Abstract
We report the long-term outcome of a multicenter, prospective study examining fludarabine and rituximab in Waldenström macroglobulinemia (WM). WM patients with less than 2 prior therapies were eligible. Intended therapy consisted of 6 cycles (25 mg/m2 per day for 5 days) of fludarabine and 8 infusions (375 mg/m2 per week) of rituximab. A total of 43 patients were enrolled. Responses were: complete response (n = 2), very good partial response (n = 14), partial response (n = 21), and minor response (n = 4), for overall and major response rates of 95.3% and 86.0%, respectively. Median time to progression for all patients was 51.2 months and was longer for untreated patients (P = .017) and those achieving at least a very good partial response (P = .049). Grade 3 or higher toxicities included neutropenia (n = 27), thrombocytopenia (n = 7), and pneumonia (n = 6), including 2 patients who died of non–Pneumocystis carinii pneumonia. With a median follow-up of 40.3 months, we observed 3 cases of transformation to aggressive lymphoma and 3 cases of myelodysplastic syndrome/acute myeloid leukemia. The results of this study demonstrate that fludarabine and rituximab are highly active in WM, although short- and long-term toxicities need to be carefully weighed against other available treatment options. This study is registered at clinicaltrials.gov as NCT00020800.
Introduction
Waldenström macroglobulinemia (WM) is a distinct B-cell lymphoproliferative disorder characterized primarily by bone marrow infiltration with lymphoplasmacytic cells, along with demonstration of an immunoglobulin M (IgM) monoclonal gammopathy.1-3 Among treatment options for patients with WM, nucleoside analogs, as well as the CD20-directed monoclonal antibody rituximab, have been commonly used. Response rates of 30% to 70% and durations of response of 20 to 24 months have been reported with the use of nucleoside analogs in WM patients.4-13 Importantly, similar response rates were reported in these studies whether nucleoside analogs were used as first line or salvage therapy. The use of rituximab has also been extensively evaluated in patients with WM. Using standard dose (ie, 4 weekly infusions at 375 mg/m2), overall response rates of 25% to 30% have been observed. More recently, an extended dose regimen giving rituximab at 375 mg/m2 per week for 4 weeks, then repeated again at week 12, has resulted in higher (40%-50%) overall response rates.4,14-19
In preclinical studies, the potential for rituximab and fludarabine to enhance each other's activity has been demonstrated and may involve a spectrum of intracellular as well as extracellular mechanisms.20-22 Moreover, in other indolent B-cell malignancies, the combination of rituximab and fludarabine has led to higher response rates than those observed with either agent alone.23-27 The potential for enhanced clinical benefit by giving rituximab with fludarabine concurrently vs sequentially has also been reported in patients with chronic lymphocytic leukemia.26 In view of these considerations, we initiated a multicenter clinical trial of fludarabine and rituximab in patients with WM, which enrolled 43 subjects from March 7, 2001, to May 2, 2003. The outcome and long-term follow-up of this study are presented in this report.
Methods
Patients with a clinicopathologic diagnosis of WM requiring therapy who were naive to fludarabine and rituximab and who had 2 or fewer prior therapies, along with CD20+ disease as determined by previous bone marrow immunohistochemistry or flow cytometry were eligible for this study. To meet eligibility, patients had to demonstrate a monoclonal IgM protein, a minimum IgM level more than 2 times the upper limit of normal, a baseline platelet count of more than 25 000/μL, an absolute neutrophil count of more than 500/μL, a serum creatinine of less than 2.5 mg/dL (unless nephropathy was attributable to their WM), a serum total bilirubin and serum glutamic oxaloacetic transaminase of less than 2.5 times the upper limit of normal, and an Eastern Cooperative Oncology Group performance status of 0 to 2. No chemotherapy, steroid therapy, or radiation therapy within 30 days of study entry was permitted. Patients who were pregnant or lactating, had serious comorbid disease, had any uncontrolled bacterial, fungal, or viral infection, or had an active second malignancy were not eligible. All men and women of reproductive potential were required to agree to use an acceptable method of birth control before, during treatment, and for 6 months after completion of study treatment.
