Abstract
Although successful in utero hematopoietic cell transplantation (IUHCT) of X-linked severe combined immune deficiency (X-SCID) with enriched stem and progenitor cells was achieved more than a decade ago, it remains applied only in rare cases. Although this in part reflects that postnatal transplantations have overall given good results, there are no direct comparisons between IUHCT and postnatal transplantations of X-SCID. The proposed tolerance of the fetal immune system to foreign human leukocyte antigen early in gestation, a main rationale behind IUHCT, has recently been challenged by evidence for a considerable immune barrier against in utero transplanted allogeneic bone marrow cells. Consequently, there is need for further exploring the application of purified stem and progenitor cells to overcome this barrier also in IUHCT. Herein, we demonstrate in a congenic setting that recently identified lymphoid-primed multipotent progenitors are superior to hematopoietic stem cells in providing rapid lymphoid reconstitution after IUHCT of X-SCID recipients, and sustain in the long-term B cells, polyclonal T cells, as well as short-lived B-cell progenitors and thymic T-cell precursors. We further provide evidence for IUHCT of hematopoietic stem cells giving superior B- and T-cell reconstitution in fetal X-SCID recipients compared with neonatal and adolescent recipients.
Introduction
X-linked severe combined immune deficiency (X-SCID), the most frequent form of SCID (46%, ∼1:20 000 births),1,2 is caused by a mutation in the gene encoding the common gamma chain, shared by multiple cytokines with important functions in the immune system.3 X-SCID is lethal unless treated by bone marrow transplantation (BMT). Human leukocyte antigen (HLA)–identical postnatal BMT has overall been very successful although associated with significant morbidity and mortality.1 Further, only a minority of patients with SCID have an available HLA-identical donor at birth. For these patients, it was considered a breakthrough when successful correction of the common gamma-chain deficiency and T-cell deficiency was achieved by retroviral-mediated gene therapy.4 However, as some patients later developed T-cell leukemia because of insertional mutagenesis of the retroviral vector in or near the LMO-2 oncogene,5,6 gene therapy of X-SCID is currently approached with caution,7-9 and the need for development of alternative therapeutic approaches is still evident.
In utero hematopoietic cell transplantation (IUHCT) has been successfully applied to a limited number of patients with X-SCID. Using stem-progenitor CD34 antigen-enriched haploidentical bone marrow (BM) cells or unrelated fetal liver cells collected early in gestation, long-term donor-derived engraftment of T and NK cells has been achieved, with no observation of graft-versus-host disease.10-12 Despite little or no evidence of stem cell engraftment, successful donor T and NK cell reconstitution was accomplished, reflecting a considerable competitive advantage over host hematopoietic cells, in expansion and differentiation toward these lineages. However, although these cases support the main rationale behind IUHCT, namely, that the fetal immune system is tolerant to foreign HLA early in gestation, IUHCT continues to be applied only in rare cases of X-SCID. The limited prenatal screening and diagnostic routines only partially account for this. Rather, it has been argued that postnatal transplantations have obtained very good results, although no comparative studies of IUHCT and postnatal transplantations have been performed. However, studies have clearly suggested that BMT of patients with SCID in the neonatal period (first month of life) gives superior results to transplantations later, with regard to both T-cell reconstitution and survival.13 It does, however, remain unclear to what degree the improved results of early transplantations reflect changes in the immune barrier, with age and/or the fetal/neonatal hematopoietic microenvironment being more permissive for homing and engraftment.
Using mouse models, several previous studies supported that the engraftment after fetal transplantations is not limited by an immune barrier.14-16 In contrast, a recent mouse study provided compelling evidence for the existence of an immune barrier to allogeneic engraftment after IUHCT, demonstrating stable multilineage reconstitution in all congenic recipients, whereas allogenic IUHCT in most cases resulted in failed long-term reconstitution.17 If so, although there might still be strong clinical justifications for IUHCT,18,19 such as reducing susceptibility to postnatal infections, the hematopoietic advantage of preimmune transplantations might be questionable. However, whereas most successful clinical IUHCT of X-SCID fetuses have been performed with stem and progenitor (CD34) enriched hematopoietic cells, the studies of Peranteau et al17 involved IUHCT of whole BM transplants with major mismatch between the donor and recipient, and notably no mouse studies have compared the reconstitution of immune-deficient or wild-type (WT) recipients with purified stem/progenitor cells in the fetal, postnatal, and adult setting. Thus, although recent data clearly support the existence of an immune barrier after IUHCT, this barrier might be less significant than that in the postnatal setting. The fact that Peranteau et al17 obtained consistent long-term multilineage chimerism in unconditioned fetal recipients of congenic BM cells, despite evidence of small congenic differences constituting a significant immunologic barrier in adult BMT,20-22 could be compatible with immunologic and/or microenvironmental advantages of IUHCT compared with postnatal transplantations, even in situations with minimal mismatch.
The present studies were designed to investigate issues not previously addressed but of considerable relevance to IUHCT in X-SCID. First, we asked whether or not engraftment with long-term hematopoietic stem cells (HSCs)23 is a requirement to obtain durable and polyclonal T- and B-cell reconstitution in fetal X-SCID (γc−/−) recipients. This was addressed by comparing the kinetics and longevity of not only peripheral B- and T-cell reconstitution but also reconstitution of short-lived B- and T-cell precursors in hematopoietic tissues, from purified (congenic) adult HSCs (Lin−SCA-1+KIT+CD34−FLT3−)23 and Lin−SCA-1+KIT+-CD34+FLT3hi lymphoid-primed multipotent progenitors (LMPPs).24-26 Importantly, unlike HSCs, LMPPs lack extensive self-renewal potential and represent the earliest identified lineage restricted MPPs, sustaining (at the single-cell level) combined granulocyte-monocyte (GM), B and T lineage potential, but little or no megakaryocyte-erythroid (MkE) potential.25 Further, unlike HSCs, LMPPs express a combined GM and lymphoid transcriptional priming25 and rapidly and robustly reconstitute B and T lymphopoiesis in lethally irradiated WT recipients, although in this setting lymphoid reconstitution does not remain stable in the long-term.24 Second, we compared the ability of congenic HSCs as well as LMPPs to reconstitute lymphopoiesis in X-SCID recipients transplanted during fetal development, neonatally (postnatal days 1-4), or in early adult life (4-6 weeks old). Performing this comparative analysis in a congenic setting, and thereby reducing the immunologic barrier to a minimum, we could, for the first time, directly assess the potential nonimmunologic advantages for transplantations of nonconditioned X-SCID recipients in the prenatal (in utero) versus postnatal (early and late) stages.
Herein we demonstrate that LMPPs are far superior to HSCs in providing rapid lymphoid reconstitution after IUHCT of X-SCID recipients. LMPPs also sustain peripheral B cells and polyclonal T cells, as well as short-lived BM B-cell precursors and thymic T-cell precursors, although HSCs might prove essential for securing stable engraftment in the long term. Our findings underscore the importance of 2 distinct populations of primitive multipotent stem and progenitors cells in rapid and long-term reconstitution of the immune system in X-SCID recipients. Most notably, we provide evidence for IUHCT of HSCs giving superior T-cell reconstitution in X-SCID recipients compared with postnatal transplantations.
Methods
Mice
X-SCID (B6.129S4-Il2rgtm1Wjl/J, CD45.2)27 mice backcrossed to C57/Bl6 background for more than 10 generations were purchased from The Jackson Laboratory (Bar Harbor, ME). Congenic WT C57/Bl6 (CD45.1) mice were used as donors. Mice were housed in pathogen-free conditions and had unrestricted access to sterilized food and autoclaved acidified water. All experiments were approved by the Malmö/Lund Animal Ethics Committee of Sweden.
