Abstract
A characteristic feature of leukemia cells is a blockade of differentiation at a distinct stage in cellular maturation. In the 1970s and 1980s, studies demonstrating the capabilities of certain chemicals to induce differentiation of hematopoietic cell lines fostered the concept of treating leukemia by forcing malignant cells to undergo terminal differentiation instead of killing them through cytotoxicity. The first promising reports on this notion prompted a review article on this subject by us 25 years ago. In this review, we revisit this interesting field of study and report the progress achieved in the course of nearly 3 decades. The best proof of principle for differentiation therapy has been the treatment of acute promyelocytic leukemia with all-trans retinoic acid. Attempts to emulate this success with other nuclear hormone ligands such as vitamin D compounds and PPARγ agonists or different classes of substances such as hematopoietic cytokines or compounds affecting the epigenetic landscape have not been successful on a broad scale. However, a multitude of studies demonstrating partial progress and improvements and, finally, the new powerful possibilities of forward and reverse engineering of differentiation pathways by manipulation of transcription factors support the continued enthusiasm for differentiation therapy of leukemia in the future.
Introduction
A characteristic abnormality of leukemia cells is that they are blocked at an early stage of their development and fail to differentiate into functional mature cells. During the 1970s and 1980s, several scientific achievements popularized the strategy of inducing malignant cells to overcome their block of differentiation and enter the apoptotic pathways as an elegant alternative to killing cancer cells by cytotoxic therapies. This intervention could theoretically limit exposure to unwanted side effects of cytotoxic chemotherapy, and more importantly, improve complete remission and cure rates. Pioneering reports included studies demonstrating the differentiating capability of dimethylsulfoxide (DMSO) on erythropoiesis,1 efforts to elucidate substances to control the differentiation of myeloid leukemia,2 and the first evidence of the differentiating properties of retinoic acid.3,4 The initial promising preclinical results of this approach prompted a review article by us 25 years ago concerning the possibilities and therapeutic implications of differentiation induction in leukemia.5 At that stage, cell line models for in vitro differentiation experiments were described. Substances such as phorbol diesters, teleocidins, polar planar drugs, cytokines, retinoids, and vitamin D metabolites showed dramatic potential to differentiate cell lines such as HL-60, KG-1, ML-3, or K562 in vitro and fueled the hope of developing a new approach to treat cancers by overcoming their blocked differentiation. Today, 25 years later, we discuss the progress and the clinical achievements of this therapeutic approach
Ligands of nuclear hormone receptors
Poster child of success: complete remissions of APL by differentiation therapy
The potential for differentiating therapy to improve cure rates in leukemia is exemplified by the development of all-trans retinoic acid (ATRA) for the targeted treatment of acute promyelocytic leukemia (APL). One of the most remarkable results of initial in vitro experiments was achieved in differentiating HL-60 cells with ATRA, which produced terminal differentiation in 90% of cells with 10−6 M retinoic acid.3 Investigators soon realized that ATRA was specifically effective in APL cells carrying a typical chromosomal translocation between chromosomes 15 and 17 [t(15;17)(q22;q21)]6 but not in other leukemias.4 The first APL patients treated with ATRA in the early 1980s achieved encouraging remissions by the new therapy.7-10
The first clinical trial of ATRA reported 16 newly diagnosed, and 8 anthracycline refractory patients who were induced with single-agent ATRA. Complete hematologic remission was induced in all patients; more than 90% of samples from these individuals demonstrated in vitro evidence of blast differentiation.10 This seminal trial changed the management and prognosis of APL, and introduced a paradigm for success of cell differentiation therapy. Subsequently, APL therapy was improved stepwise through elucidation of the most effective combination regimen of ATRA with cytotoxic chemotherapy.11-16
Shortly after ATRA therapy became standard for induction of newly diagnosed patients with APL, strategies for overcoming ATRA resistance became necessary. Arsenic trioxide (ATO) emerged as an option for relapsed patients capable of producing high rates of molecular remissions and resensitizing patients to the differentiating effect of ATRA.17-22 Today, the wealth of clinical information and improvements of targeted differentiation therapy with ATRA have led to the development of guidelines including highly effective induction and consolidation regimen including ATRA/ATO and anthracycline-based chemotherapy, achieving complete remission rates of up to 90% to 95%.23
The breakthrough in clinical oncology achieved by differentiation therapy with ATRA also sparked intensive research into mechanisms underlying the observed successes. ATRA is a ligand to retinoic acid receptors (RARs), which comprise a family of transcription factors that bind to retinoic acid response elements (RAREs) and regulate granulocytic differentiation24 (Figure 1A). The APL cells of 95% of patients have a characteristic chromosomal translocation between chromosomes 15 and 17 [t(15;17)(q22;q21)]6 leading to a fusion of the genes promyelocytic leukemia (PML) and the retinoic acid receptor alpha (RARα).25,26 The classical model describes that this fusion product acts as a dominant negative of RARα by forming homodimers, recruiting corepressors, and inhibiting expression of target genes necessary for granulocytic differentiation by binding to RAREs.27 However, because this repression of RARα target genes by PML-RARα is associated with the recruitment of DNA and histone-modifying enzymes such as histone deacetylases (HDACs), histone methyltransferases,28,29 and DNA methyltransferases,30 the leukemogenic activity of this fusion product is mediated by mechanisms beyond the simple repression of RARα-regulated genes. It leads to a highly repressive chromatin environment, which affects multiple pathways. Furthermore, the PML-RARα product may also inhibit the normal function of the PML protein as a tumor suppressor and therefore acts as a dominant negative against both proteins.31