All patients provided informed written consent, in accordance with the Declaration of Helsinki, and the institutional review board approved the protocol at each participating site. A Data and Safety Monitoring Committee (DSMC) at the Dana-Farber Cancer Institute oversaw adverse events from all participating centers connected to this study. A baseline evaluation was obtained for enrollment within 30 days before initiation of therapy and consisted of a medical history and physical examination, laboratory studies consisting of a complete blood count and differential, chemistries, serum IgM levels, bone marrow biopsy, and aspiration, and computerized tomography (CT) scans of the chest, abdomen, and pelvis. Intended therapy consisted of 8 infusions of rituximab (375 mg/m2 per week) administered at weeks 1 to 4, 17, 18, and 30, 31, along with 6 cycles of fludarabine (25 mg/m2 daily) given for 5 days at weeks 5, 9, 13, 19, 23, and 27. Patients were assessed at week 12 and were eligible for continuation of therapy if they did not have progressive disease. Dose reduction of rituximab was not permitted; however, patients who demonstrated life-threatening adverse events to rituximab infusion were allowed to have their rituximab discontinued and continued on fludarabine therapy. The use of diphenhydramine (50 mg intravenously), acetaminophen (1000 mg orally), and at the treating physician's discretion, corticosteroids (hydrocortisone 100 mg intravenously), was permitted for rituximab infusion prophylaxis. Dose modification for fludarabine was permitted on the basis of hematologic toxicity. No dose modification for hematologic grade 0 to 2 toxicity was required. For patients demonstrating grade 3 hematologic toxicity, fludarabine was withheld until the patient's platelet count, hemoglobin, and absolute neutrophil count recovered to pretreatment baseline values and then resumed omitting day 5 of fludarabine administration. For patients demonstrating grade 4 hematologic toxicity, fludarabine was withheld until patient's platelet count, hemoglobin, and absolute neutrophil count recovered to pretreatment baseline values and then resumed, omitting days 4 and 5 of fludarabine administration. Granulocyte colony–stimulating factor, erythropoietin, and transfusions of packed red blood cells or platelets were permitted to support the patients' counts during therapy. Patients with an estimated creatinine clearance of less than 50 mL/min received fludarabine at a 50% dose reduction (ie, 12.5 mg/m2 per day for 5 days). The use of herpes zoster prophylaxis was mandated for all patients after the enrollment of the first 21 patients, after the DSMC identified an increased risk of herpes zoster on this study. The DSMC strongly recommended the prophylactic use of plasmapheresis for patients demonstrating a serum viscosity level of more than 3.5 centipoises before the administration of rituximab after the enrollment of the first 15 patients after one patient had a hyperviscosity-induced intracerebral bleed from a rituximab-induced IgM spike.28,29
Patients underwent reassessment of their disease at weeks 12, 24, and 52, and thereafter every 12 weeks until progression of disease. Restaging visits consisted of a physical examination, complete blood count and differential, chemistries, serum IgM levels, a bone marrow biopsy and aspiration (to confirm complete remission), and CT scans of the chest, abdomen, and pelvis (if extramedullary disease was present at baseline). Response determinations were made using modified consensus panel criteria from the Third International Workshop on WM,30 and response rates were determined on an intent-to-treat basis. A complete response was defined as having resolution of all symptoms, normalization of serum IgM levels with complete disappearance of IgM paraprotein by immunofixation, no evidence of disease by bone marrow examination, and resolution of any adenopathy or splenomegaly. Patients achieving a very good partial response (VGPR), partial response, and a minor response were defined as achieving a more than 90%, 50% to 90%, and 25% to 50% reduction in serum IgM levels, respectively. Patients with stable disease were defined as having less than 25% change in serum IgM levels, in the absence of new or increasing adenopathy or splenomegaly and/or other progressive signs or symptoms of WM. Progressive disease was defined as occurring when a greater than 25% increase in serum IgM level occurred from the lowest attained response value or progression of clinically significant disease-related symptom(s). Time to disease progression (TTP) was calculated from the start of therapy using the Kaplan-Meier method. The primary endpoints of this study were determination of overall response, median progression-free survival, and toxicity. A 48-month landmark analysis was also performed comparing patients with untreated vs previously treated disease and for those patients achieving more than VGPR versus less than VGPR.