Isolation and purification of hematopoietic stem and progenitor cells
Hematopoietic stem and progenitor cell populations from 10- to 14-week-old C57Bl6 CD45.1 mice were isolated as previously described.23,25 Briefly, BM cells were enriched for lineage (Lin) low/negative cells by immunomagnetic beads (sheep–anti-rat Dynabeads, Dynal Biotech, Oslo, Norway) through depletion of Lin+ cells stained with purified B220/CD45R (RA3-6B2), CD4/L3T4 (H129.19), CD5 (53-7.3), CD8α (53-6.7), CD11b/Mac-1 (M1/70), Gr1 (RB6-8C5), and Ter119 (LY-76). Samples were subsequently incubated with polyclonal goat–anti-rat Tricolor (Caltag, San Francisco, CA), CD16/32 (2.4G2; Fc block, to reduce unspecific staining), anti–mouse-CD34 fluorescein isothiocyanate (RAM34), -FLT3 phycoerythrin (PE) (AZF10.1), -SCA-1 biotin (E13-161.7), and -KIT allophycocyanin (2B8) or isotype-matched control antibodies. Biotinylated antibodies were visualized with streptavidin (Sav) PE TxR (Invitrogen, Carlsbad, CA). Dead cells were excluded by 7-amino actinomycin (7-AAD; Sigma-Aldrich, St Louis, MO). Cells were sorted on a FACSDiVa (BD Biosciences, San Jose, CA). BM cells were gated as Lin−/lo7-AAD− and subsequently as SCA-1+KIT+. Within the Lin−SCA-1+KIT+ (LSK) CD34+ population, the 25% highest FLT3 expressing cells (LSKCD34+FLT3hi, purity reproducibly > 99%) and CD34−FLT3− cells (purity reproducibly > 95%) were sorted as described23,25 (see Figure 2A).
In utero transplantations
X-SCID mice were mated overnight and vaginal plugs checked the next morning (counted embryonic day E0.5). At E14 to E16, females premedicated with Temgesic (0.4 mg/kg) were anesthetized with isoflurane and a mid-abdominal incision was performed. Uterine horns were exposed and each fetus injected intraperitoneally with donor cells (5 × 105 unfractionated BM cells, 200 or 1000 LSKCD34+FLT3hi and 200 LSKCD34−FLT3−) or phosphate-buffered saline (sham-injected group) in a total volume of 2 μL per fetus. Abdominal incisions were closed in 2 layers and females allowed to complete pregnancy to term. Peripheral blood (PB) samples were collected at indicated time points. BM, thymus, and spleen were collected at termination.
Neonatal transplantations
Newborn unconditioned X-SCID mice (postnatal day D1-4) were injected intraperitoneally with 200 LSKCD34+FLT3hi or 200 LSKCD34−FLT3− cells in a volume of 20 μL per mouse and returned to the mother until weaning. PB samples were collected at 3 to 4, 6, and 16 weeks after transplantation.
Adult transplantations
Young unconditioned X-SCID mice (4-6 weeks old) were injected with 200 LSK CD34+FLT3hi or 200 LSK CD34−FLT3− cells through intravenous tail vein injection. Hematopoietic reconstitution was evaluated in PB at 4, 6, and 16 weeks
Secondary transplantations
Sublethally (650 cGy) irradiated X-SCID mice (8-12 weeks old) received by tail vein injection 1 femur equivalent of BM cells harvested 16 weeks after IUHCT transplantation of either 1000 LSK CD34+FLT3hi or 200 LSK CD34−FLT3− cells. PB donor reconstitution in secondary recipients was evaluated by fluorescence-activated cell sorting (FACS) at 12 weeks after transplantation.
Analysis of donor reconstitution
PB was collected at indicated time points after transplantation and analyzed for donor-derived reconstitution as previously described.23 BM, spleen, and thymus were collected at 36 weeks after transplantation (in case of LMPPs and HCSs transplanted fetal recipients). We used rat–anti-mouse antibodies to differentiate host (CD45.2; clone104) from donor (CD45.1; A20) cell reconstitution, and defined B cells as B220+ (clone RA3-6B2), T cells as CD4+ (clone H129.19) and CD8+ (clone 53-6.7), and myeloid cells as Mac1/CD11b+ (clone M1/70). At 36 weeks after transplantation, a more extensive FACS analysis was performed in which antibodies against CD19 (1D3), IgM (R6-60.2), TCR β (H57-597), TCR-γ (GL-3), NK1.1 (PK136), CD49b (DX5), CD3 (17A2), CD44 (1M7), and CD25 (3C7) also were included. In the case of whole BM fetal recipients, BM was harvested from transplanted mice at 16 weeks after transplantation, and analysis of donor-derived LSK cells was performed. For all analyses, cell counts were measured on a KX21 Sysmex (Sysmex Europe, Hamburg, Germany). All antibodies were from BD Biosciences. FACS analysis was performed on a FACSCalibur or FACSDiva (BD Biosciences). Data obtained were subsequently analyzed using FlowJo Software (TreeStar, Ashland, OR).
TCR Vb rearrangement analysis
The Mouse Vβ TCR Screening Panel (BD Biosciences PharMingen, San Diego, CA), which contains prediluted fluorescein isothiocyanate-conjugated antibodies specific for mouse Vβ 2, 3, 4, 5.1 + 5.2, 6, 7, 8.1 + 8.2, 8.3, 9, 10b, 11, 12, 13, 14, and 17a T-cell receptors was used to identify polyclonal subsets of T cells in spleens after transplantation. Samples were prepared at a density of 106cells/antibody reaction and stained with donor marker CD45.1 (A20) PE and T lymphocyte markers CD4 (H129.19) PE Cy5, and CD8 (53-6.7) allophycocyanin (BD Biosciences) before specific staining with the Vβ antibodies. Analysis was performed on a FACSCalibur.
Statistical analysis
Results are presented as means (SEM), and statistical significance of differences between groups was determined using a 2-tailed unpaired t test. P values less than .05 were regarded as significant. Fisher exact test was used to analyze and compare the frequencies of survival and frequencies of positively reconstituted fetally transplanted mice (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) as well as frequencies of engrafted fetal, neonatal, and adult mice (GraphPad Prism version 4; GraphPad Software, San Diego, CA).
