Abstract
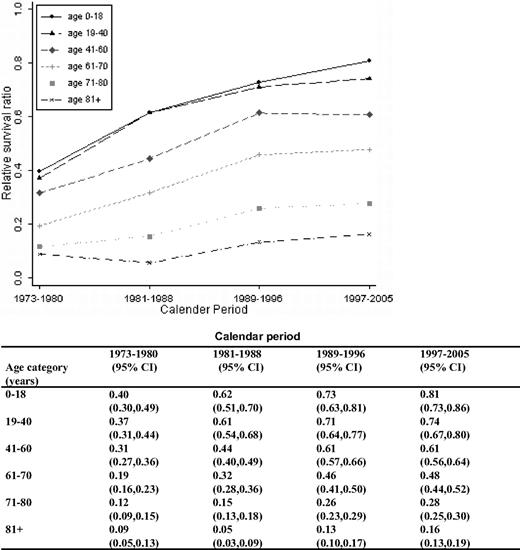
We evaluated survival patterns for all registered acute myeloid leukemia (AML) patients diagnosed in Sweden in 1973 to 2005 (N = 9729; median age, 69 years). Patients were categorized into 6 age groups and 4 calendar periods (1973-1980, 1981-1988, 1989-1996, and 1997-2005). Relative survival ratios were computed as measures of patient survival. One-year survival improved over time in all age groups, whereas 5- and 10-year survival improved in all age groups, except for patients 80+ years. The 5-year relative survival ratios in the last calendar period were 0.65, 0.58, 0.36, 0.15, 0.05, and 0.01 for the age groups 0 to 18, 19 to 40, 41 to 60, 61 to 70, 71 to 80, and 80+ years, respectively. Intensified chemotherapy, a continuous improvement in supportive care, and allogeneic stem cell transplantation are probably the most important factors contributing to this finding. In contrast, there was no improvement in survival in AML patients with a prior diagnosis of a myelodysplastic syndrome during 1993 to 2005 (n = 219). In conclusion, AML survival has improved during the last decades. However, the majority of AML patients die of their disease and age remains an important predictor of prognosis. New effective agents with a more favorable toxicity profile are needed to improve survival, particularly in the elderly.
Introduction
Acute myeloid leukemia (AML) has an age-adjusted incidence rate of 3 to 4/100 000 in Western countries,1-3 and the median age at diagnosis is approximately 70 years.3-5 It is an aggressive disease that is rapidly fatal without specific therapy, and achievement of complete remission (CR) is a prerequisite for long-term survival. Curative treatment was not available until the late 1960s, when daunorubicin and cytosine arabinoside (DA) were introduced.6,7 The combined use of these drugs made it possible to induce CR in AML patients. In a small subpopulation of patients, there was even a potential of long-term survival. Although several antileukemic drugs have been introduced since then, no other combination of drugs has proven to be more effective.8 Thus, DA remains the cornerstone of AML treatment. The concept of consolidation treatment has been developed to prevent relapse of the disease.9-12 In addition, allogeneic stem cell transplantation (allo-SCT) reduces the risk of relapse and improves survival in selected patient groups.13-15 Autologous SCT (auto-SCT) has been a part of AML treatment since the 1980s but does not seem to significantly improve survival compared with conventional consolidation treatment.16-18
In the early 1970s when DA was introduced, treatment-related mortality (TRM) was substantial because of a high incidence of severe infectious complications.19 Improvements in supportive care have reduced TRM after conventional chemotherapy as well as after allo-SCT.20-23
AML is a heterogeneous disease where treatment has to be tailored according to age, performance status, and other established prognostic factors. Older age and a preceding diagnosis of a myelodysplastic syndrome (MDS) or myeloproliferative disorder are factors associated with lower remission rates, higher rates of relapse, and shorter survival. Furthermore, patients can be divided into risk groups based on results of cytogenetic analysis,24 and decisions on allo-SCT should be based on this risk stratification.15
Acute promyelocytic leukemia (APL) is a subentity of AML with specific biologic and clinical features. It is characterized by the presence of the PML/RARα fusion gene. The introduction of all-trans retinoic acid in the early 1990s has led to increased CR rates and improved long-term survival in this group of patients.25
Recently published studies indicate that CR can be achieved in 75% to 90% of younger and in 50% to 60% of older patients with AML.26 Unfortunately, the relapse rates remain high, reflected in only 40% to 50% and 10% to 15% long-term survival in younger and older patients, respectively.26 Importantly, most survival data arrive from clinical trials that are associated with a various degree of patient selection, and elderly frail patients are often not included.27
We have conducted a population-based cohort study including 9729 AML patients diagnosed in Sweden between 1973 and 2005. The aim of the study was to define AML survival patterns in the Swedish population, regardless of clinical trial enrollment, during this 33-year period.