Statistical analysis
Comparison of pretreatment and posttreatment parameters was performed using a 2-tailed Student t test on Microsoft Excel software. Nonparametric testing of pretreatment and posttreatment variables was performed by Fisher exact t test (Vassar Stats). A P value less than .05 was deemed to be significant for these studies.
Results
Patients and disease characteristics
Forty-three patients were enrolled in this study. The baseline characteristics for these patients are outlined in Table 1. The median age for all enrolled patients was 61 years (range, 52-75 years). The median number of treatments was 0 (range, 0-2 prior therapies). Twenty-seven (63%) patients had no prior therapy. Of the 16 previously treated patients, 14 and 2 patients were relapsed or refractory from their previous therapy, which included chlorambucil alone or with steroids (n = 12), rituximab (n = 2), vincristine, doxorubicin, dexamethasone (n = 1), and autologous transplant (n = 1). Median pretherapy bone marrow involvement with lymphoplasmacytic cells was 55% (range, 5%-100%), and median serum IgM level was 3840 mg/dL (range, 655-10 500 mg/dL). Twenty-seven (63%) patients had an IgM level of more than 3000 mg/dL. The median pretherapy hematocrit and platelet count for all enrolled study patients were 31.2% (range, 23.2%-44.7%) and 252 000 (range, 55 000-597 000/mm3), respectively. Fifteen (35%) and 4 (9.3%) of the patients had a hematocrit less than 30% and a platelet count of less than 100 000/mm3, respectively. Five patients (12%) had adenopathy and/or splenomegaly.
Baseline characteristics for all patients enrolled in the study
| Characteristic . | Value . |
|---|---|
| Median age, y | 61 (52-75) |
| Median prior therapies | 0 (0-2) |
| No prior therapy | 27 (63%) |
| Median bone marrow involvement | 55% (5%-100%) |
| Adenopathy and/or splenomegaly | 5 (12%) |
| Median IgM, mg/dL | 3840 (655-10500) |
| IgM > 3000 mg/dL | 27 (63%) |
| Hematocrit < 30% | 15 (35%) |
| Platelets < 100 000/mm3 | 4 (9%) |
| Characteristic . | Value . |
|---|---|
| Median age, y | 61 (52-75) |
| Median prior therapies | 0 (0-2) |
| No prior therapy | 27 (63%) |
| Median bone marrow involvement | 55% (5%-100%) |
| Adenopathy and/or splenomegaly | 5 (12%) |
| Median IgM, mg/dL | 3840 (655-10500) |
| IgM > 3000 mg/dL | 27 (63%) |
| Hematocrit < 30% | 15 (35%) |
| Platelets < 100 000/mm3 | 4 (9%) |
Of the 43 patients enrolled in the study, 41 were eligible for and received therapy beyond week 12. One patient was removed from the study at week 4 after experiencing an intracerebral bleed resulting from hyperviscosity induced by rituximab, whereas another patient was removed for progressive disease after restaging at week 12. Thirty-five and 33 patients completed the intended 8 weekly infusions of rituximab and 6 cycles of fludarabine. The median number of cycles of fludarabine and infusions of rituximab administered were 6 (range, 1-8) and 8 (range, 4-8), respectively, and were the same for untreated and previously treated patients.