Ligands of nuclear hormone receptors. Depicts schematically the molecular mechanisms of differentiation induction of nuclear hormone receptor agonists. (A) All-trans retinoic acid (ATRA) for acute promyelocytic leukemia (APL). The characteristic chromosomal translocation t(15;17)(q22;q21) in APL leads to the production of a fusion protein between the PML protein and the retinoic acid receptor alpha (RARα). This fusion product is able to form homodimers and disrupt normal RARα signaling. It binds to retinoic acid response elements (RAREs) of target genes and recruits corepressors (Co-Rs) such as histone deacetylases (HDACs) and DNA methyltransferases (DNMTs), and sequesters retinoic X receptor (RXR) and the wild-type PML protein (PML), which finally leads to repression of genes necessary for granulocytic differentiation. Treatment with pharmacological concentrations of ATRA causes a conformational change of the PML-RARα fusion product leading to the release of the corepressors, recruitment of histone acetyl transferases (HATs), and relief of transcriptional repression. This causes the treated APL cells to undergo terminal granulocytic differentiation and finally apoptosis. (B) Biologically active vitamin D [1,25(OH)2D3] binds to the nuclear vitamin D receptor (VDR), which heterodimerizes with retinoic X receptor (RXR). This activated complex binds to vitamin D response elements (VDREs) in the promoter regions of genes inducing cell-cycle arrest, apoptosis, and differentiation in cancer cells. Furthermore, it leads to an up-regulation of the antimicrobial peptide cathelicidin (CAMP) in myeloid cells. (C) Thiazolidinediones (TZDs) bind to peroxisome proliferator activated receptor gamma (PPARγ). This activated complex acts as a transcription factor by heterodimerizing with (RXR) and binding to PPARγ-responsive elements in the promoter regions of target genes involved in cell-cycle arrest, apoptosis, growth inhibition, and differentiation of cancer cells.
Ligands of nuclear hormone receptors. Depicts schematically the molecular mechanisms of differentiation induction of nuclear hormone receptor agonists. (A) All-trans retinoic acid (ATRA) for acute promyelocytic leukemia (APL). The characteristic chromosomal translocation t(15;17)(q22;q21) in APL leads to the production of a fusion protein between the PML protein and the retinoic acid receptor alpha (RARα). This fusion product is able to form homodimers and disrupt normal RARα signaling. It binds to retinoic acid response elements (RAREs) of target genes and recruits corepressors (Co-Rs) such as histone deacetylases (HDACs) and DNA methyltransferases (DNMTs), and sequesters retinoic X receptor (RXR) and the wild-type PML protein (PML), which finally leads to repression of genes necessary for granulocytic differentiation. Treatment with pharmacological concentrations of ATRA causes a conformational change of the PML-RARα fusion product leading to the release of the corepressors, recruitment of histone acetyl transferases (HATs), and relief of transcriptional repression. This causes the treated APL cells to undergo terminal granulocytic differentiation and finally apoptosis. (B) Biologically active vitamin D [1,25(OH)2D3] binds to the nuclear vitamin D receptor (VDR), which heterodimerizes with retinoic X receptor (RXR). This activated complex binds to vitamin D response elements (VDREs) in the promoter regions of genes inducing cell-cycle arrest, apoptosis, and differentiation in cancer cells. Furthermore, it leads to an up-regulation of the antimicrobial peptide cathelicidin (CAMP) in myeloid cells. (C) Thiazolidinediones (TZDs) bind to peroxisome proliferator activated receptor gamma (PPARγ). This activated complex acts as a transcription factor by heterodimerizing with (RXR) and binding to PPARγ-responsive elements in the promoter regions of target genes involved in cell-cycle arrest, apoptosis, growth inhibition, and differentiation of cancer cells.
Pharmacological concentrations of ATRA lead to a conformation change of the multifunctional molecule complex around PML-RARα. Corepessors are released, normal regulation of RARα-responsive genes is restored, and hence terminal differentiation of APL cells is induced.32 This is supported by several recent microarray and proteomic studies, which have identified hundreds of genes that are differentially regulated in the ATRA-induced differentiation of APL cells33,34 including down-regulation of c-myc35 and up-regulation of C/EBPϵ,36 as well as PU.1.37 Furthermore, genes governing increased protein synthesis such as PDCD4 or RTP801 are down-regulated in APL cells during ATRA exposure,38,39 whereas genes associated with protein degradation are up-regulated by ATRA, leading to a degradation of the PML-RARα fusion product.40,41
The degradation of PML-RARα may also represent an intersection, where the mechanisms of action of ATO converge on those of ATRA. Initial endeavors to elucidate the molecular activities of ATO showed a dual mode of action. At low concentrations, ATO induced partial morphologic differentiation in APL cells, whereas at high concentrations, apoptosis induction predominated. Both effects were associated with a degradation of PML-RARα.42 ATO induced PML-RARα and PML degradation is associated with enhanced sumoylation of these proteins,43 indicating that effects achieved by ATO in APL may be attributed to an increased targeting of the PML moiety versus RARα targeting of ATRA. A comparison of ATO- and ATRA-induced gene expression and proteome profiles showed that both compounds regulate similar cellular factors. However, ATO's emphasis was on altering the proteome and inducing apoptosis as opposed to predominant regulation of gene expression and differentiation by ATRA.33 Interestingly, differentiation of APL cells by either ATRA or ATO is highly dependent on stimulation by myeloid growth factors as evidenced by experiments using growth factor–neutralizing antibodies.44 In addition, both substances can induce a side effect known as APL differentiation syndrome, suggesting some overlapping mechanisms of action.23 Sharing similar pathways but exhibiting different foci of action, the 2 compounds complement each other.