Methods
Central registers and patients
Information regarding patients diagnosed with a malignant disorder in Sweden is reported to a centralized nationwide Swedish Cancer Register that was established in 1958. The register contains information on diagnosis, sex, date of birth, date of diagnosis, and hospital where the diagnosis was made.1,28 Every physician and pathologist/cytologist is obliged by law to report each occurrence of cancer to the registry. Each person in Sweden is given a unique national registration number. For each person, the date of death is centrally registered. Allo- and auto-SCTs performed in Sweden are reported to the European Group for Blood and Marrow Transplantation (EBMT) register, which was established in 1974. Information on the type and number of SCT in AML patients reported from Swedish centers during the study period was obtained from the EBMT register.
Using International Classification of Diseases codes (version 7), we identified all patients diagnosed with AML between January 1, 1973 and December 31, 2005 in the Swedish Cancer Register. The diagnoses of 2050 acute myeloid leukemia, 2059 acute myelomonocytic leukemia/acute myeloid leukemia nonspecified, 2060 acute monocytic leukemia, and 2069 acute monocytic leukemia nonspecified were included. Using Systematized Nomenclature of Medicine–Clinical Terms (SNOMED) codes (introduced in 1993), we were able to define patients with APL subtype (SNOMED code 98663) diagnosed in 1993 to 2005. Analysis of patients with a preceding MDS was restricted to the same time period because MDS was not reported to the Swedish Cancer Register until the early 1990s. All patients with APL and a prior MDS were also included in the analysis of the whole AML cohort. Patients with a preceding cancer diagnosis, including a hematologic malignancy, were not excluded.
All patients were followed from the date of diagnosis until death, emigration, or end of follow-up (December 31, 2006), whichever occurred first. Patients diagnosed at autopsy only were excluded. The choice to include patients from 1973 was based on the facts that, by then, the Swedish Cancer Register had reached a high coverage28,29 and treatment with anthracyclines and cytosine arabinoside had been introduced as standard induction treatment for most patients judged eligible for aggressive treatment.30
Date of death was obtained by linking the national registration number to the Register of Causes of Death. Information was gathered for sex, date of birth, region of residence at diagnosis, and date of death. Thus, no detailed clinical information on French-American-British/World Health Organization subtype, previous treatment with chemotherapy or radiotherapy, cytogenetics, routine laboratory tests, treatment, and comorbidity, was available for the cohort.
This study was approved by the Karolinska Institutional Review Board.
Treatment
In general, treatment principles for AML in Sweden during the period 1973 to 2005 have followed those of the international Western medical community. Sweden is divided into 6 major healthcare regions. The Leukemia Group of Central/Middle Sweden (LGMS), including the Stockholm, Uppsala, and Örebro regions, has performed clinical studies and used joint treatment recommendations in AML since 1970. A Swedish 4-regional group introduced common treatment recommendations in 1997. During the 33-year study period, several clinical trials have been performed in Swedish AML patients, which to a certain degree have influenced treatment strategies.31-33 We have estimated that 450 adult patients or approximately 5% of the cohort under study were included in clinical trials during the study period. Patients younger than 18 years have been treated according to Nordic Society of Pediatric Hematology and Oncology protocols since 1984.34 A total of 949 SCTs were reported to the EBMT register during the study period, 626 allo- and 323 auto-SCTs.
Survival analysis
Relative survival ratios (RSRs) were computed as measures of AML survival.35,36 An important advantage of using relative survival is that it does not rely on the accurate classification of cause of death. Instead, it provides a measure of total excess mortality associated with a diagnosis of AML irrespective of whether the excess mortality is directly or indirectly because of the cancer. The RSR is defined as the observed survival in the patient group (where all deaths are considered as events) divided by the expected survival of a comparable group from the general population, which is assumed to be free from the cancer in question. One-year, 5-year, and 10-year RSRs can be interpreted as the proportion of AML patients who survived their malignancy at 1, 5, and 10 years, respectively. Expected survival was estimated using the Ederer II method37 from Swedish population life-tables stratified by age, sex, and calendar period. One-, 5-, and 10-year RSRs were calculated for 4 calendar periods (1973-1980, 1981-1988, 1989-1996, and 1997-2005) and 6 age categories (0-18, 19-40, 41-60, 61-70, 71-80, and older than 80 years). In addition, patients diagnosed with APL or prior MDS were studied separately in 2 calendar periods (1993-1999 and 2000-2005). For APL patients, 3-year RSRs were used as outcome variable because of reduced observation time. We constructed regression models with the goal of estimating the effect of the target factors described in “Central registers and patients” while controlling for potential confounding factors. Poisson regression was used to model excess mortality.38 The estimates of this model are interpreted as excess mortality ratios; an excess mortality ratio of 1.5, for example, for males/females indicates that males experience 50% higher excess mortality than females. All calculations were performed using Stata 9.2 (StataCorp, College Station, TX).
Results
A total of 9729 AML patients (4954 males and 4775 females; median age, 69 years) were included in the study (Table 1). There was a moderate increase in the incidence of AML during the study period. In addition, there was a gradual increase in median age at diagnosis over time, being 65 years in the first period and 71 years in the last period. The age-adjusted incidence remained higher for men than women throughout the study period (Table 1). Forty percent of the patients were diagnosed in LGMS regions (Table 1). A total of 949 SCTs were reported to the EBMT register during the study period, 626 allo- and 323 auto-SCTs. Of these, more than half were carried out during the last calendar period with allo-SCT dominating (Table 1).
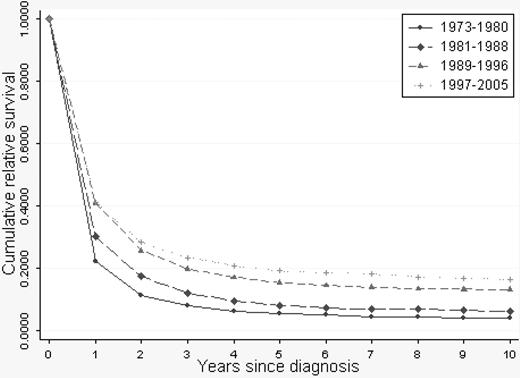
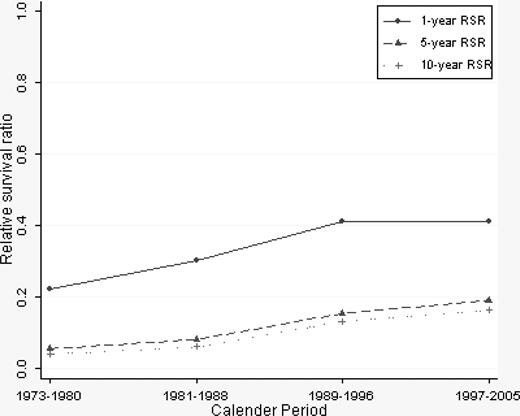
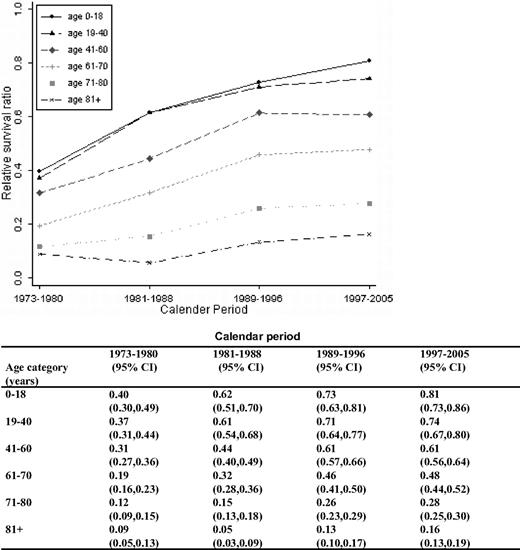
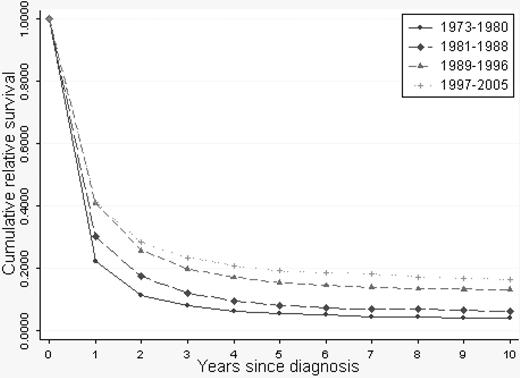
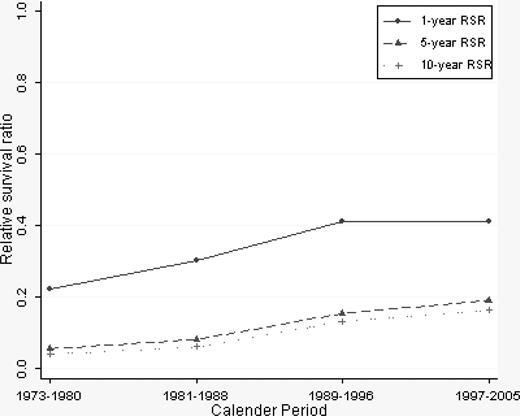
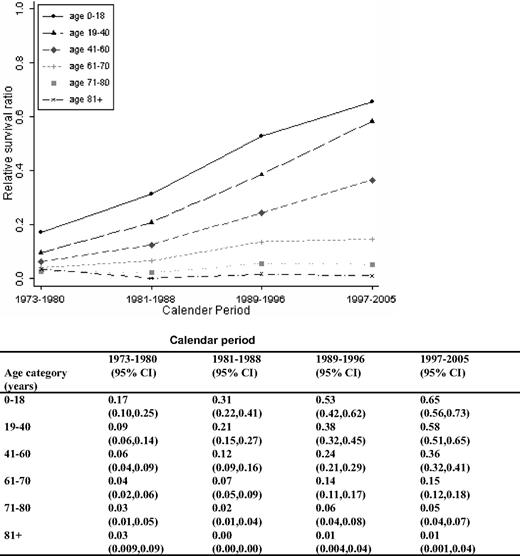
A continuous overall improvement in RSRs was seen over the study period (Figures 1, 2). One-year RSR (Figure 3) was higher in younger patients. However, during the study period, it increased by almost 2-fold in all age categories, and the increase was found to be most pronounced in the age categories 61 to 70 and 71 to 80 years (by 2.53 and 2.33, respectively).
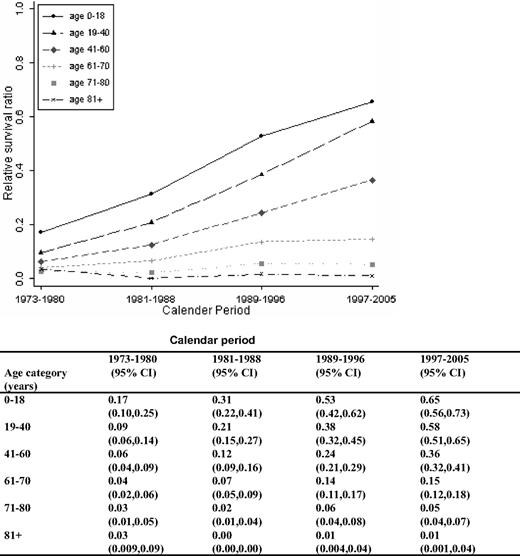
As for 1-year RSRs, the 5-year RSRs were higher and excess mortality lower in younger patients (Figure 4; Table 2). Five-year RSRs were substantially lower than 1-year RSRs. However, the improvement in 5-year RSRs was more pronounced than in 1-year RSRs, and most conspicuous in patients 19 to 40 years (6.4-fold). In patients 61 to 80 years of age, the better outcome was not observed until after the mid 1980s. For the younger age categories, the improvement seemed to be more constant over the entire study period. The 5-year RSRs were not improved in patients older than 80 years (Figure 4). Ten-year RSRs differed only little from 5-year RSRs (Figures 1, 2).
Males had a 5% higher mortality compared with females during the first 5 years after diagnosis (P = .032; Table 2). Overall, patients resident in LGMS counties had a 6% lower mortality during the first 5 years after diagnosis (P = .006; Table 2), mainly confined to differences in the first calendar period. There was no difference in excess mortality ratios between patients diagnosed at university compared with nonuniversity hospitals (data not shown).
A total of 111 patients with APL (median age, 54 years; 56 and 47 years in the 2 calendar periods under study, respectively) were diagnosed between 1993 and 2005, constituting 2.5% of all AML cases during this period. Overall 3-year RSR was 0.61 being 0.53 (95% confidence interval [CI], 0.38-0.66) in patients diagnosed in 1993 to 1999 and 0.69 (95% CI, 0.55-0.79) in patients diagnosed 2000 to 2005. Forty-eight (43%) of the APL patients had died at follow-up. One-month mortality rate was 23%: 27% in 1993 to 1999 and 18% in 2000 to 2005.
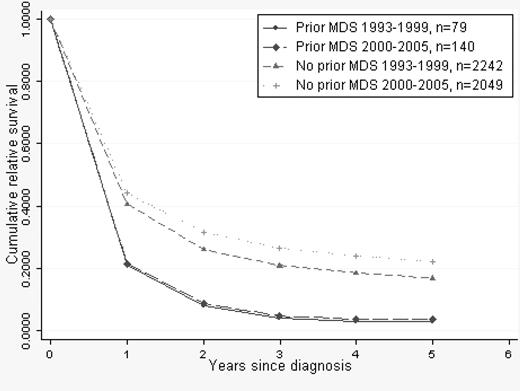
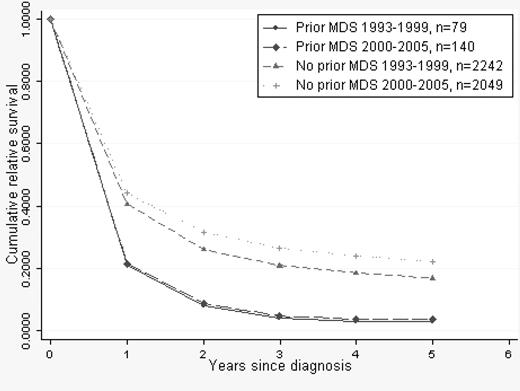
There were 219 patients (median age, 72 years) with a preceding MDS diagnosis. Mortality of patients with prior MDS was 51% higher than in patients with de novo AML when adjusted for age (Table 2), and RSRs in this group of patients did not improve during the second calendar period (Figure 5).
Cumulative relative survival in AML stratified by prior MDS/de novo AML. Median age of MDS patients was 72 years in both calendar periods.
Cumulative relative survival in AML stratified by prior MDS/de novo AML. Median age of MDS patients was 72 years in both calendar periods.
Discussion
In this large population-based cohort study including virtually all patients with AML diagnosed in Sweden during a 33-year period, we observed significant improvements in survival. It is important to note that 1-year survival increased across all age categories, whereas the improvement in 5- and 10-year AML survival was confined to patients younger than 80 years and was most prominent in the younger (< 60 years) patient population. Consequently, there are still few long-term survivors among patients older than 60 years at diagnosis. Among people diagnosed with APL, we found a numerically lower short-term mortality and longer survival in the later calendar period, although the differences were not significant because of a small number of patients. There was no evidence of improvement in survival among AML patients with a preceding MDS diagnosis over time (1993-2005).
There are few population-based studies on survival in AML,3,39,40 and most survival data arrive from clinical trials, which are associated with various degrees of patient selection. Pulte et al40 recently published a report on relative survival in AML patients reported to the Surveillance, Epidemiology, and End Results program of the US National Cancer Institute. In that study, the authors found evidence of increasing 5- and 10-year relative survival in patients younger than 75 years.40 Their observation is in concordance with our findings. In addition, we found that 1-year RSRs increased in all age groups, also in patients older than 80 years. Interestingly, 5- and 10-year RSRs in our cohort were higher in all age categories (data not shown for 10-year RSRs), although patients with a preceding diagnosis of cancer or hematologic malignancy were included. These patients were excluded in the report by Pulte et al.40 Other differences between these 2 studies include the fact that we used the total Swedish population as reference group when calculating RSR estimates. In contrast, in the study by Pulte et al,40 AML survival from the included states was related to the entire US population survival. In addition, these authors used a period analysis; whereas in the present study, we used a cohort analysis. Unlike cohort estimates, period estimates do not represent the survival experience of a well-defined cohort of patients. It is not unlikely that we would have seen slightly higher survival estimates if we had used period analysis; the trends would however be similar. Survival appears also to be superior to that reported in a limited population-based study performed in Southeast England.3 There are several potential explanations for the observed superior survival in the present cohort. First, Sweden has a well-established government-funded public healthcare system in which all residents by law are entitled to equal access to health services. Second, patients with AML are almost exclusively diagnosed, treated, and followed clinically by physicians at nonprivate hospital-based hematology units. As a consequence, treatment decisions including allo-SCT are based on patient- and disease-related factors only without any financial consequences for the person. We realize that the 5-year RSR of 0.58 in the last calendar period in patients 19 to 40 years of age may compare favorably to the 0.52 (in patients 15-35 years of age) reported by Pulte et al.40 Although the number of patients in each period was somewhat modest, we think that the CIs for the 5-year RSRs reflect a reasonable level of precision. In addition, the proportion of patients in this age group undergoing allo-SCT was 14% in 1981 to 1988, 32% in 1989 to 1996, and 58% in 1997 to 2005. However, the RSR results should still be cautiously interpreted. Superior survival in elderly patients may be explained by a higher proportion of patients receiving treatment with curative intent in Sweden compared with most other countries. In 1997 to 2001, 54% of Swedish AML patients 70 to 79 years of age received treatment with a curative intent5 ; whereas in a report based on Surveillance, Epidemiology, and End Results data (1991-1999), 49% of patients 65 to 74 years of age received any kind of chemotherapy, not specified whether for palliation or with a curative intent.41 In regions of Sweden where a higher proportion of patients 70 to 79 years of age received treatment with a curative intent, the survival was superior compared with regions where a smaller proportion of patients were treated intensively.5
Most probably, a combination of factors related to diagnostics and treatment have contributed to the observed improvements in survival. Improvements in 1-year RSRs reflect higher CR rates because of more effective induction treatment and better management of short-term toxicity. In the early 1970s when DA was introduced, there were only modest improvements in survival because of a high rate of lethal infectious complications.19 TRM decreased substantially when empirical use of broad-spectrum antibiotics and antifungal therapy were introduced.19,21,42 Altogether, in modern AML treatment, short-term mortality resulting from infectious complications has been reported to be as low as 4%.43
The introduction of consolidation treatment44,45 has lowered the relapse rate and contributes in part to the improvement in 5- and 10-year RSRs. Especially important was the introduction of high-dose cytosine arabinoside consolidation treatment in the late 1980s.10,11 In addition, allo-SCT reduces the risk of relapse in certain high- and intermediate-risk patients.15,16 In Sweden the fraction of patients treated with allo-SCT increased substantially during the study period, whereas the number of auto-SCT performed reached a peak in the early 1990s and then decreased mainly because of reports stating no superiority of this procedure to prevent relapse.13,14 Allo-SCT was then introduced associated with a considerable TRM. Refinement of conditioning regimens, the use of larger amounts of stem cells, and improvement in supportive care have reduced TRM.22,23 In addition, risk stratification based on cytogenetic analyses has significantly contributed an improved identification of patients who benefit from allo-SCT.24 The fraction of older patients treated with antileukemic therapy in Sweden increased over the years.5 This more generous attitude toward administering intensive treatment to elderly probably explains the improvements seen in 1- and 5-year RSRs in patients older than 60 years after the year 1986. Notwithstanding this, survival remains substantially lower than in younger patients. Many elderly patients are not fit for intensive induction chemotherapy because of comorbidities and poor performance status leading to low CR rates. Postremission therapy is often restricted and allo-SCT is rarely a treatment option in patients older than 60 years, contributing to the observed higher relapse rate. In addition, there is an aggregation of adverse prognostic factors, such as high-risk cytogenetics, antecedent MDS or myeloproliferative disorder, and overexpression of genes involved in drug resistance in elderly patients with AML.26,46
Only 2.5% of the patients in this study were diagnosed with APL. This reflects the lower incidence of this AML subtype in Scandinavia compared with that seen in southern Europe, Latin America, and Asia.47 Patients diagnosed with APL were younger (median age, 54 years) than those diagnosed with other subtypes of AML, which is consistent with the published literature.47 In this group of patients, 52% with a fatal outcome died within the first month of diagnosis. Bearing the limited number of APL patients in mind, short-term mortality appears lower in the later calendar period. An improved immediate management of this patient group, including the introduction of all-trans retinoic acid, has probably contributed to this finding.25,48 Rather discouraging in a subgroup analysis of AML patients with a preceding MDS, no survival improvement was observed in the most recent calendar period.
In good agreement with previous observations,2,3 the age-adjusted AML incidence was higher in men. We found a slightly better survival for women when adjusted for age and calendar period. A female predominance among long-term survivors has been reported previously.49 However, in the study by Phekoo et al,3 there was no difference in survival by sex, and information on outcome in relation to sex was not reported in the study by Pulte et al.40
In our study, we used a register-based cohort design, which ensured a population-based setting and generalization of our findings. The Swedish Cancer Register has a validity of more than 90% in routinely diagnosed hematologic malignancies.1,28 We recently performed a validation study on lymphoproliferative diseases reported to the register and were able to show an overall 93% diagnostic accuracy and 95% completeness for multiple myeloma.29 We have no reason to believe that the accuracy and completeness of the registry differ between multiple myeloma and AML. However, both underdiagnosing and underreporting, especially of elderly/frail patients, probably explain the increased age-adjusted AML incidence between the first and second calendar period under study. The increasing number of patients diagnosed with AML during the more recent calendar periods is mainly explained by a larger and ageing population. An increasing median age at diagnosis has also been reported by other authors.39,50 In all, underreporting and diagnostic misclassifications may only marginally bias the results of our study.
We computed RSR estimates as measures of AML survival, which is in contrast to reports on survival from clinical trials.49 As previously mentioned, an RSR estimate reflects the ratio of the observed survival divided by the expected survival of a cohort of the general population, possessing similar characteristics with respect to age, sex, and era of diagnosis. A major advantage of working with RSR estimates includes the fact that data on cause of death are not required, which circumvents difficulties with inaccuracy or lack of death certificates. The crucial assumption in working with RSR estimates is that one can accurately estimate expected survival. For most cancers (including AML), patients are representative of the general population, so their expected survival can be estimated using general population survival rates. In contrast to reports based on patients treated in clinical trials, the large majority of patients in this study (95%) were treated in routine clinical practice. Thus, elderly patients and patients not treated with curative intent were included in the analysis. This study therefore supplies a more complete picture of the survival of AML patients.
Limitations include lack of clinical data for individual patients and changes in diagnostic practices over time, that is, the World Health Organization's redefinition of AML.51 On the other hand, it is not probable that the observed improved survival is influenced by an increased access to health care and earlier detection of the disease over time (ie, lead-time bias) because AML is a disease that comes to medical attention within a short period of time. The Swedish AML register, which was established in 1997, collects information on clinical data, including cytogenetics and molecular markers (since 2007), and data from this register will be of future interest.
In conclusion, we found that AML patients younger than 80 years have gained significantly from the developments in management of the disease during a 33-year period. However, there are still few long-term survivors among patients older than 60 years. A high relapse rate mainly contributes to the sharp decline in 5-year relative survival compared with 1-year relative survival. Few relapses after 5 years lead to only a marginal further decrease in 10-year relative survival. The somewhat lower 10-year relative survival may also be explained by late TRM. Age remains an important predictor of prognosis: why innovative agents and procedures suitable for the older patient are greatly needed. In addition, more individualized management based on accurate risk stratification will hopefully significantly improve the outlook for the whole AML patient population.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Feresthe Ebrahim (National Board of Health and Welfare, Stockholm, Sweden) for important efforts in setting up this database and Vanderson Rocha and Emanuelle Polge (European Board for Blood and Marrow Transplantation) for providing information regarding stem cell transplantations.
This work was supported by grants from the Swedish Cancer Society, Stockholm County Council, and the Karolinska Institutet Foundations.
Authorship
Contribution: Å.R.D., M.B., P.W.D., S.Y.K., and O.L. designed the study; Å.R.D., M.B., P.W.D., and S.Y.K. obtained and analyzed data; T.M.-L.A. and P.W.D. performed statistical analyses; Å.R.D. and M.B. wrote the paper; and all authors read, gave comments, and approved of the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Åsa Rangert Derolf, Division of Hematology, Department of Medicine, Karolinska University Hospital Solna and Karolinska Institutet, SE-171 76 Stockholm, Sweden; e-mail: asa.derolf@karolinska.se.