Response
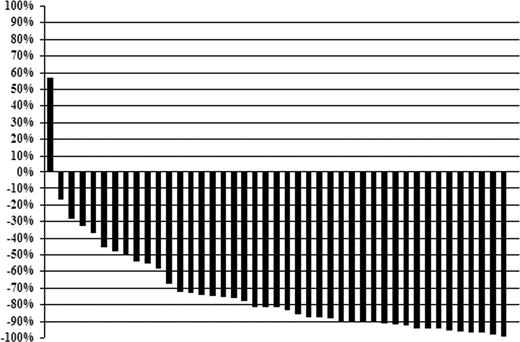
The individual changes in serum IgM levels at best response for all patients are shown in Figure 1. Median IgM levels for all 43 patients declined from 3840 mg/dL (range, 655-10 400 mg/dL) pretherapy to 443 mg/dL (range, 43-7040 mg/dL) at best response (P < .001). Pretherapy, 27 of 43 (62.8%) patients demonstrated an IgM level more than 3000 mg/dL; after treatment, only 3 of 43 (6.9%) had an IgM level more than 3000 mg/dL (P < .001). Bone marrow involvement also decreased after therapy, with a decline in the median percentage of tumor cell involvement from 55% (range, 5%-100%) to 5% (range, 0%-95%) (P < .001). Categorical responses were as follows: complete response (n = 2), VGPR (n = 14), partial response (n = 21), minor response (n = 4), for an overall and major response rate of 95.3% and 86.0%. The overall (96.3% vs 93.8%; P = 1.0) and major (88.9% vs 81.3%; P = .65) response rates were similar for untreated and previously treated patients, respectively. Among responders, the median time to at least a 25% reduction in serum IgM was 3.9 months (range, 0.6-34.1 months), whereas the median time to best response for responding patients was 19.2 months (range, 4.2-61 months).
Individual changes (percentage) in serum IgM levels for after treatment with fludarabine and rituximab at best response.
Individual changes (percentage) in serum IgM levels for after treatment with fludarabine and rituximab at best response.
Time to progression
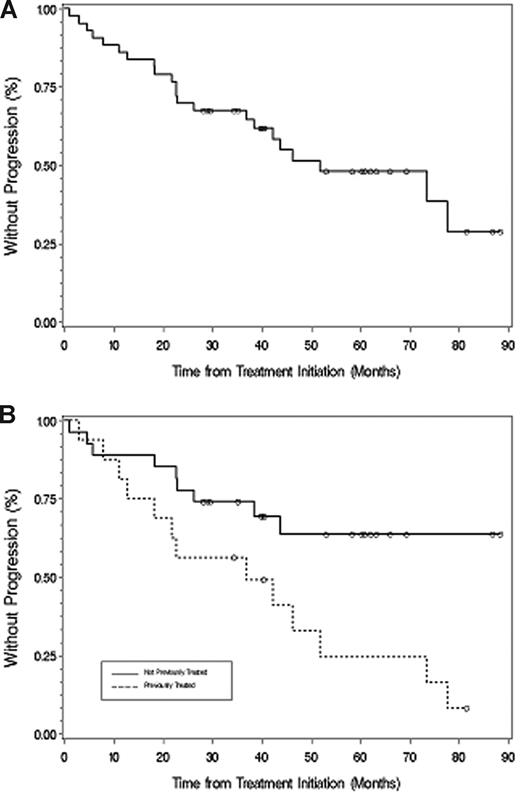
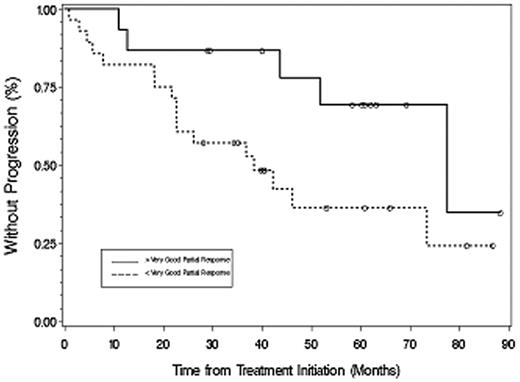
The median time to progression for all patients was 51.2 months (Figure 2), which was longer for untreated (77.6 months) versus previously treated (38.4 months) patients (P = .049). With a median follow-up of 40.3 months (range, 1-88.3+ months), 31 patients are alive and 21 patients remain free of disease progression. Among responding patients (Figure 3), TTP was longer for patients achieving at least VGPR (> 88.3 months) versus less than VGPR (36.9 months; P = .017). For the 2 patients achieving a complete response, the TTP as of December 31, 2007, was 60.9+ and 88.3+ months. A 48-month landmark analysis was also performed for progression-free survival for those patients who were untreated versus previously treated, and for those patients achieving at least VGPR versus less than VGPR. Eighteen of 27 (67%) untreated and 6 of 16 (38%) previously treated patients were free of disease progression at 48 months (P = .028). Twelve of 15 patients (80%) achieving at least VGPR versus 12 of 28 (42.9%) achieving less than VGPR remained free of disease progression at 48 months (P = .021).