Taken together, therapy of APL with ATRA and ATO is to date the most successful example of differentiation therapy and its scientific history serves as a template for subsequent development of similar treatments in other leukemias and cancers.
Vitamin D compounds
Concurrent with the first observations of the differentiating action of retinoids on selected myeloid cell lines, similar promising effects were also demonstrated for the physiologically active form of vitamin D, 1,25 dihydroxy vitamin D3 [1,25(OH)2D3]. This seco-steroid potently differentiated cells of the myeloid lineage in vitro and ex vivo,5,45 which led to early clinical trials to test the ability of 1,25(OH)2D3 to treat myelodysplastic syndromes (MDSs) or acute myeloid leukemia (AML).46,47 Although 1,25(OH)2D3 induced partial differentiation of hematopoietic blast cells in some of these patients, clinical improvements such as blood counts or survival were modest. The same was true for studies that assessed the effect of 1,25(OH)2D3 in combination with other agents used to treat either MDS or AML.48-50 Initial studies also suggested that 1,25(OH)2D3 had cancer-preventive properties in prostate and colon cancers51-54 and exerted positive antitumor effects by regulation of proliferation, apoptosis, and angiogenesis.55-58
As for the mechanism of action, 1,25(OH)2D3 binds and activates the vitamin D receptor (VDR), which heterodimerizes with the retinoic X receptor and binds to vitamin D–responsive elements (VDREs) in the promoter regions of target genes59-61 (Figure 1B). One of the main mechanisms of antiproliferative and differentiating action of 1,25(OH)2D3 is the induction of cell-cycle arrest by regulation of genes such as p21 and p27, which harbor VDREs.62-64 However, despite greater insight into the fundamental signaling of vitamin D compounds, the responses in individual tumor types are very heterogeneous so that a common understanding of how it mediates its antiproliferative activity is yet to be established.51
A limiting factor in the clinical application of 1,25(OH)2D3 is hypercalcemia.65 Pharmacokinetic and pharmacodynamic optimization has shown that this can be mitigated by intermittent, high doses of 1,25(OH)2D3 and therapeutic support with glucosteroids.66 In addition, a large number of vitamin D analogs and vitamin D receptor modulators have been synthesized in the hope of gaining greater antitumor effects while deceasing their hypercalcemic activity.51 Paricalcitol or doxercalciferol have partly achieved this goal. However, summarizing clinical trials testing them against hematologic malignancies such as MDS or AML, the results are still rather disappointing.67,68
Another important potential activity of 1,25(OH)2D3 is the ability to transcriptionally induce the expression of the antimicrobial peptide cathelicidin (CAMP).69,70 The ability of vitamin D compounds to enhance the expression of antimicrobial peptides has created an exciting new field of research to elucidate the role of vitamin D compounds as boosters of the immune system to fight infectious diseases.71 However, regarding hematologic malignancies, vitamin D compounds have not had a major impact on their clinical management at this stage.
PPARγ receptor ligands
Peroxisome proliferator activated receptor gamma (PPARγ) is also a member of the superfamily of nuclear hormone receptors (NHRs) with an important role in the regulation of fatty acid metabolism and a variety of endogenous ligands such as 15-deoxy-delta 12, 14-prostaglandin J2, and polyunsaturated fatty acids.72 In 1995, investigators discovered that PPARγ was the molecular target of thiazolidinediones (TZDs),73,74 a group of synthetic substances widely used in the treatment of type 2 diabetes. Reports soon noted that PPARγ agonists had the ability to prevent either the development or growth of tumors and to induce differentiation using various model systems.75-78 In the wake of this, PPARγ agonists were tested in vitro and in animal tumor models against a plethora of tumors including colon, breast, and prostate cancers and acute leukemias. The PPARγ agonists displayed various antiproliferative and differentiating potency in many cancer types,75 especially in models of myeloid, lymphoid, and chronic myelogenous leukemia.79-83
PPARγ as a nuclear hormone receptor functions as a transcription factor (Figure 1C). It heterodimerizes with the retinoid X receptor (RXR) and then binds to PPARγ response elements in the promoter regions of target genes.84 Antitumor effects of PPARγ agonists have been associated with exit from the cell cycle by up-regulation of inhibitors of cyclin-dependent kinases (CDKs) such as p18, p21, or p27 associated with reduced phosphorylation of the retinoblastoma protein (Rb).85 The apoptosis pathways are also modestly activated by down-regulation of the antiapoptotic Bcl-2 protein and up-regulation of the proapoptotic proteins Bax and Bad.86,87 Furthermore, PPARγ agonists increased the expression of other tumor suppressor genes such as PTEN and BRCA1.88,89 Several lines of evidence also suggest “off-target” effects of PPARγ agonists, which are not dependent on the PPARγ receptor. Experiments in PPARγ−/− murine cells demonstrated that PPARγ ligands continued to have inhibitory activity on inflammation pathways in macrophages and to cause cell-cycle arrest independently of PPARγ.90-92
Paradoxically, PPARγ has proneoplastic activity in certain contexts.93 For example, mice carrying a heterozygous mutation of the adenomatous polyposis coli gene (APC) (Apc+/Min mice) demonstrated increased tumor number and size when treated with TZDs.94 This obervation was supported in other animal tumors models of spontaneous colon cancers or genetically induced mammary gland tumors, which showed increased incidence or growth of tumors with TZD treatment or activation of PPARγ.95,96 Nevertheless, the majority of basic research reports attribute antitumor effects to activation of PPARγ and prompted clinical trials of these substances in several human malignancies. Therefore, initial observations of adipocyte maturation in liposarcoma patients78 could not be confirmed in a later trial with 9 patients, which could neither demonstrate terminal differentiation of tumor cells nor achievement of any clinical benefit through treatment with TZDs.97
Given evidence that TZDs could cause terminal differentiation of breast cancer cell lines, clinical breast cancer studies were undertaken. Among 22 patients with metastatic, refractory breast cancer, no clinical benefit was observed.98 In a study of 38 women with newly diagnosed, early stage breast cancer, neoadjuvant TZD treatment showed that the postoperative pathologic tissue did not show evidence of enhanced differentiation of tumor cells.99 Clinical trials in prostate, colon, lung, and thyroid cancer also did not find a meaningful benefit of TZD therapy.100-105
In summary, the clinical effects achieved by PPARγ agonists have not been resounding and probably do not merit a primary role for them in cancer therapy. However, their low toxicity profile and the observation that they can achieve additive or synergistic effects combined with other anticancer agents such as retinoids, histone deacetylase (HDAC) inhibitors, or TRAIL ligands86,100-104 make them possible candidates to include in adjuvant or combination therapy trials.106
Cytokines
The differentiation of hematopoietic stem cells into mature blood cells is intricately controlled by an array of hematopoietic cytokines.107 The discovery of growth factors involved in the regulation of hematopoiesis dates back to the 1960s when the first models for in vitro culturing of hematopoietic progenitors were developed.108,109 Soon, these factors were isolated and produced as recombinant substances for clinical application.107,110 The physiological signal transduction of hematopoietic cytokines consists of their binding to their specific receptor and subsequent activation of downstream pathways such as protein kinase C (PKC), mitogen-activated protein kinase (MAPK), Janus kinases (JAKs), Src kinase pathways, and STATs. Activation of these pathways, in turn, induces transcriptional activation and repression of genes governing the differentiation and lineage commitment of hematopoietic progenitors.
The quickly increasing knowledge about the mechanisms of action of cytokines in the differentiation of hematopoietic progenitors also induced high hopes of using these factors in the treatment of leukemia.111 Some of the hematopoietic leukemia cell lines of myeloid origin such as K562, U937, HL-60, CS-1, KG-1, MUTZ-3, or ex vivo AML or chronic myeloid leukemia (CML) blasts were modestly permissive to induction of in vitro differentiation by EPO, G-CSF, GM-CSF, IL-4, IL-6, SCF, or synergistic combinations of several cytokines.111-115 Molecular mechanisms of cytokine-induced leukemic differentiation were also elucidated. Several prominent proto-oncogenes such as c-myb, c-myc, c-fos, and Ets family transcription factors such as ets-1, fli-1, and TEL2 were found to be differentially regulated upon cytokine-induced differentiation of leukemic cells.116-120 However, when translated to a clinical setting, the approach to treat leukemia by trying to differentiate the malignant cells with cytokines remained rather modest. In single case reports, AML patients have been shown to enter complete hematologic remissions upon treatment with either G-CSF or GM-CSF.121-125 However, in larger trials assessing the therapeutic impact in terms of differentiation of leukemia cells in AML or MDS, hematopoietic cytokines have had limited success.126
A niche for hematopoietic cytokines in differentiation therapy exists in the treatment of congenital neutropenia disorder. In this disease, several decades of progress have identified pertubations of the G-CSF receptor signaling as one of the underlying causes, and the administration of G-CSF to these patients has overcome a block of myeloid differentiation leading to a substantial prolongation of their survival.127 In summary, the concept of using cytokines as a differentiation treatment against leukemia has been disappointing. Nevertheless, cytokines have gained many other important domains of action such as supportive therapy during cytotoxic chemotherapy or treatment of congenital neutropenia.
