to untreated (A) or neuraminidase-treated (B) cells after incubation with 0, 1, 3, or 10 μg/mL SSL5 for 15 minutes. (C) Binding of α-CXCR1 and α-CXCR2 mAbs to neutrophils and α-CCR1 and α-CCR2 mAbs to monocytes was measured after incubation with (□) or without (■) 10 μg/mL SSL11 for 15 minutes. Results are relative to buffer-treated cells and are expressed as mean plus or minus SEM of 3 independent experiments.
SSL5 targets chemokine and anaphylatoxin receptors. (A,B) Binding of α-CXCR1 (●), α-CXCR2 (▾), and α-C5aR (*) mAbs to neutrophils, α-CXCR4 (♦) mAb to Jurkat cells, and α-CCR1 (■) and α-CCR2 (▴) mAbs to monocytes was measured to untreated (A) or neuraminidase-treated (B) cells after incubation with 0, 1, 3, or 10 μg/mL SSL5 for 15 minutes. (C) Binding of α-CXCR1 and α-CXCR2 mAbs to neutrophils and α-CCR1 and α-CCR2 mAbs to monocytes was measured after incubation with (□) or without (■) 10 μg/mL SSL11 for 15 minutes. Results are relative to buffer-treated cells and are expressed as mean plus or minus SEM of 3 independent experiments.
GPCRs are known to be glycosylated, and glycosylation of CXCR419 and CCR520 has been shown to be important for chemokine binding. These glycans also play a role in SSL5 binding to the C5a and chemokine receptors because neuraminidase treatment abrogated the ability of SSL5 to compete with the mAbs against these receptors (Figure 4B). Antibody binding was not affected by neuraminidase treatment (Figure S1). Although SSL11 targets glycoproteins, it had no effect on antibody binding, suggesting no interaction with chemokine receptors (Figure 4C).
SSL5 acts through the N-terminal domain of the C5a and chemokine receptors
Glycosylation sites relevant for ligand binding to the chemokine receptors are located at the N-termini of these GPCRs.19,20 Because the SSL5 effects were glycan dependent, we suspect that SSL5 binding at least occurs at the N-terminus of the anaphylatoxin and chemokine receptors. In addition, SSL5 competed with the anti-CXCR2 and anti-C5aR mAbs that are directed against the N-terminal domain of CXCR2 and C5aR, respectively. To further test this hypothesis, a C-terminal peptide of C5a was used. This 10-amino acid peptide activates the C5aR solely through the extracellular loops, whereas full-length C5a additionally requires binding to the N-terminus. Although SSL5 inhibits neutrophil activation by C5a, it had no effect when cells were stimulated with the C5a C-terminal peptide (Figure 5A), indicating that SSL5 only interferes with stimulus binding at the N-terminus of the C5aR. Finally, we also tested the effect of SSL5 on neutrophil stimulation by PGP. This collagen-derived tripeptide can be mapped in CXCL821 and supposedly activates CXCR1 and CXCR2 independently of the N-terminus. SSL5 did not inhibit stimulation by PGP (Figure 5B), confirming that SSL5 primarily acts through the N-terminus of GPCRs.
SSL5 binds glycan dependently to the N-termini of GPCRs. (A,B) Neutrophils were treated with or without 10 μg/mL SSL5 and stimulated with 1 μM C5a C-terminal peptide (A) or 100 μg/mL PGP (B). Calcium mobilization was measured over time. (C-E) HEK cells were transfected with (C) the N-terminus of CXCR1, CXCR2, CXCR4, CCR1, CCR2, CCR5, C3aR, or C5aR, and with (E) FPR, FPRL1, P2Y2, LTB4R, or PAFR. Histograms depict binding of 30 μg/mL SSL5-FITC to untreated (gray) or neuraminidase-treated (thick line) cells. The thin line represents SSL5-FITC binding to untransfected cells, and black histograms symbolize background fluorescence of transfected cells. Results are representative plots of at least 3 independent experiments. (D) Amino acid sequence of the cloned and expressed N-termini with putative (gray) and described (black) glycosylation sites. References are mentioned between square brackets. (F,G) HEK cells transfected with the N-terminus of wild-type CXCR1 or CXCR2, and CXCR1 or CXCR2 glycosylation mutants were incubated with (line) or without (gray) 10 μg/mL SSL5 for 15 minutes before staining with anti-CXCR1 (F) or anti-CXCR2 (G). (H) Binding of 10 μg/mL HIS-SSL5 was measured to untreated (■) or neuraminidase-treated (□) N-terminal peptides of CXCR1, CXCR4, and C5aR fused to the C-terminus of GH. GH protein without a C-terminal GPCR sequence served as a control.
SSL5 binds glycan dependently to the N-termini of GPCRs. (A,B) Neutrophils were treated with or without 10 μg/mL SSL5 and stimulated with 1 μM C5a C-terminal peptide (A) or 100 μg/mL PGP (B). Calcium mobilization was measured over time. (C-E) HEK cells were transfected with (C) the N-terminus of CXCR1, CXCR2, CXCR4, CCR1, CCR2, CCR5, C3aR, or C5aR, and with (E) FPR, FPRL1, P2Y2, LTB4R, or PAFR. Histograms depict binding of 30 μg/mL SSL5-FITC to untreated (gray) or neuraminidase-treated (thick line) cells. The thin line represents SSL5-FITC binding to untransfected cells, and black histograms symbolize background fluorescence of transfected cells. Results are representative plots of at least 3 independent experiments. (D) Amino acid sequence of the cloned and expressed N-termini with putative (gray) and described (black) glycosylation sites. References are mentioned between square brackets. (F,G) HEK cells transfected with the N-terminus of wild-type CXCR1 or CXCR2, and CXCR1 or CXCR2 glycosylation mutants were incubated with (line) or without (gray) 10 μg/mL SSL5 for 15 minutes before staining with anti-CXCR1 (F) or anti-CXCR2 (G). (H) Binding of 10 μg/mL HIS-SSL5 was measured to untreated (■) or neuraminidase-treated (□) N-terminal peptides of CXCR1, CXCR4, and C5aR fused to the C-terminus of GH. GH protein without a C-terminal GPCR sequence served as a control.
SSL5 binds directly to GPCRs
To demonstrate a direct interaction between SSL5 and the receptors for anaphylatoxins and chemokines, we performed transfection studies. For this purpose, HEK cells were transfected with the N-termini of the CXCR1, CXCR2, CXCR4, CCR1, CCR2, CCR5, C3aR, and C5aR. Subsequently, SSL5 binding to untransfected and transfected cells was assessed. SSL5 bound somewhat to untransfected HEK cells, indicating these cells synthesize glycans that can be recognized by SSL5 (Figure 5C). More importantly, transfection of HEK cells with the chemokine and anaphylatoxin receptors induced clear SSL5 binding to these cells, indicating direct binding of SSL5 to these receptors. Furthermore, this interaction was glycan dependent because neuraminidase treatment of transfected HEK cells abolished SSL5 binding. Because not only chemokine receptors but all GPCRs are presumably glycosylated (Figure 5D), SSL5 binding to the N-termini of the FPR, FPRL1, P2Y2, LTB4R, and PAFR was examined as well. Activation of these receptors by fMLP, MMK-1, ATP, LTB4, and PAF, respectively, was not affected by SSL5. However, transfection of HEK cells with these receptors induced binding of SSL5 to these cells (Figure 5E). Only slight binding was observed for the LTB4R. Importantly, SSL5 binding was abrogated on treatment of the cells with neuraminidase. Thus, SSL5 binds to all GPCRs tested in a glycan-dependent manner.
We further investigated whether other forms of interactions exist between SSL5 and the GPCR N-termini. Therefore, SSL5 binding to the N-termini of wild-type GPCRs and GPCRs devoid of glycosylation sites was examined. For this purpose, N-CXCR1 and N-CXCR2 mutants were generated by altering asparagines, serines, or threonines that are part of putative or described glycosylation sites. To focus on SSL5 binding to the GPCR N-termini, the ability of SSL5 to inhibit antibody binding to these receptors was examined. As shown in Figure 5F,G, antibody binding to the GPCR glycosylation mutants was clearly less affected by SSL5 compared with wild-type GPCRs, indicating that glycosylation of the GPCR N-terminus itself plays an important role in SSL5 binding. However, because some competition remained on the GPCR mutants, other forms of interaction cannot be excluded yet. SSL5 binding to other glycoproteins on the HEK cell surface possibly plays a role in its binding to the GPCR N-termini. To test this hypothesis, soluble excreted GPCR N-termini of the CXCR1, CXCR4, and C5aR were expressed and isolated from HEK cells. The GPCR N-termini were fused to the C-terminus of GH to enable proper expression and isolation. The GH protein lacking a C-terminal GPCR sequence served as a control. A clear binding of SSL5 was observed to the GH-GPCR N-termini in an enzyme-linked immunoabsorbent assay (ELISA) setting, whereas SSL5 did not bind to the control GH protein (Figure 5H). Neuraminidase treatment of the GH-GPCR N-termini completely abolished SSL5 binding. Therefore, SSL5 binding to the GPCR N-termini is glycan dependent, binding to other glycoproteins is not needed, and without glycosylation other possible forms of interaction are not sufficient to sustain binding.
SSL5 increases chemokine binding to cells
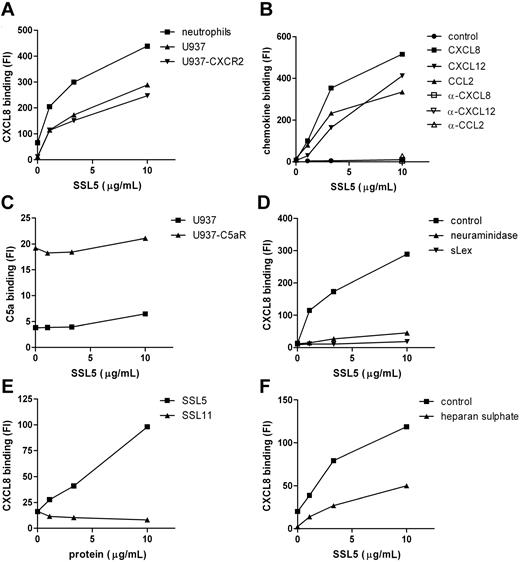
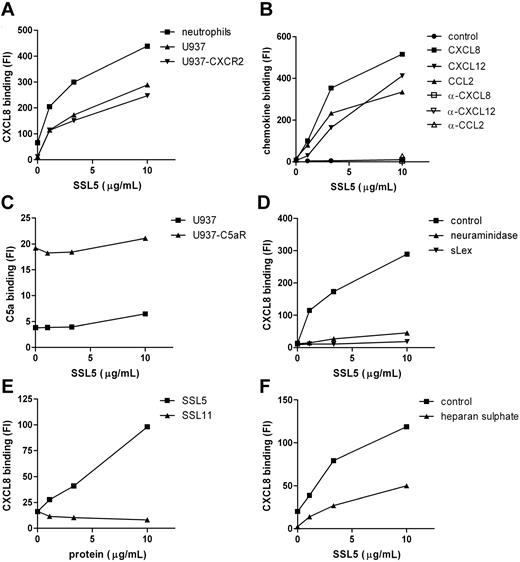
The inhibitory effects of SSL5 on cell activation by chemokines and anaphylatoxins may not only be exerted through binding of SSL5 to their receptors but could also be achieved through binding to the stimuli. SSL5, however, did not bind directly to the stimuli as was measured by several solid-phase assays (ie, surface plasmon resonance and ELISA) and experiments in solution, such as gel filtration of putative SSL5/CXCL8 complexes before analysis by ELISA and chemical crosslinking of putative SSL5/CXCL8 complexes before analysis by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Subsequently, we investigated the effect of SSL5 on chemokine binding to cells. For this purpose CXCL8-biotin binding to neutrophils was investigated in the presence or absence of SSL5. To our surprise, SSL5 dose dependently increased binding of CXCL8 to neutrophils (Figure 6A). To investigate the importance of the receptors for CXCL8 here, we made use of U937 cells that are devoid of chemokine receptors and U937-CXCR2 cells that stably express the CXCL8 receptor CXCR2 (Figure S2). SSL5 equally bound to both cell types (data not shown). As was the case with neutrophils, SSL5 dose dependently increased CXCL8 binding to U937-CXCR2 cells (Figure 6A). More importantly, SSL5 also induced CXCL8 binding to U937 cells, showing that SSL5 binding to chemokine receptors does not play a role here. In addition, the same effect was observed for CXCL12 and CCL2 (Figure 6B). SSL5 had no influence on binding of a control biotinylated protein, and its effect on chemokine binding was lost when the chemokines were pretreated with blocking chemokine-specific antibodies (Figure 6B). Thus, SSL5 induces chemokine binding to cells independently of chemokine receptors.
SSL5 induces chemokine binding to glycoproteins independent of chemokine receptors. (A) Binding of CXCL8-biotin was measured to neutrophils (■), U937 (▴), and U937-CXCR2 (▾) cells after treatment with SSL5 for 15 minutes. (B) U937 cells were incubated for 15 minutes with SSL5. Binding of biotin-labeled control protein (●), CXCL8 (■), CXCL12 (▾), or CCL2 (▴) untreated or preincubated with blocking α-CXCL8 (□), α-CXCL12 (▿), or α-CCL2 (▵), respectively, was measured. (C) C5a-FITC binding to U937 (▴) or U937-C5aR (■) cells was measured after incubation with SSL5. (D) Binding of CXCL8-biotin was measured to untreated (■) or neuraminidase-treated (▴) U937 cells after incubation with SSL5 that was preloaded without or with sLex (▾) for 15 minutes. (E) Binding of CXCL8-biotin was measured to U937 cells after treatment with SSL5 (■) or SSL11 (▴) for 15 minutes. (F) Cells were treated with SSL5 for 15 minutes and binding of CXCL8-biotin preincubated with (▴) or without (■) heparan sulfate for 30 minutes was measured. Results are representative of 3 independent experiments.
SSL5 induces chemokine binding to glycoproteins independent of chemokine receptors. (A) Binding of CXCL8-biotin was measured to neutrophils (■), U937 (▴), and U937-CXCR2 (▾) cells after treatment with SSL5 for 15 minutes. (B) U937 cells were incubated for 15 minutes with SSL5. Binding of biotin-labeled control protein (●), CXCL8 (■), CXCL12 (▾), or CCL2 (▴) untreated or preincubated with blocking α-CXCL8 (□), α-CXCL12 (▿), or α-CCL2 (▵), respectively, was measured. (C) C5a-FITC binding to U937 (▴) or U937-C5aR (■) cells was measured after incubation with SSL5. (D) Binding of CXCL8-biotin was measured to untreated (■) or neuraminidase-treated (▴) U937 cells after incubation with SSL5 that was preloaded without or with sLex (▾) for 15 minutes. (E) Binding of CXCL8-biotin was measured to U937 cells after treatment with SSL5 (■) or SSL11 (▴) for 15 minutes. (F) Cells were treated with SSL5 for 15 minutes and binding of CXCL8-biotin preincubated with (▴) or without (■) heparan sulfate for 30 minutes was measured. Results are representative of 3 independent experiments.
SSL5 does not affect binding of anaphylatoxins
To investigate whether SSL5 has the same effect on anaphylatoxins, we tested C5a-FITC binding to U937 cells and U937-C5aR cells, which stably express the C5aR. C5a-FITC specifically bound to U937-C5aR cells (Figure 6C). This binding was not interfered nor increased by SSL5. SSL5 also did not induce binding of C5a-FITC to U937 cells. Thus, SSL5 does not affect anaphylatoxin binding to cells.
Increase in chemokine binding by SSL5 is dependent on sLex at the cell surface
The importance of glycans in the SSL5 effect on chemokine binding was investigated. Neuraminidase treatment completely abolished SSL5-induced binding of CXCL8 to U937 cells (Figure 6D). In addition, preincubating SSL5 with sLex before U937 cell staining with CXCL8 no longer induced CXCL8 binding to U937 cells (Figure 6D). In conclusion, presence of sLex at the cell surface is crucial for the effect of SSL5 on chemokine binding.
Because SSL11 also targets glycoproteins and is structurally highly homologous to SSL5, its effect on chemokine binding was investigated. Although SSL11 bound to U937 cells (data not shown), Figure 6E shows that SSL11 did not induce binding of CXCL8 to U937 cells, showing specific chemokine effects for SSL5.
SSL5 and heparan sulfate compete for CXCL8 binding
Chemokines are generally retained and presented on endothelial cells and extracellular matrix by glycosaminoglycans (GAGs) such as heparan sulfate. To examine whether SSL5 interacts with the GAG-binding site of chemokines, CXCL8-biotin was first incubated with heparan sulfate and then added to SSL5-treated U937 cells. Figure 6F shows that the increased binding of CXCL8 by SSL5 was affected when heparan sulfate–loaded CXCL8 was used. SSL5 binding to U937 cells, however, was not influenced by heparan sulfate (data not shown). Consequently, SSL5 and heparan sulfate share binding sites for CXCL8.
Discussion
As an immediate response to the invading pathogen, leukocytes are recruited to the site of infection. This multistep process is highly coordinated and mediated by several adhesion molecules and host- or bacteria-derived chemoattractants. Initial tethering and rolling is mediated by PSGL-1. Subsequent leukocyte activation by chemokines allows for firm adhesion through integrins. After transmigration, leukocytes are directed to the site of inflammation by a gradient of chemoattractants such as fMLP, anaphylatoxins, or chemokines. We have recently described that SSL5 binds PSGL-1 and thereby inhibits neutrophil rolling on endothelial cells. Here, we show that SSL5 is also a broad-spectrum chemokine and anaphylatoxin inhibitor because it inhibits all classes of chemokines and anaphylatoxins. SSL5 thus not only attacks the initial leukocyte adherence but also inhibits subsequent leukocyte activation and migration.
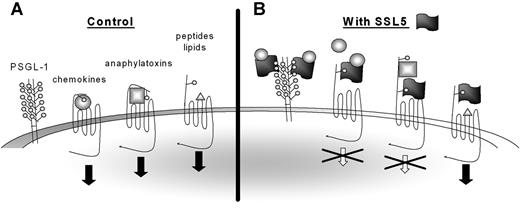
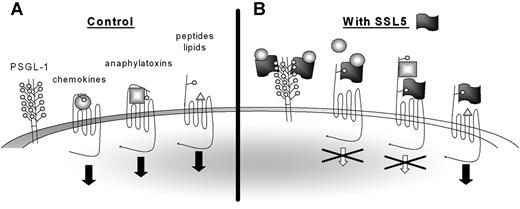
SSL5 potently and specifically inhibits leukocyte activation by chemokines and anaphylatoxins, whereas activation by chemoattractants fMLP, LTB4, PAF, and ATP is not affected. Nevertheless, SSL5 not only targets the chemokine and anaphylatoxin receptors but binds to the N-terminus of all GPCRs tested. Inhibition of this subset of ligands therefore seems to be inherent in the ligand (Figure 7). LTB4,30,31 PAF,32,33 fMLP,34,35 MMK-1,28,36 and ATP37 are lipid-, bacteria-, peptide-, and nucleotide-derived stimuli that primarily interact with the transmembrane (TM) regions of their target GPCRs. The N-terminal region did not seem to play an important role for either of these ligands. However, a 2-step binding model has been proposed for chemokines and anaphylatoxins. Chemokines11 and anaphylatoxins38 bind the N-terminus of their receptors whereupon they are positioned to interact with the pocket formed by the TM domains, which is necessary for activation of the receptor. Small synthetic peptides derived from chemokines (eg, PGP) and anaphylatoxins (eg, C-terminal peptide of C5a) can bypass the N-terminus and activate target GPCRs only through the TM region. SSL5 only inhibited cell activation by full-length chemokines and anaphylatoxins. SSL5 thus only inhibits ligands of protein nature that also require binding to the N-terminus of their target GPCR.
Model for SSL5 inhibition of chemokines and anaphylatoxins. (A) Chemokines and anaphylatoxins activate their target receptors through binding to both the N-terminus and the transmembrane regions, whereas peptides and lipid-derived stimuli only require the transmembrane region for activation. Some chemokines require glycosylation of the N-terminus of the target receptors for binding, whereas glycosylation is dispensable for binding of anaphylatoxins. (B) SSL5 binds all GPCRs through their glycosylation sites. However, SSL5 only inhibits cell activation by chemokines and anaphylatoxins. It thus acts on receptors that depend on their N-terminus for cell activation. Furthermore, SSL5 binds chemokines through glycans expressed on glycoproteins as PSGL-1. Thereby, it scavenges the chemokines from the chemokine receptors to prevent cell stimulation. SSL5 probably also binds chemokines through glycans presented on the chemokine receptors.
Model for SSL5 inhibition of chemokines and anaphylatoxins. (A) Chemokines and anaphylatoxins activate their target receptors through binding to both the N-terminus and the transmembrane regions, whereas peptides and lipid-derived stimuli only require the transmembrane region for activation. Some chemokines require glycosylation of the N-terminus of the target receptors for binding, whereas glycosylation is dispensable for binding of anaphylatoxins. (B) SSL5 binds all GPCRs through their glycosylation sites. However, SSL5 only inhibits cell activation by chemokines and anaphylatoxins. It thus acts on receptors that depend on their N-terminus for cell activation. Furthermore, SSL5 binds chemokines through glycans expressed on glycoproteins as PSGL-1. Thereby, it scavenges the chemokines from the chemokine receptors to prevent cell stimulation. SSL5 probably also binds chemokines through glycans presented on the chemokine receptors.
Glycosylation is an important posttranslational modification of GPCRs. N-linked glycosylation sites can be found within the N-terminal domain and second extracellular loop (ECL) of GPCRs. They are often described to be important for intracellular trafficking and cell-surface expression, as described for the FPR,27 PAFR,39 and CXCR2.23 In addition, glycosylation can also be important for ligand binding. N-linked glycans on CXCR4 were shown to be important for optimal binding of CXCL12.19 Threonine- and serine-rich sites typical for O-linked glycans can also be found in the N-terminal region of GPCRs. Major O-glycans containing sialic acid, Gal, and GalNac on serines of the CCR5 were important for binding of CCL3 and CCL4.20 N-glycosylation of the C5aR was, however, dispensable for C5a binding.26 Figure 5D shows that the N-termini of all examined GPCRs contain already identified or putative N- or O-linked or both glycosylation sites. Because SSL5 targets glycosylation sites,8,9 it binds the N-termini of all examined GPCRs. Ligands that require binding to the N-terminus for receptor activation as chemokines and anaphylatoxins are, therefore, inhibited by SSL5. In addition to SSL5, SSL11 is described to interact with sLex and target glycosylated proteins.10 In this study, SSL11 did not inhibit leukocyte activation by several tested chemoattractants, nor did it compete with antibodies against several chemokine receptors. SSL11 was also not able to induce chemokine binding though glycoproteins. SSL11 binding to target cells, however, was glycan dependent. Sequence comparison of SSL5 and SSL11 used in these experiments shows no difference in amino acid residues important for sLex binding, suggesting that not only glycosylation sites but also additional recognition sites are required for SSL5 effects. Although other interactions may thus be important, transfection studies with the N-terminal glycosylation mutants show that the glycans are crucial. As mentioned, some GPCRs show putative N-linked glycosylation sites in their second ECL. Although the transfection studies and isolated GPCR-N-termini showed that the N-termini of GPCRs are sufficient for SSL5 binding, we cannot exclude the possibility that SSL5 also targets the glycosylation site found in the second ECL of GPCRs. SSL5 competes with the anti-CXCR4 antibody that is directed against the second ECL of CXCR4. Consequently, SSL5 may bind this region of CXCR4, or the results may be explained through steric hindrance. Nonetheless, SSL5 binds at least the N-terminus of GPCRs glycan dependently.
SSL5 not only binds GPCRs but also scavenges chemokines such as CXCL8, CXCL12, and CCL2 independently of their target chemokine receptor through glycoproteins. On binding to glycoproteins, SSL5 possibly interacts directly with chemokines, or it enables the interaction between chemokines and glycoproteins. PSGL-1 is a heavily glycosylated protein that also requires sulfation for ligand binding. Because PSGL-1 and GPCRs have common posttranslational modifications, interaction between PSGL-1 and chemokines has been investigated. Several reports have shown an interaction between PSGL-1 and certain chemokines, but, depending on the chemokine and cell type used, this interaction was glycan dependent40,41 or independent.42 SSL5 binds PSGL-1 in a glycan-dependent manner, and here we show that it induces binding of chemokines to properly glycosylated cells, whereas it does not directly interact with chemokines. SSL5 could indirectly mediate an interaction between PSGL-1 and chemokines by altering the display of its glycans to chemokines. However, for chemokines that require glycans on PSGL-1, this is unlikely. SSL5 binding to PSGL-1, or other surface-expressed glycoproteins, rather induces a change in SSL5 that enables its subsequent binding and sequestration of chemokines. Significant structural/conformational changes are unlikely because the 2-domain structure of SSL5 is quite stable6 ; only but small flexibilities at the ends of the beta sheets and the loop that connects the 2 domains can be found. The sLex-binding site is described to reside in the C-terminal domain of SSL5.9 The binding site for chemokines might therefore be located in the N-terminal oligonucleotide/oligosaccharide-binding (OB) fold. Further research is needed to elucidate the exact interplay between glycoproteins, SSL5, and chemokines.
SSL5 inhibits cell activation by chemokines and anaphylatoxins through binding of GPCRs and through chemokine sequestration, both in a glycan-dependent manner. To our knowledge, both mechanisms are novel. Chemokine inhibition is known primarily in virology. Several viruses have developed strategies to down-regulate GPCR expression in the host. In addition, viruses produce chemokine-like molecules that interact with human chemokine receptors to modulate activity.43,44 Some herpesviruses and poxviruses, however, can bind chemokines through proteins that mimic sites of the host chemokine receptors or GAGs and thereby scavenge chemokines from host cells.43 This mechanism is also used by the host through DARC, which is an endogenous chemokine scavenger receptor that prevents overstimulation of host cells.45 SSL5 also sequesters chemokines from target chemokine receptors, but not through receptor mimicry. SSL5 binding to glycoproteins enables chemokine binding independent of chemokine receptors. Because SSL5 targets the GAG-binding site common among all chemokines, it is directed against all chemokines. In addition, chemokines share high structural homology. Anaphylatoxins are structurally different from chemokines, and they do not have a GAG-binding site,46 which may account for the inability of SSL5 to sequester anaphylatoxins. For bacteria, several species attain chemoattractant inhibition by secreting proteases that disable chemoattractants. Proteases from Pseudomonas aeruginosa, Serratia marcescens, Porphyromonas gingivalis, Streptococcus pyrogenes, and group A, B, and G streptococci degrade anaphylatoxins and chemokines.47 We have previously described CHIPS from S aureus that inhibits leukocyte activation by fMLP and C5a.2 CHIPS, however, binds distinct and specific sites on the FPR and C5aR, whereas SSL5 targets glycosylation sites common among all GPCRs.
We have recently demonstrated SSL5 binding to PSGL-1. Thereby, SSL5 inhibits P-selectin–dependent neutrophil rolling on endothelial cells. Here, we report SSL5 to be a potent modulator of leukocyte activation and chemotaxis by a subset of chemoattractants. SSL5 binds GPCRs, and thereby it inhibits the initial activation and chemotaxis of leukocytes by chemokines and anaphylatoxins. SSL5 thus targets several stages of leukocyte extravasation and is therefore a potential therapeutic in inflammatory pathologies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Dutch Technology Foundation STW (UKG-06609), Netherlands Organization for Scientific Research (NWO)–TOP (9120.6020), and the European Union (FP6-512093).
Authorship
Contribution: J.B. designed and performed research and wrote the article; H.A. performed activation experiments; I.G.J.B. performed binding experiments; R.A.R. cloned and expressed GH-N-GPCR fusion proteins; K.P.M.v.K., A.M.W., J.A.G.v.S., and C.J.C.d.H. participated in designing research; and all authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jovanka Bestebroer, Medical Microbiology, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands; e-mail: j.bestebroer@umcutrecht.nl.