Time to progression. (A) All patients. (B) Those patients who were untreated or had received treatment before their therapy with fludarabine and rituximab. ○ represents patients who had not progressed at last follow-up.
Time to progression. (A) All patients. (B) Those patients who were untreated or had received treatment before their therapy with fludarabine and rituximab. ○ represents patients who had not progressed at last follow-up.
Time to progression for all responding patients based on achieving more than VGPR or less than VGPR after therapy with fludarabine and rituximab. ○ represents patients who had not progressed at last follow-up.
Time to progression for all responding patients based on achieving more than VGPR or less than VGPR after therapy with fludarabine and rituximab. ○ represents patients who had not progressed at last follow-up.
Changes in hematologic parameters in treated WM patients
A significant increase in the median hematocrit was noted for all patients from 31.2% (range, 23.2%-44.7%) before therapy to 38.0% (range, 24.9-45.9%) after treatment (P < .001), with 30 of the 43 (70%) patients demonstrating a hematocrit increase of more than 2%. Conversely, the median platelet count decreased after treatment from 252 000/mm3 (range, 55 000-597 000/mm3) to 204 000/mm3 (range, 21 000-365 000/mm3) (P = .002), although for most patients this decrease was not clinically significant. Before therapy, 15 (35%) and 4 (9.3%) of the 43 patients demonstrated a hematocrit less than 30% and a platelet count of less than 100 000/mm3, respectively. After therapy, 6 (13.9%) and 6 (13.9%) of the 43 patients demonstrated a hematocrit of less than 30% and platelet count of less than 100 000/mm3 (P = .043 and .74, respectively).
Adverse events
Toxicities encountered were mainly hematologic and infectious. Encountered toxicities contributed to dose reduction (n = 5) and/or truncation of intended therapy (n = 9) in 13 patients. Intolerance to rituximab because of infusion-related reactions (n = 3) and rituximab-mediated hyperviscosity (n = 1) resulted in discontinuation of therapy for 4 of these patients, whereas prolonged myelosuppression on the basis of neutropenia (n = 7) and/or thrombocytopenia (n = 2) and/or peripheral neuropathy (n = 2) from fludarabine resulted in discontinuation of therapy for 9 patients. Among the first 21 patients, 3 patients had grade 2 herpes zoster, prompting institution of prophylaxis with either acyclovir or famcyclovir for the duration of therapy, and thereafter for 1 year at the recommendation of the DSMC. After institution of herpes zoster prophylaxis, no further cases of herpes zoster were observed. Grade more than 3 toxicities included neutropenia (n = 27); thrombocytopenia (n = 7); pneumonia (n = 6), including in 2 patients who died of non–Pneumocystis carinii pneumonia interstitial pneumonia; peripheral neuropathy (n = 2); limbic encephalitis (n = 1); and hemolytic anemia (n = 1). The rate of grade more than 3 toxicities was similar among untreated and previously treated patients (P = .48). With a median follow-up of 40.3 months, we observed transformation to aggressive lymphoma (n = 3), myelodysplasia (n = 1), acute myelogenous leukemia (n = 2), bladder carcinoma (n = 1), and carcinoma of unknown primary (n = 1) among 8 patients. The median time from initiation of protocol therapy to diagnosis of transformation was 21.1 months (range, 6-34.4 months) and for myelodysplastic syndrome/acute myeloid leukemia was 39.4 months (range, 11.9-40.8 months). Among patients who developed myelodysplastic syndrome or acute myeloid leukemia, one previously received chlorambucil and another had previously undergone high-dose chemotherapy with an autologous transplantation. Among the 3 patients who had transformation to diffuse large cell lymphoma, only one was previously treated and had received cyclophosphamide followed by rituximab. Among the 2 patients who developed adenocarcinoma, 1 was previously treated with chlorambucil.