Results
Undetectable myeloid and stem cell reconstitution after congenic transplantation of WT adult bone marrow cells to X-SCID fetuses
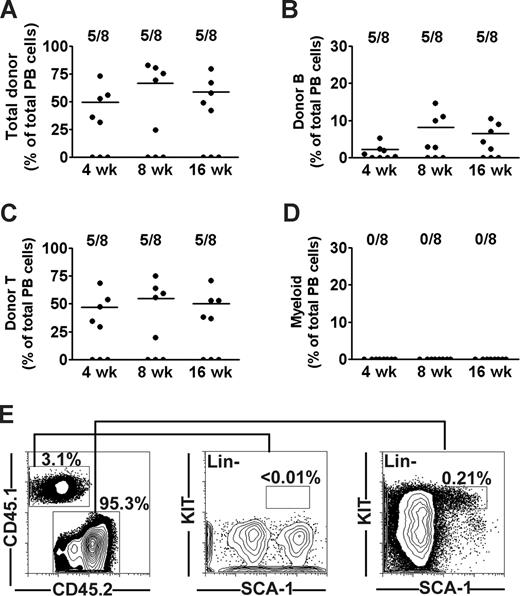
It has been suggested that the long-term engraftment and immune correction of X-SCID recipients transplanted in utero might require HSC reconstitution,28,29 although direct evidence for this is limited. Thus, we first investigated the potential of unfractionated adult BM cells from CD45.1 C57Bl/6 mice to multilineage reconstitute lymphopoiesis and myelopoiesis in fetal (E14-E16) CD45.2 X-SCID mice (also on C57Bl/6 background). Of a total of 24 fetal recipients, 8 (33%) were alive at time of analysis; and of these, 5 (62%) were positive for donor engraftment at all analysis time points. At 4 weeks after transplantation of 5 × 105 CD45.1 congenic BM cells, donor-derived contribution to total peripheral blood (PB) white blood cells was as much as 49% (Figure 1A), of which B and T cells accounted for 2.1% and 46%, respectively. Eight weeks after transplantation, levels of donor chimerism increased to 66%, and B- and T-cell reconstitution reached 8.1% and 54%, respectively. These levels were sustained also in the long-term (16 weeks; Figure 1B,C). However, no evidence of significant (above background staining) myeloid reconstitution was found at any time point (Figure 1D). Because no previous IUHCT studies had directly investigated the potential reconstitution of the HSC compartment itself, we next analyzed the BM of transplanted mice for the presence of donor-derived HSCs as defined by a Lin−SCA-1+KIT+ (LSK) phenotype.25 Notably, and in seeming contrast to the high levels of chimerism in the periphery, the BM contained only 3.5% CD45.1 cells (Figure 1E), and no evidence of donor contribution to the LSK HSC compartment was observed (Figure 1E), whereas the host (CD45.2) frequency of LSK cells in BM was within the expected range. Thus, in agreement with earlier studies,14,15 fetally transplanted WT congenic BM cells sustained B- and T-lymphoid reconstitution, without evidence for corresponding myeloid and/or HSC reconstitution.
Long-term lymphoid but not myeloid or stem cell reconstitution in X-SCID mice BM transplanted during fetal development. Twenty-four fetal (E14.5) X-SCID (CD45.2) mice were transplanted with 5 × 105 whole BM cells from congenic (CD45.1) adult donors. Eight (33%) live born transplanted mice were analyzed for reconstitution at 4, 8, and 16 weeks after birth. Contribution of donor cells to (A) total nucleated cells, (B) B cells, (C) T cells, and (D) myeloid cells as percentage of total blood cells. Data points represent individual mice; horizontal bars represent mean values of positively engrafted mice (> 0.1% for each parameter). Results are from 2 independent experiments (E). FACS analysis of donor-derived LSK cells in BM of one representative animal at 16 weeks after birth. Left panel shows typical donor and host contribution to total BM cells. Middle and right panels show donor and recipient-derived LSK cells, respectively, gated as lineage negative, and investigated for expression of the HSC markers SCA-1 and KIT. Numbers represent percentages of total BM cells (0.01%; detection level).
Long-term lymphoid but not myeloid or stem cell reconstitution in X-SCID mice BM transplanted during fetal development. Twenty-four fetal (E14.5) X-SCID (CD45.2) mice were transplanted with 5 × 105 whole BM cells from congenic (CD45.1) adult donors. Eight (33%) live born transplanted mice were analyzed for reconstitution at 4, 8, and 16 weeks after birth. Contribution of donor cells to (A) total nucleated cells, (B) B cells, (C) T cells, and (D) myeloid cells as percentage of total blood cells. Data points represent individual mice; horizontal bars represent mean values of positively engrafted mice (> 0.1% for each parameter). Results are from 2 independent experiments (E). FACS analysis of donor-derived LSK cells in BM of one representative animal at 16 weeks after birth. Left panel shows typical donor and host contribution to total BM cells. Middle and right panels show donor and recipient-derived LSK cells, respectively, gated as lineage negative, and investigated for expression of the HSC markers SCA-1 and KIT. Numbers represent percentages of total BM cells (0.01%; detection level).
Rapid and sustained lymphoid engraftment of X-SCID recipients transplanted with LMPPs in utero
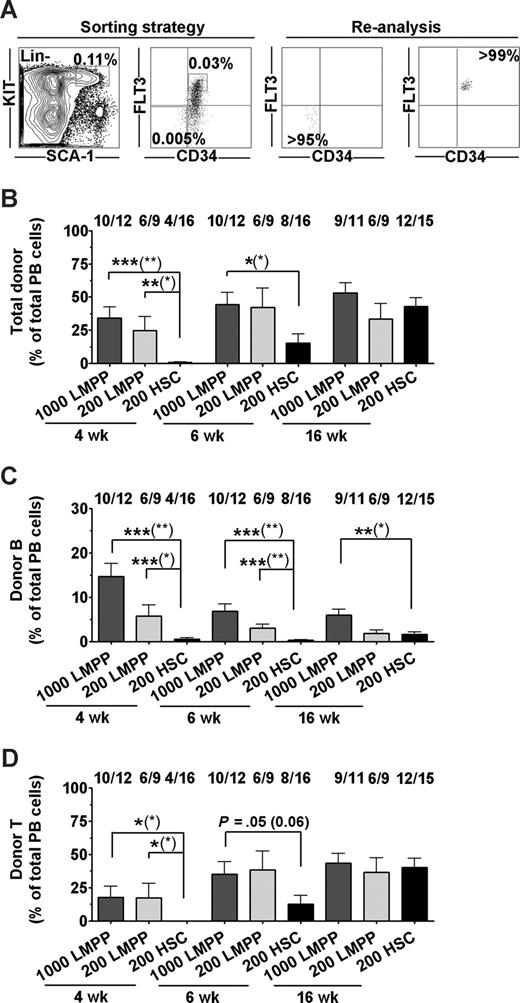
As transplantation of unfractionated BM cells gave robust lymphoid reconstitution without evidence of HSC engraftment, we next addressed whether the non–self-renewing LMPPs could potentially promote and sustain immune reconstitution in nonablated X-SCID fetal recipients or whether HSCs are required for sustained reconstitution. Thus, highly purified CD45.1 HSCs (LSKCD34−FLT3−, 200 per recipient)23 or LMPPs (LSKCD34+FLT3hi, 200 or 1000 per recipient)25 (Figure 2A) were transplanted into fetal (E14-16) CD45.2 X-SCID recipients. Importantly, LMPPs were transplanted at the same cell number as LSKCD34−FLT3− HSCs to compare their reconstitution potencies on a cell-to-cell basis, but also at 5-fold higher numbers, as they are present in WT BM at a 5-fold higher frequency than LSKCD34−FLT3− HSCs.23,25
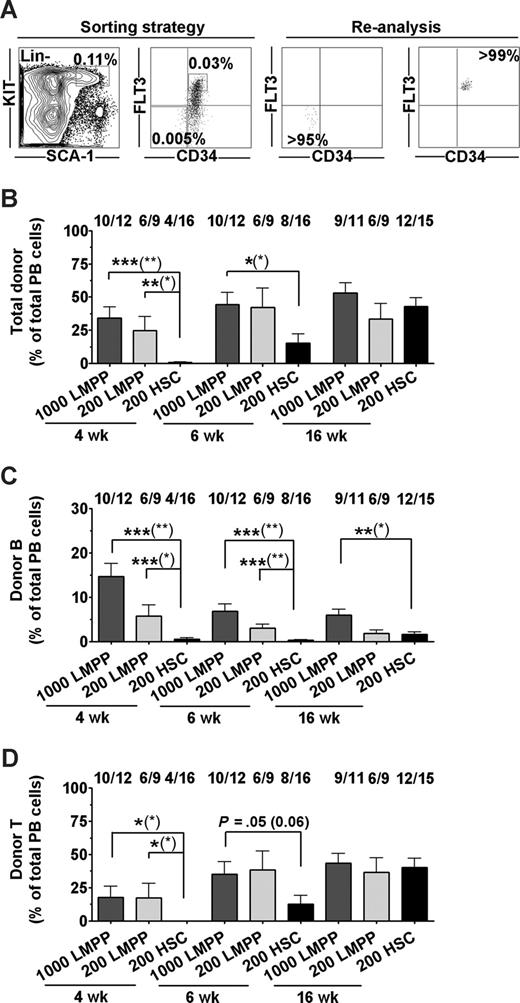
LSK CD34+Flt3hi LMPPs provide rapid and sustained B- and T-cell engraftment of fetal X-SCID recipients. (A) Sorting strategy and purity of sorted Lin−SCA-1+KIT+CD34+FLT3hi LMPPs and Lin−SCA-1+KIT+CD34−FLT3− HSCs. Numbers in gates/quadrants indicate mean frequencies of total BM cells. (B-D) LMPPs (1000 or 200) and HSCs (200) were transplanted to fetal X-SCID recipients. PB analysis was performed at 4, 6, and 16 weeks after transplantation. (B) Donor-derived total reconstitution as percentage of total nucleated cells. Indicated above bars are frequencies of mice reconstituted (> 0.1% for each parameter) by transplanted test populations at the analysis time point, of all mice analyzed. (C) Donor-derived B lymphocytes as percentage of total PB cells. (D) Donor-derived T lymphocytes as percentage of total PB cells. All bar graphs represent mean (SEM) reconstitution levels of positively reconstituted mice. Statistical significance in reconstitution levels between LMPP and HSC transplanted recipients was evaluated, comparing only reconstituted mice, as well as comparing all recipients alive (positive and negative for reconstitution) at analysis time point (P values within parentheses). ***P < .001; **P < .005; *P < .05.