Transcription factors and agents affecting the epigenetic landscape
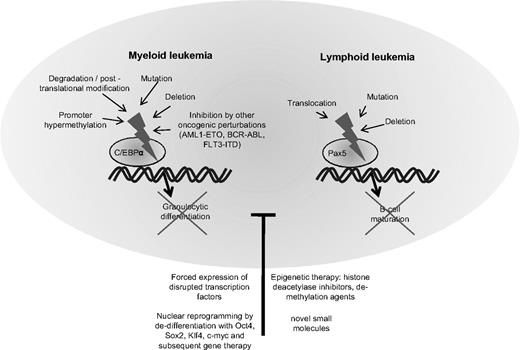
In the last few years, increasing information has emerged about the transcription factors governing the differentiation of hematopoietic cells. The function of several of these transcription factors is frequently disrupted in leukemia cells.128 Examples include the CCAAT/enhancer binding protein alpha (C/EBPα) in AML or the paired box gene 5 (Pax5) in ALL (Figure 2).
Block of differentiation by disruption of hematopoietic transcription factors in myeloid and lymphoid leukemia. Depicts the molecular mechanisms leading to disruption of the transcription factor CCAAT/enhancer binding protein alpha (C/EBPα) in myeloid leukemia and the paired box gene 5 (Pax5) in lymphocytic leukemia. Disruption of these transcription factors blocks hematopoietic cells in their early stages of differentiation. Treatment with substances altering the epigenetic settings such as histone deacetylase inhibitors or demethylating agents may partly overcome the block in differentiation. Forced expression of the normal counterpart of the disrupted transcription factor can often re-establish differentiation. In the future, synthesis of small molecules specifically targeting transcription factors or nuclear reprogramming and gene therapy may provide useful tools for correcting defective differentiation in hematologic malignancies.
Block of differentiation by disruption of hematopoietic transcription factors in myeloid and lymphoid leukemia. Depicts the molecular mechanisms leading to disruption of the transcription factor CCAAT/enhancer binding protein alpha (C/EBPα) in myeloid leukemia and the paired box gene 5 (Pax5) in lymphocytic leukemia. Disruption of these transcription factors blocks hematopoietic cells in their early stages of differentiation. Treatment with substances altering the epigenetic settings such as histone deacetylase inhibitors or demethylating agents may partly overcome the block in differentiation. Forced expression of the normal counterpart of the disrupted transcription factor can often re-establish differentiation. In the future, synthesis of small molecules specifically targeting transcription factors or nuclear reprogramming and gene therapy may provide useful tools for correcting defective differentiation in hematologic malignancies.
In physiological hematopoiesis, C/EBPα is an important factor for the development of granulocytes129,130 and targeted disruption of C/EBPα leads to a selective block in granulocyte maturation.131 The normal function of C/EBPα is disturbed by a variety of events in AML.132 Several groups have detected spontaneous mutations of the C/EBPα gene, and a summary of these studies revealed that C/EBPα was mutated in 11% of 1565 AML samples.133-135 Mutations in the amino end of the gene produce a dominant negative form of the protein and those in the carboxyl end disturb its DNA binding and heterodimerization ability with other family members.133 Often, some AML cells harbor both kinds of mutations, each affecting a different allele. Interestingly, the presence of CEBPα mutations in AML cells confers a favorable prognosis.135 Other mechanisms of perturbation of C/EBPα include down-regulation of its mRNA levels by AML1-ETO in t(8;21)-positive AMLs,136 hypermethylation of its promoter region, silencing its expression,137 posttranslational inhibition by phosphorylation,138 or dysregulated proteasomal degradation.139 Regardless of the cause, the dysregulation of normal C/EBPα signaling more or less culminates in an arrest of granulocytic differentiation and accumulation of immature blasts in AML. As expected, forced expression of C/EBPα in leukemia cells can reverse the block of differentiation and induce terminal maturation of AML cells.140,141
A comparable candidate in ALL is the B-lineage–specific transcription factor Pax5. In a recent study analyzing the genomes of 242 cases of childhood ALLs with high-density SNP arrays, Pax5 was the most commonly altered gene, either by deletion or by mutation.142 Both the Downing group (Mullighan et al)142 and we143 showed that Pax5 mutant proteins or Pax5 fusion products displayed reduced transcriptional activity or dominant negative effects. The physiological role of Pax5 lies in the regulation of B-cell gene expression during development of lymphocytes. Its expression is up-regulated beginning at the pro-B progenitor stage and is maintained at high levels throughout B-cell development until it is silenced during the final transition to plasma cells.144 Studies of hematopoiesis in Pax5-deficient mice demonstrated arrested differentiation at very early stages of hematopoietic development before the emergence of B-cell progenitors or B cell–specific gene expression.145,146 Experimental manipulation of Pax5 has shown its effects on the development of lymphocytes.147 In an experiment to challenge the paradigm of irreversible lineage commitment during physiological hematopoiesis, a murine model with a conditional Pax5 deletion allowed mature B cells from peripheral lymphoid organs to dedifferentiate back to early uncommitted progenitors in the bone marrow, which rescued T lymphopoiesis in the thymus of T cell–deficient mice.148 Furthermore, ectopic expression of Pax5 in normal hematopoietic progenitors or myeloid malignancies has yielded heterogeneous results concerning its function and potential to reverse a malignant phenotype.149-151 The mechanisms of leukemogenesis caused by perturbation of Pax5 in lymphoid malignancies is unclear. The leukemogenic effects of Pax5 fusion products and the consequences of re-establishing physiological levels of functional Pax5 in ALL cells with Pax5 deletions is currently being investigated by us and other laboratories.
Given the powerful potential of hematopoietic transcription factors such as C/EBPα and Pax5 to control normal hematopoiesis and their frequent dysregulation in hematologic malignancies, they are obvious therapeutic targets. However, development of drugs that precisely manipulate specific transcription factors has not been achieved successfully. Although, hope lies in large drug screens for small molecules or small interfering RNA molecules that specifically modify endogenous expression or function of transcription factors.135
As mentioned before, signaling of important hematopoietic transcription factors can be impaired by an aberrant epigenomic environment.137,152 Epigenetic silencing of tumor suppressors and transcription factors governing differentiation occurs in many cancers. In recent years, many compelling mechanisms have been identified, which epigentically alter the genome. The 2 most characterized have led to the development of clinically applicable substances to reverse dysregulated epigenomic changes. One is the aberrant methylation of cytidine-phosphate-guanosine (CpG) dinucleotides, which are accumulated in “CpG islands” of genomic DNA in the promoter regions of genes (DNA methylation). The other is the deacetylation of histones, which results in a positive charge of these proteins, consequently a stronger binding of negatively charged DNA to them and ultimately producing a transcriptional repression by hindering access of transcription complexes to the DNA (histone deacetylation).
Already in the early 1980s, investigators demonstrated that cytidine analogs such as 5′-aza-cytidine or 5′-aza-2′-deoxycytidine (decitabine) were able to differentiate mouse embryo cells to muscle cells by inhibiting methylation of DNA.153 In the last few years, these drugs have established themselves as effective alternatives to cytotoxic chemotherapy in MDS and AML. Both compounds integrate into DNA as alternative nucleotides and trap DNA methyltransferases resulting in the formation of demethylated DNA.154 Due to this mechanism, hypermethylation of DNA in malignant cells is reversed in the course of several cell divisions.155 On the grounds of this gradual process, unlike chemotherapy, demethylating agents are not applied at a maximum tolerable dose but rather in smaller portions over a longer duration to induce differentiation and inhibit proliferation of the malignant cells.153,156 Recent clinical trials using these substances for the treatment of advanced MDS and AML have been promising157 with improvements of survival rates achieved with 5′-aza-cytidine over conventional therapeutic regimens in MDS.158
Another class of epigenetically active substances are histone-deacetylase (HDAC) inhibitors. HDAC inhibitors manipulate cell growth and differentiation by inhibiting deacetylation of histones and proteins including transcription factors, and thereby reversing transcriptional repression of tumor suppressors or factors responsible for normal differentiation.159 Four classes of histone deacetylases with specific functions are known, and inhibitors against either single classes or a broader spectrum are available. HDAC inhibitors generally display low toxicity and some can be administered orally such as suberoylanilide hydroxamic acid (SAHA, vorinostat) or valproic acid (VPA), which has also widely been used as an anticonvulsive agent. SAHA was originally discovered while screening for differentiation-inducing compounds similar to the polar/planar compounds dimethylsulfoxide (DMSO) and hexamethylene bisacetamide (HMBA). SAHA has demonstrated antitumor effects in various cell lines and in vivo models of leukemia and solid tumors160,161 ; and phase 1 trials have shown efficacy of the drug in a panel of hematologic diseases.162 Furthermore, Vorinostat has successfully been used as a treatment for refractory cutaneous T-cell lymphoma,163 leading to its FDA approval. VPA has been shown to work synergistically with ATRA in cell lines to induce differentiation.164 A pilot study combining the 2 agents in 8 refractory or high-risk AML patients demonstrated clinical benefit in 7 of the patients, with evidence of hyperacetylation of histones and myelomonocytic differentiation of circulating blasts.165 A recently published phase 1 trial of an oral isotype–specific HDACi (MGCD0103, MethylGene, Quebec, QC; Celgene, Summit, NJ) in 29 patients with either AML or MDS, all previously treated with at least 1 prior chemotherapy regimen, produced complete hematologic remissions in 3 patients.166
In summary, the manipulation of dysregulated transcription factors responsible for hematopoietic differentiation represents a powerful tool to be harnessed for the differentiation therapy of leukemia, pending development of targeted substances. Compounds that already partly achieved this by influencing the epigenetic landscape in favor of growth control and differentiation are already successfully being used clinically and will be developed further, to reduce their toxicity and improve their efficacy.
Tyrosine kinase inhibitors and their off-target activities
One of the most seminal achievements in cancer research of the last decade was the development of the tyrosine kinase inhibitor (TKI) imatinib for the treatment of CML with its characteristic Philadelphia chromosome as a drugable target.167 The unsurpassed success of imatinib in CML has led to great efforts to apply the approach of targeted inhibition of tyrosine kinases in other malignancies.
Targeting the dysregulated signaling of the epidermal growth factor receptor (EGFR) pathways has become a major strategy for the treatment of solid tumors. Two “small molecule” agents, which inhibit the intracellular tyrosine kinase activity of EGFR, have reached FDA approval. Gefinitib and erlotinib have shown efficacy for the treatment of a wide range of solid tumors.168 Recently, 2 groups demonstrated that these agents also have the ability to induce apoptosis and differentiation in AML cell lines and primary blasts even though these cells do not express EGFR.169-171 Microarray gene-expression analysis, immunophenotyping, and morphologic assessment have shown that these 2 tyrosine kinase inhibitors induce a differentiation program in myeloid leukemia cells that corresponds to neutrophil maturation. Moreover, a selective induction of apoptosis in CD34+ progenitors derived from either MDS or AML was observed, whereas CD34+ cells from healthy individuals were spared.171 Efforts to elucidate the observed off-target effects have shown that they are mediated at least in part by inhibition of the autophosphorylation of the oncogenic JAK2 kinase.171 A recent case report of a patient suffering from a non–small cell lung cancer (NSCLC) as well as myelogenous leukemia, who was treated with erlotinib monotherapy for 3 months and subsequently displayed a complete remission of his leukemia,172 corroborates the notion that the antileukemic efficacy of erlotinib or gefitinib may not be limited to laboratory experiments. Given the favorable profile of side effects of these substances and the need for milder antileukemic treatments for elderly patients, small molecule tyrosine kinase inhibitors and their off-target effects may represent a new treatment approach for leukemia, which merits further clinical testing.
Future perspectives
In the past 3 decades, the arsenal and interrogational power of molecular methods to study malignancy has exploded. Aberrant epigenetic profiles in cancer are assessed with whole-genome CpG methylation and histone modification array techniques.152 Whole-genome analysis of DNA copy number changes and loss of heterozygosity is being performed with high-density single nucleotide polymorphism (SNP) arrays,142,143,173 comparative genomic hybridization, and whole-genome sequencing, which has uncovered mutations in cytogenetically normal AML.174 Candidate genes identified by these powerful screening methods can be analyzed for their function in murine knockout systems, by small interfering RNA inhibition, retroviral and lentiviral overexpression systems, and many other sophisticated new tools, which are constantly accelerating the pace of scientific progress in cancer research. Specifically concerning differentiation therapy, the most important recent development may be the revision of the prevailing conception that differentiation is a unidirectional process. In recent experiments, Takahashi and Yamanaka175 and Jaenisch and Young176 achieved a breakthrough by demonstrating that it is possible to dedifferentiate adult somatic cells to “inducible pluripotent stem cells” (IPS cells) by forced expression of 4 transcription factors: Oct4, Sox2, Klf4, and c-Myc. This nuclear reprogramming was first performed in murine fibroblasts; and after optimization of the method, IPS cells were generated, which were epigenetically and developmentally indistinguishable from embryo-derived stem cells.177-179 When these reprogrammed cells are transferred into a host blastocyst, they take part in normal differentiation to give rise to all 3 embryonic germ layers.176 By further manipulation of transcription factors such as transduction of C/EBPα or knockdown of Pax5, even mature B cells can be reprogrammed into IPS cells.180 This new molecular approach has immense therapeutic potential as it could ultimatively be used to create IPS cells from adult somatic tissue, manipulate, or “repair” these cells in an appropriate fashion and reintroduce them into the patient. Recent studies have shown a proof of principle of this notion by treating murine models of sickle cell anemia, hemophilia, as well as a rat model of Parkinson disease with transplantation of genetically engineered IPS cells.181-183 Recently, IPS cells have successfully been differentiated into hematopoietic progenitor cells184 and reprogrammed cells can be engrafted into irradiated severe combined immunodeficient (SCID) mice.185 Many questions remain such as the oncogenic potential of the factors introduced to reprogram the somatic cells into IPS cells or the fact that the epigenetic profile of the original aberrant somatic cells can be reprogrammed but the abnormal genomic template remains. However, this could even be used as a way to elucidate the relevance and contributions of epigenetic changes in cancer cells bearing specific genomic alterations. Intriguingly, the reprogramming effect of the transcription factors to produce IPS cells is necessary only for a limited time in the dedifferentiation process186 and methods are being refined to induce conditionally the necessary factors or even transiently, thereby ultimately eliminating many of the negative effects in the tissue arising from the IPS cells.
Conclusions
The paradigm that leukemias are characterized by the alteration of 2 sets of genes, those that give the malignancy a proliferative advantage and those associated with a block of differentiation, is still as valid today as it was 3 decades ago (Figure 3). To date, the hope that a variety of common chemicals would be identified that could induce differentiation of leukemia cells similar to the successes of ATRA in APL has not come to fruition on a broad scale. This can be attributed to the fact that candidate substances, which have been assessed for differentiation therapy subsequent to ATRA, have been unable to target a disease-specific lesion comparable with the PML-RARα fusion product in APL or the BCR-ABL chimeric protein in CML. Agents other than ATRA, which have been described in this review, are able to exert therapeutic differentiating effects on multiple deregulated pathways within the leukemic cells and therefore may be useful for improving combination therapies, especially because they often have few side effects. However, these differentiation agents are not targeted and potent enough to achieve the seminal successes of ATRA and imatinib. The future of differentiation therapy may lie in the manipulation of aberrant transcription factors in leukemia as these have emerged as powerful and common abnormalities in AML, ALL, and other cancers. As more knowledge is gathered about their mechanisms of action and their targets, new substances may be developed, which mimic the physiological action of transcription factors or compete for binding sites of mutated leukemogenic factors.
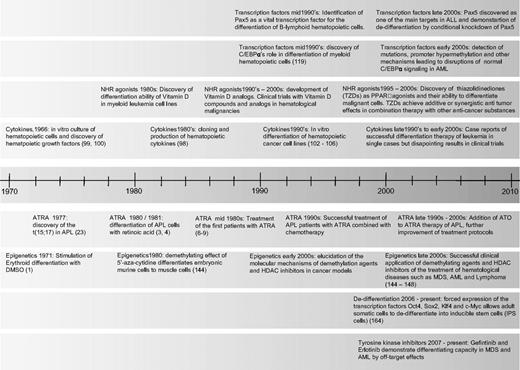
Timeline of milestones in differentiation therapy of leukemia. NHR indicates nuclear hormone receptor; TZD, thiazolidinediones; ATRA, all-trans retinoic acid; ATO, arsenic trioxide; and HDAC, histone deacetylase.
Timeline of milestones in differentiation therapy of leukemia. NHR indicates nuclear hormone receptor; TZD, thiazolidinediones; ATRA, all-trans retinoic acid; ATO, arsenic trioxide; and HDAC, histone deacetylase.
Acknowledgments
This work was supported by the Parker Hughes Fund (Los Angeles, CA) and by grants from the National Institutes of Health (Bethesda, MD). D.N. was supported by a research grant from the Deutsche Forschungsgemeinschaft (DFG, Bonn, Germany; NO 817/1-1). H.P.K. holds the Mark Goodson Chair in Oncology Research at Cedars Sinai Medical Center (Los Angeles, CA) and is a member of the Jonsson Cancer Center and the Molecular Biology Institute of UCLA.
National Institutes of Health
Authorship
Contribution: H.P.K. designed the review; and D.N., D.S., and H.P.K. wrote and proofread the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel Nowak, Division of Hematology and Oncology, Cedars Sinai Medical Center, UCLA School of Medicine, 8700 Beverly Blvd, Los Angeles, CA 90048; e-mail: daniel.nowak@cshs.org.

![Figure 1. Ligands of nuclear hormone receptors. Depicts schematically the molecular mechanisms of differentiation induction of nuclear hormone receptor agonists. (A) All-trans retinoic acid (ATRA) for acute promyelocytic leukemia (APL). The characteristic chromosomal translocation t(15;17)(q22;q21) in APL leads to the production of a fusion protein between the PML protein and the retinoic acid receptor alpha (RARα). This fusion product is able to form homodimers and disrupt normal RARα signaling. It binds to retinoic acid response elements (RAREs) of target genes and recruits corepressors (Co-Rs) such as histone deacetylases (HDACs) and DNA methyltransferases (DNMTs), and sequesters retinoic X receptor (RXR) and the wild-type PML protein (PML), which finally leads to repression of genes necessary for granulocytic differentiation. Treatment with pharmacological concentrations of ATRA causes a conformational change of the PML-RARα fusion product leading to the release of the corepressors, recruitment of histone acetyl transferases (HATs), and relief of transcriptional repression. This causes the treated APL cells to undergo terminal granulocytic differentiation and finally apoptosis. (B) Biologically active vitamin D [1,25(OH)2D3] binds to the nuclear vitamin D receptor (VDR), which heterodimerizes with retinoic X receptor (RXR). This activated complex binds to vitamin D response elements (VDREs) in the promoter regions of genes inducing cell-cycle arrest, apoptosis, and differentiation in cancer cells. Furthermore, it leads to an up-regulation of the antimicrobial peptide cathelicidin (CAMP) in myeloid cells. (C) Thiazolidinediones (TZDs) bind to peroxisome proliferator activated receptor gamma (PPARγ). This activated complex acts as a transcription factor by heterodimerizing with (RXR) and binding to PPARγ-responsive elements in the promoter regions of target genes involved in cell-cycle arrest, apoptosis, growth inhibition, and differentiation of cancer cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/16/10.1182_blood-2009-01-198911/7/m_zh80190934660001.jpeg?Expires=1767738711&Signature=pa8l7up0jKr8vM9nPP6K~c8PW88Rw0b7zlRadMXaw6zCcM4qOneeNguju~ONXZ4Wx-5Obs1VsnDYcL3zUFkNeMLDaUAEhz6ErJU0BrFQVU2yMsMzvgSjlQyJwD9t~N~EVs4x3orC3krg0tFl1XEEzfGB338BgykaeKgLVqJesdlTfwmp0sTzrhZH8KLIv1Ss8LSHgRs-puJIXdmkmJlhWlUSW9lrLnspkzwIpTjIU-ekTn2Fw8DS3wc6--4gcKc6iag6SSdPf1y0N5M0hn6WjjjPgu1I67T~lqSL78x4B50h7ZAL1KpoKnoCZZGyO7XLsX~JGo5T9IKtwJXRIv~Brg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)