Discussion
We examined the use of fludarabine in combination with rituximab in patients with WM given preclinical evidence suggestive of additive, and possibly synergistic, mechanisms of action in various preclinical lymphoma models and clinical activity in other lymphoid malignancies. To our knowledge, this study represents one of the longest in terms of follow-up in the therapy of WM and provides an important opportunity to assess both short- and long-term outcomes of this treatment. Intended therapy consisted of 6 cycles (25 mg/m2 per day for 5 days) of fludarabine and 8 infusions (375 mg/m2 per week) of rituximab over 31 weeks. On an intent-to-treat basis, we observed an overall and major response rate of 95.3% and 86.0%, respectively. Moreover, the median time to progression for all patients in this study was 51.2 months, which compares favorably to those previously reported with either fludarabine or rituximab alone, wherein response rates of 30% to 50% and median time to progression of 27 to 36 months have been reported. Median TTP was considerably longer (> 88.3 vs 36.9 months) for previously untreated vs treated patients, respectively, suggesting that fludarabine and retuximab may represent a particularly good combination for initial long-term disease control in suitable patients with WM.
An interesting observation in this study was that, for responding patients achieving at least a very good partial response (ie, at least a 90% reduction in disease burden), TTP was significantly greater vs patients attaining less than a VGPR (77.6 vs 38.4 months, respectively). Although the importance of attaining a VGPR has been reported as a predictive variable for progression-free survival in multiple myeloma,31,32 this study, to our knowledge, constitutes the first report of such potential benefit in WM. Studies addressing the impact of achieving at least a VGPR with other therapies would therefore seem appropriate to clarify whether VGPR attainment is categorical or therapy specific in terms of TTP benefit.
An intriguing finding in this study was the late response activity, as evidenced by the median time to best response, which was 19.2 months (range, 4.2-61 months). Continued declines in serum IgM beyond one year have previously been reported by our group19 and Dimopoulos et al18 among patients receiving rituximab monotherapy. One possibility for this finding is that fludarabine and rituximab may more effectively target earlier members of the WM clone, that is, mature B cells and lymphoplasmacytic cells, and spare (at least initially) more differentiated plasma cell members. Indeed, the relative resistance of malignant plasma cells to rituximab and/or fludarabine has previously been reported by us and others33,34 and may account for the frequent detection of residual plasma cells in the absence of earlier B-cell precursors in the bone marrow of patients treated with either agent alone or in combination. The eventual clonal extinction of IgM-producing plasma cells might then follow the initial elimination of precursor B cells by fludarabine and rituximab, thereby accounting for the slow, but continued, declines in serum IgM observed in these studies.
An important consideration in this study was the differential impact of combined fludarabine and rituximab therapy on hematologic parameters. A significant increase in the median hematocrit was noted for all patients from 31.2% to 38.0% after treatment, with 30 of the 43 (70%) patients demonstrating a hematocrit increase of more than 2%. Although the impact on hematocrit proved to be a positive outcome for patients, some patients experienced significant neutropenia and thrombocytopenia, which led to treatment discontinuation in 9 (21%) patients. Among these patients, 3 subjects experienced grade 4 neutropenia lasting more than 6 months. In 2 of these subjects, we observed a prompt recovery in neutrophil count after switch in cytokine support from granulocyte colony-stimulating factor to granulocyte-monocyte colony-stimulating factor, suggesting that the stimulation of earlier lineage leukocytes may overcome nucleoside analog-related prolonged neutropenia in certain patients.