LSK CD34+Flt3hi LMPPs provide rapid and sustained B- and T-cell engraftment of fetal X-SCID recipients. (A) Sorting strategy and purity of sorted Lin−SCA-1+KIT+CD34+FLT3hi LMPPs and Lin−SCA-1+KIT+CD34−FLT3− HSCs. Numbers in gates/quadrants indicate mean frequencies of total BM cells. (B-D) LMPPs (1000 or 200) and HSCs (200) were transplanted to fetal X-SCID recipients. PB analysis was performed at 4, 6, and 16 weeks after transplantation. (B) Donor-derived total reconstitution as percentage of total nucleated cells. Indicated above bars are frequencies of mice reconstituted (> 0.1% for each parameter) by transplanted test populations at the analysis time point, of all mice analyzed. (C) Donor-derived B lymphocytes as percentage of total PB cells. (D) Donor-derived T lymphocytes as percentage of total PB cells. All bar graphs represent mean (SEM) reconstitution levels of positively reconstituted mice. Statistical significance in reconstitution levels between LMPP and HSC transplanted recipients was evaluated, comparing only reconstituted mice, as well as comparing all recipients alive (positive and negative for reconstitution) at analysis time point (P values within parentheses). ***P < .001; **P < .005; *P < .05.
LMPPs rapidly engrafted the majority of unconditioned fetal X-SCID recipients, and reconstituted mice showed at 4 weeks after IUHCT mean total reconstitution levels of 34% derived from 1000 LMPPs and 24% from 200 LMPPs, compared with only 0.6% from 200 HSCs (Figures 2B, S1A). At 6 weeks, mean total reconstitution levels were 44% and 42% for mice transplanted fetally with 1000 and 200 LMPPs, respectively, compared with the HSC recipient group with a mean reconstitution level of 15% (Figures 2B, S1B).
Notably, high levels of PB reconstitution were maintained at 16 weeks in SCID recipients transplanted with 1000 or 200 LMPPs (53% and 33%, respectively), whereas HSC-derived reconstitution increased to 43% (Figures 2B, S1C).
At 4 weeks, 200 LMPPs contributed substantially to reconstitution of the B- and T-cell lineages (mean of 5.7% and 17% of total blood cells, respectively; Figures 2C,D and S1A). These levels of LMPP-derived lymphoid reconstitution were in the case of B cells sustained and for T cells further improved (to 36% for 200 cells) at 16 weeks after transplantation. Although initially severely delayed in their reconstitution compared with LMPPs, HSCs contributed to 1.6% B cells and 40% T cells (of total PB cells) in X-SCID recipients at 16 weeks after IUHCT.
Previous studies of IUHCT in mouse models have been associated with considerable loss of transplanted recipients (typically 70%-80%), in part related to the surgical procedure itself and partially because of postnatal loss of mice resulting from cannibalism and neglect by the mothers.15,16,30 In our studies, a total of 59 fetuses were transplanted with 1000 LMPPs; 34 fetuses received 200 LMPPs and 47 fetuses received 200 HSCs. Survival ratios at the time of weaning varied from 34% (200 HSC) to 20% (1000 LMPPs) and 26% (200 LMPPs; Table S1). These differences were not statistically different, and the loss of recipients appeared unrelated to transplanted cells, as in control experiments of sham- and BM-injected fetal recipients, comparable survival was observed (37% and 33%, respectively; Table S1).
Sustained generation of lymphoid progenitors and mature lymphocytes in fetal recipients of both HSCs and LMPPs
LMPPs have been demonstrated to have limited self-renewing capacity when transplanted in lethally irradiated WT hosts.23-25 Herein we found LMPPs to not only engraft more rapidly and with higher efficiency than HSCs, but also to sustain high levels of B- and in particular T-cell reconstitution in unconditioned X-SCID fetal recipients up to 16 weeks after transplantation. To investigate the potential of LMPPs to not only sustain mature B and T progeny, which can also be maintained through peripheral expansion,31-33 but also to replenish more short-lived T- and B-cell progenitors in the host hematopoietic tissues, we investigated the hematopoietic organs of reconstituted mice transplanted with 200 LMPPs or 200 HSCs, 36 weeks after transplantation. PB analysis at this time point showed comparable total reconstitution levels for mice transplanted fetally with as little as 200 LMPPs or the same number of HSCs, and also the total number of donor-derived (CD4+ and CD8+) T cells and (B220+CD19+IgM+) B cells were comparable (Figure 3A,B). All circulating LMPP-derived CD4+ and CD8+ T lymphocytes expressed the beta chain of the T-cell receptor (TCR-beta; Figure 3B).
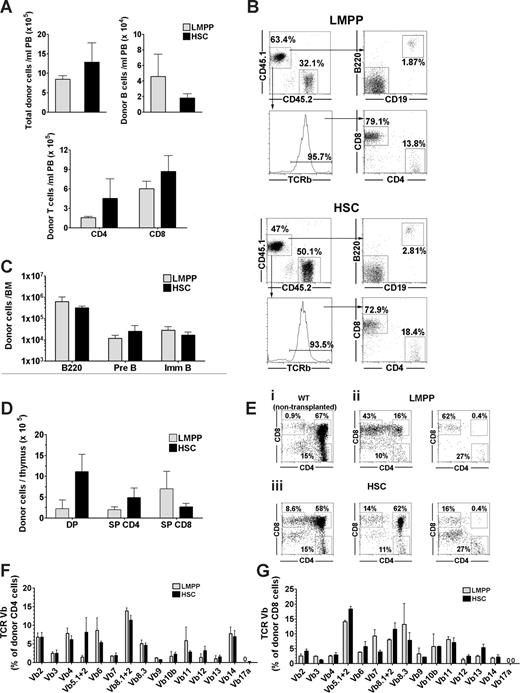
Long-term reconstitution of lymphoid progenitors and mature lymphocytes in unconditioned X-SCID mice after fetal transplantation of LMPPs and HSCs. (A-G) Fetal X-SCID mice transplanted with 200 LSK CD34+FLT3hi LMPPs ( ) or 200 LSK CD34−FLT3− HSCs (
) or 200 LSK CD34−FLT3− HSCs ( ) were analyzed for donor-derived lymphoid progenitors and mature lymphocytes in PB, thymus, BM, and spleen 36 weeks after transplantation. (A) Mean (SEM) donor-derived contribution to total nucleated blood cells (top left panel), B cells (B220+C19+; top right panel), and T cells (CD4+ and CD8+; bottom panel) from 5 reconstituted mice. (B) FACS profiles of PB analysis from typical fetal X-SCID recipient transplanted with 200 LMPPs (top FACS plots) and 200 HSCs (bottom FACS plots). For each of the 2 populations, top panels show donor-derived CD45.1 (top left) B220+CD19+ B cells (top right). Bottom left panel shows frequency of TCR β+ T cells among total CD45.1+ cells, and bottom right panel shows distribution of TCR β+ cells into CD4+ and CD8+ blood cells. Gated numbers represent frequencies of total donor cells. (C) Mean (SEM) donor contribution to total B220 cells (B220+), pre B (B220+CD43−IgM−), and immature/mature B cells (B220+CD43−IgM+) in BM (per 2 femurs and 2 tibias) of 5 reconstituted mice. (D) Mean (SEM) donor contribution to DP, SP CD4, and SP CD8 cells in thymus from 5 mice, transplanted 36 weeks earlier. (E) Representative CD4 and CD8 FACS profiles of thymus (gated on CD45.1+ donor cells) in mice transplanted with LMPPs (ii) and HSCs (iii) 36 weeks earlier, showing variable reconstitution of CD4+CD8+ DP thymocytes, and compared with same profiles in WT control (nontransplanted) mouse (i). Percentages for LMPP and HSC transplanted mice are relative to total donor cells. (F,G) TCR V-beta rearrangement of (F) CD4+ and (G) CD8+ donor (CD45.1+) derived cells in spleen. Bar graphs represent mean (± SEM) percentage of cells (of 6 mice analyzed) expressing different V-β subtypes of total donor (F) CD4 and (G) CD8 cells. For all panels, all differences between LMPP and HSC transplanted mice were nonsignificant (P > .05).
) were analyzed for donor-derived lymphoid progenitors and mature lymphocytes in PB, thymus, BM, and spleen 36 weeks after transplantation. (A) Mean (SEM) donor-derived contribution to total nucleated blood cells (top left panel), B cells (B220+C19+; top right panel), and T cells (CD4+ and CD8+; bottom panel) from 5 reconstituted mice. (B) FACS profiles of PB analysis from typical fetal X-SCID recipient transplanted with 200 LMPPs (top FACS plots) and 200 HSCs (bottom FACS plots). For each of the 2 populations, top panels show donor-derived CD45.1 (top left) B220+CD19+ B cells (top right). Bottom left panel shows frequency of TCR β+ T cells among total CD45.1+ cells, and bottom right panel shows distribution of TCR β+ cells into CD4+ and CD8+ blood cells. Gated numbers represent frequencies of total donor cells. (C) Mean (SEM) donor contribution to total B220 cells (B220+), pre B (B220+CD43−IgM−), and immature/mature B cells (B220+CD43−IgM+) in BM (per 2 femurs and 2 tibias) of 5 reconstituted mice. (D) Mean (SEM) donor contribution to DP, SP CD4, and SP CD8 cells in thymus from 5 mice, transplanted 36 weeks earlier. (E) Representative CD4 and CD8 FACS profiles of thymus (gated on CD45.1+ donor cells) in mice transplanted with LMPPs (ii) and HSCs (iii) 36 weeks earlier, showing variable reconstitution of CD4+CD8+ DP thymocytes, and compared with same profiles in WT control (nontransplanted) mouse (i). Percentages for LMPP and HSC transplanted mice are relative to total donor cells. (F,G) TCR V-beta rearrangement of (F) CD4+ and (G) CD8+ donor (CD45.1+) derived cells in spleen. Bar graphs represent mean (± SEM) percentage of cells (of 6 mice analyzed) expressing different V-β subtypes of total donor (F) CD4 and (G) CD8 cells. For all panels, all differences between LMPP and HSC transplanted mice were nonsignificant (P > .05).
Long-term reconstitution of lymphoid progenitors and mature lymphocytes in unconditioned X-SCID mice after fetal transplantation of LMPPs and HSCs. (A-G) Fetal X-SCID mice transplanted with 200 LSK CD34+FLT3hi LMPPs ( ) or 200 LSK CD34−FLT3− HSCs (
) or 200 LSK CD34−FLT3− HSCs ( ) were analyzed for donor-derived lymphoid progenitors and mature lymphocytes in PB, thymus, BM, and spleen 36 weeks after transplantation. (A) Mean (SEM) donor-derived contribution to total nucleated blood cells (top left panel), B cells (B220+C19+; top right panel), and T cells (CD4+ and CD8+; bottom panel) from 5 reconstituted mice. (B) FACS profiles of PB analysis from typical fetal X-SCID recipient transplanted with 200 LMPPs (top FACS plots) and 200 HSCs (bottom FACS plots). For each of the 2 populations, top panels show donor-derived CD45.1 (top left) B220+CD19+ B cells (top right). Bottom left panel shows frequency of TCR β+ T cells among total CD45.1+ cells, and bottom right panel shows distribution of TCR β+ cells into CD4+ and CD8+ blood cells. Gated numbers represent frequencies of total donor cells. (C) Mean (SEM) donor contribution to total B220 cells (B220+), pre B (B220+CD43−IgM−), and immature/mature B cells (B220+CD43−IgM+) in BM (per 2 femurs and 2 tibias) of 5 reconstituted mice. (D) Mean (SEM) donor contribution to DP, SP CD4, and SP CD8 cells in thymus from 5 mice, transplanted 36 weeks earlier. (E) Representative CD4 and CD8 FACS profiles of thymus (gated on CD45.1+ donor cells) in mice transplanted with LMPPs (ii) and HSCs (iii) 36 weeks earlier, showing variable reconstitution of CD4+CD8+ DP thymocytes, and compared with same profiles in WT control (nontransplanted) mouse (i). Percentages for LMPP and HSC transplanted mice are relative to total donor cells. (F,G) TCR V-beta rearrangement of (F) CD4+ and (G) CD8+ donor (CD45.1+) derived cells in spleen. Bar graphs represent mean (± SEM) percentage of cells (of 6 mice analyzed) expressing different V-β subtypes of total donor (F) CD4 and (G) CD8 cells. For all panels, all differences between LMPP and HSC transplanted mice were nonsignificant (P > .05).
) were analyzed for donor-derived lymphoid progenitors and mature lymphocytes in PB, thymus, BM, and spleen 36 weeks after transplantation. (A) Mean (SEM) donor-derived contribution to total nucleated blood cells (top left panel), B cells (B220+C19+; top right panel), and T cells (CD4+ and CD8+; bottom panel) from 5 reconstituted mice. (B) FACS profiles of PB analysis from typical fetal X-SCID recipient transplanted with 200 LMPPs (top FACS plots) and 200 HSCs (bottom FACS plots). For each of the 2 populations, top panels show donor-derived CD45.1 (top left) B220+CD19+ B cells (top right). Bottom left panel shows frequency of TCR β+ T cells among total CD45.1+ cells, and bottom right panel shows distribution of TCR β+ cells into CD4+ and CD8+ blood cells. Gated numbers represent frequencies of total donor cells. (C) Mean (SEM) donor contribution to total B220 cells (B220+), pre B (B220+CD43−IgM−), and immature/mature B cells (B220+CD43−IgM+) in BM (per 2 femurs and 2 tibias) of 5 reconstituted mice. (D) Mean (SEM) donor contribution to DP, SP CD4, and SP CD8 cells in thymus from 5 mice, transplanted 36 weeks earlier. (E) Representative CD4 and CD8 FACS profiles of thymus (gated on CD45.1+ donor cells) in mice transplanted with LMPPs (ii) and HSCs (iii) 36 weeks earlier, showing variable reconstitution of CD4+CD8+ DP thymocytes, and compared with same profiles in WT control (nontransplanted) mouse (i). Percentages for LMPP and HSC transplanted mice are relative to total donor cells. (F,G) TCR V-beta rearrangement of (F) CD4+ and (G) CD8+ donor (CD45.1+) derived cells in spleen. Bar graphs represent mean (± SEM) percentage of cells (of 6 mice analyzed) expressing different V-β subtypes of total donor (F) CD4 and (G) CD8 cells. For all panels, all differences between LMPP and HSC transplanted mice were nonsignificant (P > .05).
To compare the ability of LMPPs and HSCs to sustain active B lymphopoiesis in the BM, we also investigated, at 36 weeks after transplantations, the persistence of B-cell precursors in the BM. Notably, the absolute numbers of LMPPs and HSCs derived total B220+ cells as well as B220+CD43−IgM− pre-B cells and CD43−B220+IgM+ immature/mature B cells were comparable (Figure 3C), demonstrating the ability of as few as 200 LMPPs to sustain active B lymphopoiesis in the long term in X-SCID mice transplanted during fetal development.
Within the thymus, donor-derived reconstitution remained high in LMPP- and HSC-transplanted fetal recipients, respectively, at 36 weeks after transplantation. Importantly, in both cases, this reconstitution included naive double-positive (DP) CD4+CD8+ thymocytes and single-positive (SP) CD4+ and CD8+ thymocytes (Figure 3C,D), although the frequency of DP thymocytes was higher in HSC-transplanted recipients. Because thymic progenitors must be replaced daily from the BM to sustain active thymopoiesis,34 this also established the ability of fetally transplanted LMPPs to long-term replenish thymic progenitors, although probably less efficiently than HSCs.
To establish that the sustained long-term peripheral T-cell reconstitution on LMPP transplantation was polyclonal and not the result of oligoclonal peripheral expansion, we investigated, at 36 weeks after transplantation, the expression of 15 different TCR V beta subtypes in CD4 as well as CD8 SP LMPP-derived T cells. All except one (TCR Vb17a) of the subtypes analyzed could be found expressed in CD4+ and CD8+ T cells, with no difference observed between mice transplanted with LMPPs and HSCs during fetal development (Figure 3F,G), and with a repertoire of different TCR Vb subtypes comparable with that of splenic T cells in nontransplanted WT control mice, suggesting a polyclonal and balanced origin of T cells from low numbers of LMPPs transplanted into fetal X-SCID recipients.
Although the above data demonstrated the ability of low numbers of LMPPs to sustain B- and T-cell progenitors for considerable time, they also implied that reconstitution with self-renewing HSCs might in the long-term be required for stable B- and T-cell reconstitution in nonconditioned X-SCID recipients.
To compare the self-renewal ability of LMPP and HSC donor-derived BM cells in unconditioned X-SCID recipients, we performed secondary transplantation experiments. BM cells from a total of 11 fetal primary recipients of 1000 LMPPs or 200 HSCs with comparable B- and T-cell reconstitution levels at 16 weeks after transplantation (Figure 4) were transplanted into sublethally irradiated X-SCID adult mice. At 12 weeks after secondary transplantation, both total B- and T-cell donor-derived reconstitution levels were considerably higher in secondary HSC than LMPP recipients (Figure 4), despite fetal recipients being originally transplanted with 5 times more LMPPs than HSCs.
HSCs are superior to LMPPs in reconstituting unconditioned X-SCID recipients with self-renewing stem and progenitor cells. BM cells were collected 16 weeks after primary transplantation from (n = 11) unconditioned X-SCID fetal recipients transplanted with 1000 CD45.1+ LMPPs ( ) or 200 HSCs (
) or 200 HSCs ( ). For each primary (first-degree) recipient, 1 femur equivalent was transplanted into 1 or 2 secondary (second-degree) sublethally (650 cGy) irradiated (8-10 weeks old) X-SCID (CD45.2) recipients (n = 18). Reconstitution analysis was performed at 12 weeks after second-degree transplantation. (A-C) Donor-derived contribution in first-degree and second-degree X-SCID recipients toward (A) total PB cells, (B) B lymphocytes, and (C) T lymphocytes, all presented as mean (SEM) percentages of total PB cells from 3 independent experiments. *P < .05.
). For each primary (first-degree) recipient, 1 femur equivalent was transplanted into 1 or 2 secondary (second-degree) sublethally (650 cGy) irradiated (8-10 weeks old) X-SCID (CD45.2) recipients (n = 18). Reconstitution analysis was performed at 12 weeks after second-degree transplantation. (A-C) Donor-derived contribution in first-degree and second-degree X-SCID recipients toward (A) total PB cells, (B) B lymphocytes, and (C) T lymphocytes, all presented as mean (SEM) percentages of total PB cells from 3 independent experiments. *P < .05.
HSCs are superior to LMPPs in reconstituting unconditioned X-SCID recipients with self-renewing stem and progenitor cells. BM cells were collected 16 weeks after primary transplantation from (n = 11) unconditioned X-SCID fetal recipients transplanted with 1000 CD45.1+ LMPPs ( ) or 200 HSCs (
) or 200 HSCs ( ). For each primary (first-degree) recipient, 1 femur equivalent was transplanted into 1 or 2 secondary (second-degree) sublethally (650 cGy) irradiated (8-10 weeks old) X-SCID (CD45.2) recipients (n = 18). Reconstitution analysis was performed at 12 weeks after second-degree transplantation. (A-C) Donor-derived contribution in first-degree and second-degree X-SCID recipients toward (A) total PB cells, (B) B lymphocytes, and (C) T lymphocytes, all presented as mean (SEM) percentages of total PB cells from 3 independent experiments. *P < .05.
). For each primary (first-degree) recipient, 1 femur equivalent was transplanted into 1 or 2 secondary (second-degree) sublethally (650 cGy) irradiated (8-10 weeks old) X-SCID (CD45.2) recipients (n = 18). Reconstitution analysis was performed at 12 weeks after second-degree transplantation. (A-C) Donor-derived contribution in first-degree and second-degree X-SCID recipients toward (A) total PB cells, (B) B lymphocytes, and (C) T lymphocytes, all presented as mean (SEM) percentages of total PB cells from 3 independent experiments. *P < .05.
Enhanced lymphoid reconstitution after fetal transplantation of X-SCID recipients with purified congenic HSCs
It has been proposed that the preimmune status and/or the microenvironment of the fetus might result in enhanced hematopoietic engraftment compared with postnatal transplantations.14,15 However, previous comparative studies of stem cell transplantations of fetal, postnatal, and adult unconditioned recipients have all been performed in the allogeneic setting, and with whole BM transplants,15,16 rather than with enriched stem/progenitor populations as in successful clinical IUHCT of patients with X-SCID.10,11 Thus, we here also investigated the ability of purified congenic LMPPs and HSCs (in both cases, 200 cells per recipient) to reconstitute B and T lymphopoiesis at the early postnatal stage (neonatal mice D1-4) and in young adult (4-6 weeks old) unconditioned X-SCID recipients, compared with the results obtained through IUHCT.
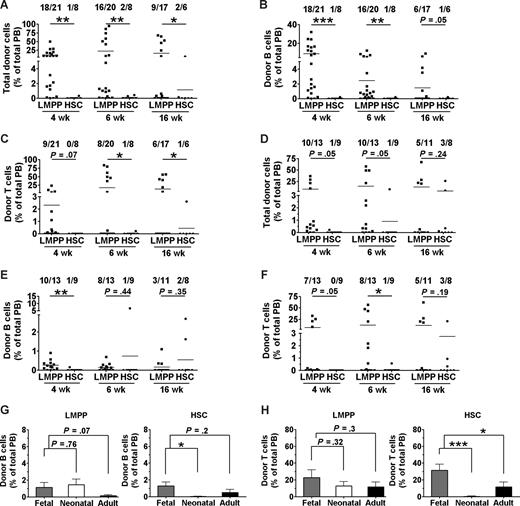
Most X-SCID mice transplanted with 200 LMPPs at the neonatal stage showed significant donor-derived reconstitution at 4 (18 of 21) and 6 (16 of 20) weeks after transplantation (Figure 5A), but only 35% of neonatal X-SCID recipients of LMPPs sustained B- and T-cell reconstitution, at 16 weeks after transplantation (Figure 5B,C). Most notably, the reconstitution frequencies as well as reconstitution levels in neonatal X-SCID recipients of HSC transplants were much lower than in LMPP recipients, as only 2 of 8 neonatal recipients ever showed any reconstitution, and at 16 weeks only 1 of 6 mice showed evidence of very low levels B- and T-cell engraftment (Figure 5A-C). This was in contrast to fetal recipients, in which 16-week reconstitution frequencies and levels were comparable in recipients of LMPPs and HSCs (Figure 2B-D).
Fetal X-SCID recipients are more permissive to congenic HSC reconstitution of the T-cell lineage than postnatal and adult recipients. Neonatal (postnatal days 1-4) and young adult (4-6 weeks old) X-SCID mice were transplanted without any conditioning with 200 congenic LMPPs (LSK CD34+ FLT3hi) or 200 HSCs (LSK CD34− FLT3−). PB reconstitution analysis of total, B cells, and T cells was performed at 3 to 4, 6, and 16 weeks after transplantation. Shown are results from 4 neonatal (A-C) and 3 adult (D-F) transplantation experiments. Numbers above bars indicate frequencies of transplanted mice with more than 0.1% total reconstitution at each time point. Statistical significance in reconstitution levels between LMPP and HSC transplanted recipients was evaluated, comparing all recipients alive (positive and negative for reconstitution) at analysis time point. ***P < .001; **P < .005; *P < .05. (G,H) Donor contribution of 200 congenic LMPPs (left panel) and 200 HSCs (right panel) to (G) B-cell and (H) T-cell reconstitution analyzed at 16 weeks after transplantation in fetal ( ), neonatal (
), neonatal ( ), and adult (
), and adult ( ) recipients. Statistical differences comparing reconstitution levels in all transplanted and alive recipients are indicated above bars. ***P < .001; **P < .005; *P < .05.
) recipients. Statistical differences comparing reconstitution levels in all transplanted and alive recipients are indicated above bars. ***P < .001; **P < .005; *P < .05.
Fetal X-SCID recipients are more permissive to congenic HSC reconstitution of the T-cell lineage than postnatal and adult recipients. Neonatal (postnatal days 1-4) and young adult (4-6 weeks old) X-SCID mice were transplanted without any conditioning with 200 congenic LMPPs (LSK CD34+ FLT3hi) or 200 HSCs (LSK CD34− FLT3−). PB reconstitution analysis of total, B cells, and T cells was performed at 3 to 4, 6, and 16 weeks after transplantation. Shown are results from 4 neonatal (A-C) and 3 adult (D-F) transplantation experiments. Numbers above bars indicate frequencies of transplanted mice with more than 0.1% total reconstitution at each time point. Statistical significance in reconstitution levels between LMPP and HSC transplanted recipients was evaluated, comparing all recipients alive (positive and negative for reconstitution) at analysis time point. ***P < .001; **P < .005; *P < .05. (G,H) Donor contribution of 200 congenic LMPPs (left panel) and 200 HSCs (right panel) to (G) B-cell and (H) T-cell reconstitution analyzed at 16 weeks after transplantation in fetal ( ), neonatal (
), neonatal ( ), and adult (
), and adult ( ) recipients. Statistical differences comparing reconstitution levels in all transplanted and alive recipients are indicated above bars. ***P < .001; **P < .005; *P < .05.
) recipients. Statistical differences comparing reconstitution levels in all transplanted and alive recipients are indicated above bars. ***P < .001; **P < .005; *P < .05.
The results in young adult X-SCID recipients were similar to those of neonatal recipients, in that most LMPP recipients showed initial donor engraftment (Figure 5D-F), but only 27% and 45% were positive for B- and T-cell reconstitution, respectively, at 16 weeks after transplantation, and in adult recipients the long-term (16 weeks) results were comparable in HSC recipients (Figure 5D-F). Thus, for HSC transplantations, compared with neonatal and adult recipients, IUHCT was clearly superior, both with regard to B-cell (Figure 5G) and in particular for the critical T-cell reconstitution (Figure 5H) of X-SCID recipients. Although a similar tendency could be observed for recipients transplanted with LMPPs, the reconstitution levels achieved after IUHCT and in particular neonatal transplantations of LMPPs were much more comparable than observed after HSC transplantations (Figure 5G,H). Thus, IUHCT of purified HSCs results in superior B- and T-cell reconstitution in unconditioned X-SCID recipients, compared with neonatal and adult transplantations.
Discussion
Although neonatal hematopoietic transplantations of patients with SCID appear to give better outcome than later transplantations, neonatal and fetal transplantations have yet to be compared in patients with X-SCID in the clinical setting, largely because the clinical outcome with neonatal X-SCID transplantations has overall been very good,13 accounting in part for the limited application of IUHCT in patients with X-SCID.18,19 Using mice with the same γc-deficiency as patients with X-SCID, we provide here evidence for fetal transplantations of congenic HSCs giving considerably higher and more consistent B- and T-cell reconstitution than transplantations at the early postnatal stage or in young adult unconditioned X-SCID recipients, and for the critical T-cell lineage similar findings (although not statistically significant) were observed when transplanting LMPPs. Although our studies do not conclusively establish the mechanism for the competitive advantage of purified HSCs (or LMPPs) in fetal over postnatal X-SCID recipients, they do demonstrate that, even when using congenic donors and purified HSCs capable of crossing fully allogeneic barriers,35 a considerable reconstitution advantage of HSCs is achieved through intrauterine transplantation. As we also demonstrate that HSC reconstitution might be critical for the long-term replenishment of T cells in X-SCID recipients, this strongly suggests that improved results with intrauterine transplantations of nonconditioned X-SCID recipients might at least in part be achieved because of a more favorable microenvironment in fetal hematopoietic tissues. Whether or not this specifically relates to enhanced access to suitable stem and progenitor niches remains to be investigated.
Although it is possible that normalizing the cell dose to the body weight of X-SCID recipients at different ages could have partially corrected the differences in reconstitution levels observed, we think this is unlikely to be a main reason for the observed differences in reconstitution levels, in part because not only adult but also neonatal recipients transplanted with the same dose of HSCs showed impaired reconstitution compared with fetally transplanted mice, and importantly the high levels of reconstitution in fetally transplanted mice were sustained into adulthood.
Conditioning (with irradiation or chemotherapy) of the X-SCID recipients could also have minimized the differences seen in engraftment levels observed at different ages, but a major goal in the current studies, and in hematopoietic transplantations in general, is to reduce or preferably alleviate the need for cytotoxic conditioning because of its serious side effects and resulting long-term morbidity.
Although recent studies demonstrated the existence of a considerable immune barrier, also after intrauterine transplantations of allogeneic whole BM cells in WT recipients,17 the data herein establish the superiority of intrauterine transplantations in immune reconstitution of unconditioned X-SCID recipients with purified HSCs. It is in that regard of relevance that (CD34) enriched stem and progenitor cell populations also have been used successfully in clinical IUHCT of X-SCID.10,11 Whether or not such enrichment is required or beneficial beyond reducing potential graft-versus-host disease, however, remains to be established.
The observed striking differences in kinetics of B- and T-cell reconstitution by transplanted purified HSCs and LMPPs in X-SCID recipients is of probable clinical relevance for IUHCT (and postnatal transplantation) of X-SCID recipients, and it also provides new insights into the biology and potential importance of LMPPs in replenishment of B- and T-cell progenitors in unconditioned recipients. From a clinical viewpoint, because one of the goals and claimed advantages of IUHCT in X-SCID is to achieve rapid immune reconstitution postnatally,19 it would be important to ensure that multipotent progenitors equivalent to the LMPPs would be included in stem/progenitor cell-enriched grafts. In that regard, whereas transplantation of as few as 200 LMPPs gave high B- and T-cell reconstitution already 4 weeks after IUHCT, 200 HSCs gave limited B- and almost no T-cell reconstitution at 4 weeks, and also much less at 6 weeks after transplantation, compared with X-SCID recipients transplanted with LMPPs. This difference was even more striking after transplantations in neonatal and young adult X-SCID recipients. Importantly, this difference in kinetics of reconstitution was observed when transplanting the same number of LMPPs and HSCs. As the compartment of LSKCD34+Flt3hi LMPPs is approximately 5 times larger than LSKCD34−Flt3− HSCs,24-26 the relative importance of LMPPs over HSCs in rapid reconstitution of lymphopoiesis in X-SCID recipients is, if anything, underappreciated in these experiments.
Because the identity of human LMPPs has yet to be established, one risks depleting these multipotent progenitors in different enrichment methods, although they are probably part of the CD34+ stem/progenitor compartment. Markers that might identify a human LMPP counterpart are probably largely distinct from that of mouse LMPPs as, for instance, Sca-1 does not exist in humans. Regardless, the present findings underscore the importance and relevance of seeking to identify and characterize the human LMPP or equivalent human multipotent progenitors.
In light of LMPPs possessing quite limited self-renewal ability when transplanted into lethally irradiated WT recipients,25 it was of considerable interest that LMPPs in the long-term (16 and even 36 weeks after transplantation) after IUHCT sustained peripheral B and T cells at a high level, comparable with that of HSCs. Importantly, as mature B and T cells can be sustained in the long-term through peripheral expansion,31-33 we investigated and demonstrated, as late as 36 weeks after transplantation, that IUHCT of as few as 200 LMPPs is sufficient to sustain replenishment of short-lived B-cell progenitors in the BM and short-lived T-cell progenitors in the thymus. The functional importance of this was further substantiated by the demonstration of polyclonal origin of LMPP-derived peripheral T cells at 36 weeks after transplantation. Thus, although our studies, including secondary transplantations, are most compatible with HSC reconstitution being essential for optimal and stable long-term replenishment of B and T lymphopoiesis in X-SCID recipients, LMPP-like cells appear required for rapid reconstitution and also efficient at contributing to mid- or long-term replenishment of polyclonal B and T lymphopoiesis. These conclusions were also supported by other recent studies36 in which progenitors with full multilineage potential37 were transplanted into adult γc−/− mice also deficient for RAG2 expression, giving a more severe immune deficiency than X-SCID. In this model, multipotent progenitors were demonstrated to be sufficient to give rapid and sustained reconstitution of peripheral B cells. However, these studies did not directly establish whether this also included maintenance of short-lived B-cell progenitors, and most importantly reconstitution of T-cell progenitors and polyclonally derived T cells was not directly demonstrated, although a functional antibody response that is T cell–dependent was demonstrated.36 Because, in humans, isolated γc-deficiency results in reduced T cells but not B cells, whereas γc−/− mice have reduced B and T cells, our demonstration of the ability of LMPPs to reconstitute in a rapid and sustainable manner polyclonal T cells is, from a clinical viewpoint, of particular significance.
The recent identification of LMPPs through different approaches25,26,38-40 implicates a novel pathway in hematopoiesis in which MkE potential is lost before GM potential in the process of lineage restriction toward lymphopoiesis.41 The present studies extend the potent ability of LMPPs to replenish lymphopoiesis to a clinically relevant setting. They also shed new light on the probable physiologic role of LMPPs as early lineage-restricted (compared with HSCs) but multipotent progenitors of B and T lymphopoiesis. Whereas the original studies of transplanted LMPPs in maximally myeloablated recipients demonstrated their lack of self-renewal ability,23,24 the present studies demonstrate that as few as 200 LMPPs, despite this, have extensive ability to not only replenish peripheral B and T cells in lymphopenic mice but also their short-lived progenitors, at least for up to 36 weeks.
LMPPs might also prove to be attractive candidates for gene therapy of X-SCID, not only because of their potent ability to reconstitute X-SCID recipients but also in part because they are more actively cycling than HSCs, and might therefore be more accessible to transduction with viral vectors than HSCs. Because of their limited self-renewal ability, they might also prove less susceptible to leukemic transformation on transduction with γc-expressing viral vectors,5 although this remains to be investigated.
The low survival rate in our fetal transplantation experiments is a considerable concern for these types of studies, although the survival rate was not lower than what has previously been reported after IUHCT transplantation in immune-deficient mouse models, typically ranging from 20% to 30%.15,16,30 Recently, Peranteau et al42 described a new transplantation procedure through the vitelline vein, apparently less traumatic, resulting in somewhat improved survival, but still only 35% to 37%. Our experience is in agreement with others in that the high loss of fetal transplant recipients is in part a consequence of the surgical procedure and transplantation of small mouse fetuses but is further aggravated by perinatal maternal cannibalism and neglect. Importantly, our control experiments with sham-transplanted fetuses demonstrated that the transplanted hematopoietic cells themselves have little or no influence on this mortality. More importantly, this high mortality appears to have limited relevance for human fetal transplantations. The expected loss in the human setting would be less than 3%, based on calculation of the cumulative risk of prenatal diagnosis (< 1%) and fetal cord blood transfusion (< 2%).43 Even when retrospectively considering the report of a total of 4 procedure-related deaths of total 39 IUHCTs performed for various inborn hematologic/metabolic defects (1989-2001), the mortality does not exceed 10%.18
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lilian Wittman, Anna Fossum, and Zhi Ma for expert technical support, Ewa Sitnicka for valuable discussions, and David Bryder and Karel Marsal for helpful feedback on the manuscript.
This work was supported by grants from the Swedish Research Council, Göran Gustaffson Foundation, Juvenile Diabetes Research Foundation, the Swedish Foundation for Strategic Research, the Swedish Cancer Society, all in Stockholm, Sweden, and EuroStemCell (EU project LHSB-CT-2003-503005). The Lund Stem Cell Center is supported by a Center of Excellence grant in life sciences from the Swedish Foundation for Strategic Research. S.-E.W.J. was supported through a strategic appointment from the Medical Research Council, United Kingdom.
Authorship
Contribution: K.L. designed and performed research, collected and analyzed data, and wrote the manuscript; C.J.H.P. performed research and collected and analyzed data; S.R.W.S. performed research and analyzed data; and S.-E.W.J. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sten-Eirik W. Jacobsen, Haematopoietic Stem Cell Lab, Weatherall Institute of Molecular Medicine, University of Oxford, Oxford, United Kingdom; e-mail: Sten.Jacobsen@imm.ox.ac.uk.