In addition to hematologic toxicities, infectious complications were encountered in this study and may have contributed to the death of 2 patients who died of non–P carinii pneumonia–related interstitial pneumonia, including one patient who was in a complete remission at the time of death. Similar complications to nucleoside analog therapy have previously been described.35 In addition, we observed herpes zoster in 3 of the first 21 patients prompting institution of herpes zoster prophylaxis with acyclovir or famcyclovir for the duration of treatment plus one year with good effect. The increased incidence of herpes zoster has also been reported by Czuczman et al23 in patients with follicular non-Hodgkin lymphoma treated with fludarabine and rituximab, prompting prophylaxis in their study as well. Therefore, prophylaxis with an antiviral agent appears warranted among WM patients receiving fludarabine and rituximab. Lastly, in one patient, we observed a case of limbic encephalitis, whose pathogenic basis remains unknown despite extensive workup but suspected to be on the basis of a herpetic infection. Viral encephalitis suspected on the basis of a herpetic infection was previously reported in a WM patient receiving treatment with cladribine.8
In addition to the aforementioned short-term toxicities, we also observed the development of diffuse large cell lymphoma (n = 3), myelodysplasia and acute myelogenous leukemia (n = 3), and carcinomas (n = 2) among patients treated in this study with a median follow-up period of 40.3 months. Although previous alkylator therapy may have been contributory in some of these cases to the development of secondary malignancies, only half of these patients received such therapy. The increased incidence of transformation to aggressive lymphoma and/or development of myelodysplasia and acute myelogenous leukemia after nucleoside analog therapy have previously been reported by us36 and others,37 and raises concerns about the long-term consequences of these agents. Therefore, careful consideration of the candidacy for individual WM patients must be undertaken to discern whether nucleoside analog therapy is appropriate, as has also been recommended by the recent consensus panels on treatment of WM.4,38 The results of this study may also call into question whether a full 6-cycle course of fludarabine using 5 days of therapy per cycle is warranted, particularly because 20% of patients experienced cytopenias resulting in treatment cessation. In most of these cases, treatment was truncated at or after 4 cycles of fludarabine. The impact of using abbreviated courses of nucleoside analogs is therefore worthy of further study, as is also its potential impact on mitigating secondary malignancies.
Lastly, the sequencing of rituximab to fludarabine may be an important factor both from an efficacy standpoint, as well as avoidance of the IgM flare that is commonly observed after rituximab28,29 and may have life-threatening consequences, as was the case in one patient in this study who experienced a hyperviscosity-related intracerebral bleed after the first 4 weekly infusions of rituximab. Byrd et al26 reported that the concurrent sequencing of fludarabine and rituximab produced a higher overall and complete remission response rate versus fludarabine followed by rituximab in chronic lymphocytic leukemia patients. The concurrent administration of these agents vs rituximab first followed by fludarabine as administered in this study might therefore lead to a better outcome in the treatment of WM patients. Moreover, as reported by Nichols et al39 and Tedeschi et al,40 the concurrent administration of fludarabine and rituximab may also be associated with a decreased frequency of IgM flaring.
In conclusion, the results of this study demonstrate that fludarabine and rituximab are an active regimen in WM, although the ideal schedule and length of treatment for this combination need to be better studied and short- and long-term toxicities need to be carefully weighed against other available treatment options. The sequencing, combination, and duration of therapy to best optimize the combination of purine nucleoside analogs and rituximab remain to be defined by larger randomized studies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Research Fund for Waldenström's at the Dana-Farber Cancer Institute, Berlex Pharmaceuticals, Genentech, Biogen IDEC, the Bing Fund for Waldenström macroglobulinemia, and a National Institutes of Health Career Development Award (K23CA087977-03) (S.P.T.).
National Institutes of Health
Authorship
Contribution: S.P.T. designed the trial, recruited and treated patients, and analyzed the data; A.R.B., L.I., J.D.S., and C.J.P. served as clinical research coordinators for this trial, and collected and analyzed data; P.W., C.E., S.R.F., A.L., P.M., J.M., S.A.G., and E.K. were site principal investigators and recruited patients, collected data, and reviewed study outcome; B.T. was the biostatisician and provided data analysis for this study; and S.P.T. wrote the manuscript.
Conflict-of-interest disclosure: S.P.T., C.E., and S.A.G. have received honoraria from Genentech BioOncology and/or Biogen IDEC. S.R.F. is presently an employee and shareholder of Hoffman-La Roche. The remaining authors declare no competing financial interests.
Correspondence: Steven P. Treon, Bing Center for Waldenström's Macroglobulinemia, Dana-Farber Cancer Institute, Harvard Medical School, M547, 44 Binney St, Boston, MA 02115; e-mail: steven_treon@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal