Abstract
Chronic immune thrombocytopenic purpura (ITP) is characterized by low platelet counts and mucocutaneous bleeding. In previous studies romiplostim (AMG531), a thrombopoiesis-stimulating protein, increased platelet counts in most patients with chronic ITP. This ongoing, long-term open-label, single-arm study investigated safety and efficacy in patients who completed a previous romiplostim study and had platelet counts less than 5 × 109/L. One hundred forty-two patients were treated for up to 156 weeks (mean, 69 weeks). Platelet responses (platelet count ≥ 50 × 109/L and double baseline) were observed in 87% of all patients and occurred on average 67% of the time in responding patients. In 77% of patients, the romiplostim dose remained within 2 μg/kg of their most frequent dose at least 90% of the time. Ninety patients (63%) received treatment by self-administration. Treatment-related serious adverse events were reported in 13 patients (9%). Bone marrow reticulin was observed in 8 patients; marrows were not routinely performed in this study, so the true incidence of this event cannot be determined. Severe bleeding events were reported in 12 patients (9%). Thrombotic events occurred in 7 patients (5%). In conclusion, romiplostim increased platelet counts in most patients for up to 156 weeks without tachyphylaxis and had an acceptable safety profile. (ClinicalTrials.gov Identifier NCT00116688).
Introduction
Immune thrombocytopenic purpura (ITP) in adults is a chronic autoimmune disorder characterized by low platelet counts and mucocutaneous bleeding.1-5 A growing body of evidence suggests that ITP is not only a disease of autoantibody-mediated platelet destruction but also a disease of impaired platelet production.6-8 Suboptimal platelet production is thought to be a direct effect of autoantibodies on the megakaryocyte,9,10 which may be affected further by failure to substantially increase circulating thrombopoietin (TPO) levels despite often marked thrombocytopenia.11-14
Treatments for patients with ITP have focused either on inducing short-term increases in platelet counts, through administration of agents such as steroids, intravenous immunoglobulin (IVIG), and intravenous anti-D,15-20 or on long-term maintenance of platelet counts through splenectomy or other treatments such as rituximab, danazol, azathioprine, or even prolonged steroid treatment.5,21-26 These treatments are effective in many patients, but may also have drawbacks related to the durability of the response and adverse effects.
Currently, available therapies increase platelet counts in patients with ITP primarily by decreasing platelet destruction.27,28 Romiplostim (AMG 531, Nplate) and eltrombopag (SB-497115, Promacta) are thrombopoietic agents stimulating platelet production that have recently been shown to induce increases in platelet counts in both healthy adults29,30 and patients with ITP.31-34
Romiplostim is a unique thrombopoiesis-stimulating protein, referred to as a peptibody, which is composed of 2 IgG Fc domains coupled with 4 copies of a TPO mimetic peptide.29 Romiplostim stimulates platelet production by a mechanism similar to that of endogenous TPO (eTPO),35 but has no amino acid sequence homology with TPO.32 Administration of a single dose of 1 to 10 μg/kg romiplostim to thrombocytopenic patients with ITP increased platelet counts to 50 × 109/L or more, although counts generally fell to less than 50 × 109/L by day 15.32,34 Weekly administration of romiplostim increased and often maintained platelet counts over a 6-week course of therapy.32 Repeated weekly dosing of romiplostim over a 24-week period in two phase 3 placebo-controlled studies in splenectomized and nonsplenectomized chronic patients ITP led to an overall response (platelet counts ≥ 50 × 109/L during ≥ 4 weeks on study) in 83% of patients and a durable response in 49% of patients.36
This report describes a single-arm, long-term safety and efficacy study of weekly treatment with romiplostim in patients who had previously completed a romiplostim ITP study. Patients in the current study continued to receive romiplostim or initiated romiplostim treatment if they had previously received placebo. This is an interim report of data collected as of July 13, 2007, from 142 patients treated with romiplostim, some for as long as 156 weeks.
Methods
Study design
This is an ongoing, open-label extension study conducted to evaluate the safety and efficacy of long-term dosing of romiplostim in thrombocytopenic patients with ITP. The study protocol was approved by the Institutional Review Boards at the participating centers before any patients enrolled into the study at that site and informed consent was obtained from the patients in accordance with the Declaration of Helsinki before entering them into the study. This study began enrolling patients in June 2004, and the planned completion date is currently December 2009.
Patients
Patients could be enrolled in the study if they had completed a previous study of romiplostim for the treatment of ITP, whether they had received romiplostim or placebo. Almost all patients were from previously completed studies that have been described elsewhere.32,34,36 Patients were enrolled at 36 centers in the United States and the European Union beginning on August 2, 2004; enrollment is continuing. Written informed consent was obtained from all patients before any screening procedures were performed. The previously completed studies enrolled thrombocytopenic patients at least 18 years of age with ITP as defined by the American Society of Hematology,37 whether they had or had not previously undergone splenectomy. After completing their prior study of romiplostim, patients could be enrolled in this long-term extension study once their platelet counts were less than or equal to 50 × 109/L. Patients were excluded from the long-term extension study if they had any bone marrow stem cell disorders or new active malignancies diagnosed since enrollment in the previous romiplostim study. Patients were also ineligible if fewer than 4 weeks had elapsed since participation in trials of any other investigational drug or the last administration of alkylating agents.
Treatment
All patients were to receive romiplostim once weekly by subcutaneous injection, and all were to receive their initial romiplostim injections at the study center. Patients who had received romiplostim in a prior study entered this study at the same romiplostim dose received in that study, unless more than 24 weeks had elapsed since their last dose of romiplostim, in which case treatment was initiated at 1 μg/kg per week. Patients who had received placebo in the prior study started romiplostim at a dose of 1 μg/kg per week in this study. Throughout this study, the dose of romiplostim was adjusted on the basis of the patient's platelet count using prespecified rules for adjustment (Table 1). The target platelet count range was 50 to 250 × 109/L.
The maximum allowed dose of romiplostim was initially 30 μg/kg and was later reduced in 2 stages to the current maximum dose of 10 μg/kg after it was determined that few patients derived additional clinical benefit from increasing doses above this level.36 Before the maximum dose was reduced, patients whose weekly dose was greater than 10 μg/kg, either because they entered the study at a dose greater than 10 μg/kg or whose dose was increased to greater than 10 μg/kg, could remain on that dose. After the maximum allowed dose was reduced, these patients could not increase their doses, and those whose dose was reduced could not subsequently increase it until their dose was less than 10 μg/kg.
Patients whose romiplostim dose was the same for at least 3 weeks were permitted to self-administer romiplostim and return to the study center for evaluation at designated visits. Patients were trained by the study center staff to prepare and administer self-injections including use of a specially prepared instructional video. Patients who had both been receiving a dose of romiplostim greater than or equal to 10 μg/kg and whose platelet count was less than 20 × 109/L for 4 consecutive weeks were considered treatment failures and were discontinued from the study unless the investigator felt the patient was clinically benefiting and the sponsor gave permission for the patient to continue.
Patients could continue to receive concurrent ITP medications (corticosteroids, danazol, and azathioprine) that had been administered at a constant dose and schedule before start of initial study. These medications could be reduced or discontinued at any time during the study after platelet counts reached 50 × 109/L. Rescue medications could be administered when platelets counts were below 10 × 109/L, when there was bleeding or wet purpura, or when deemed medically necessary by the investigator. Rescue medication was defined as any medication administered to increase the platelet count; the permitted rescue medications were IVIG, platelet transfusions, intravenous anti-D, steroids, and antifibrinolytics. Rituximab, alkylating agents, and other investigational agents could not be used as rescue medications. Increase in the dose of a concurrent ITP medication to levels above those used at baseline was also considered rescue medication.
Assessments and outcome measures
All patients underwent a screening evaluation within 30 days before enrollment, which included physical examination, measurement of vital signs, and complete blood count. The results of assessments of anti-romiplostim or anti-TPO antibody status from the patients' previous end-of-study visit were used for this study. In addition, platelet counts were performed for all patients before administration of the first dose of romiplostim.
All patients returned to the clinic weekly through week 4. Thereafter, patients who met the criteria for self-administration and elected to self-administer were required to return for ongoing evaluation at designated visits every 4 weeks; all other patients were required to return weekly for their designated visits. Assessments of platelet counts, concurrent medications, and adverse events were made at each designated visit. Patients provided samples for complete blood counts and blood chemistry determinations every 4 weeks, and physical examinations were performed at week 1 and every 12 weeks thereafter. Antibodies to romiplostim were assessed at weeks 1 and 12 and every 24 weeks thereafter according to previously described methods.32
Nine patients from this study also participated in a supplemental prospective study designed to evaluate the effect of romiplostim treatment on bone marrow morphology. Bone marrow biopsies were taken at baseline (before initiating romiplostim therapy) and after receiving romiplostim for 3 (n = 4) or 9 (n = 5) months. Reticulin assessments performed on biopsies taken from these patients were provided by a pathologist from the central laboratory. Except for biopsies taken as part of the supplemental prospective study, bone marrow examinations were not routine and were performed at the investigator's discretion; however, a biopsy was recommended if abnormalities in the peripheral blood smear (eg, nucleated red blood cells) were observed or there was loss of response despite increasing doses of romiplostim. The exact number of marrows performed in patients on this study is unknown. The results of all available bone marrow examinations are reported here.
Efficacy was evaluated on the basis of platelet response and the proportion of patients able to reduce or discontinue concurrent ITP therapies. Platelet response was defined as a platelet count at a scheduled weekly visit of 50 × 109/L or more and double the platelet count at baseline for this study in the absence of rescue medication in the preceding 8 weeks.
Safety was assessed on the basis of the incidence of adverse events, collected continuously, and captured on a standard case report form, including clinically significant changes in laboratory values and the incidence of neutralizing antibody formation. The severity of adverse events was rated by the investigator on a scale of 1 equals mild, 2 equals moderate, 3 equals severe, 4 equals life-threatening, and 5 equals fatal. An event was defined as mild if the patient was aware of the sign or symptom, but tolerated it easily; moderate if it caused enough discomfort to interfere with usual activity; severe if it was incapacitating and made it impossible to work or engage in usual activities; and life-threatening if the patient was at risk of death at the time of the event. Adverse events were considered serious if they were fatal, life-threatening, required hospitalization or prolongation of existing hospitalization, or resulted in persistent or significant disability/incapacity. Serious adverse events were reported to the sponsor within one working day of discovery or notification of the event on a serious adverse event form.
Statistical analysis
Statistical analyses were descriptive. Categorical end points were summarized by the number and percentage of patients in each category. Continuous end points were summarized by number of patients, mean, standard deviation (SD), median, 25th percentile (Q1), and 75th percentile (Q3), with minimum and maximum values. Baseline was defined as the pretreatment value closest to the first dose in this study. In post-hoc analyses, descriptive statistical summaries were provided for platelet counts by patients' previous treatment and by splenectomy status. Because platelet data were not always normally distributed, median values for these data are presented in this report. This report presents the results for the weeks during which 10 or more patients were on study for all groups in a particular analysis. These periods differed according to the set of patients being evaluated and were as follows: weeks 1 through 144 for the analyses of platelet counts and responses for all patients by study week; weeks 1 through 72 for the analyses of platelet counts and responses by previous treatment; and weeks 1 through 84 for the analyses of platelet counts and responses by splenectomy status.
To discount the possible effect of rescue medications on platelet counts, patients were considered to have had no platelet-related response or excluded from calculation of descriptive statistics for 8 weeks after administration of these medications.
Dose variability was assessed in a post-hoc analysis. For each patient, the total number of weeks beyond week 13 that the patient remained in the study and the dose most frequently administered during that period were determined. The number of weeks the patient's dose changed by greater than 2 μg/kg from that patient's most frequent dose was then calculated for each patient and expressed as a percentage of the total number of weeks. On the basis of these percentages, the frequencies (number of patients) were categorized in increments of 10% from 0% to 100%.
Exposure-adjusted adverse event rates were calculated as the total number of events over the total study duration for each group and expressed as events per 100 patient-weeks. The incidence of bleeding events rated as severe, life-threatening, or fatal; and the use of rescue medication were assessed directly from case report forms on a post-hoc basis.
Results
Patient disposition
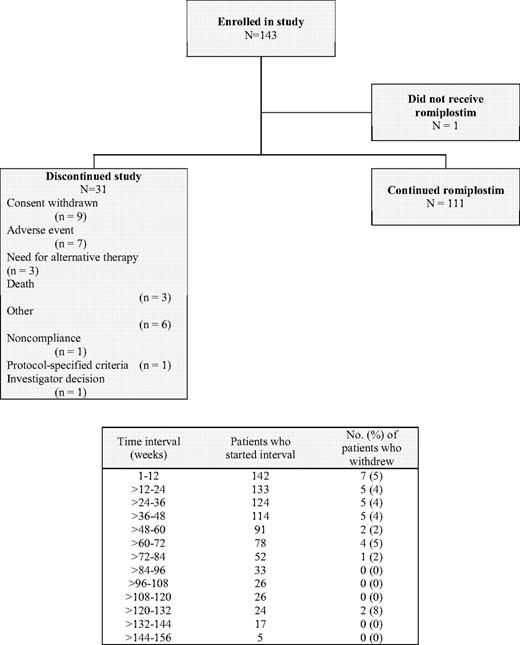
As of July 13, 2007, the cutoff date for this report, 143 patients had been enrolled in this study (Figure 1). One patient withdrew consent before treatment, 31 patients discontinued the study, and 111 (78%) are continuing. Discontinuation rates were consistently 4% or 5% during each 12-week interval over the first 72 weeks of treatment.
Disposition of patients with chronic ITP treated with romiplostim for up to 156 weeks. One patient did not receive romiplostim because he withdrew consent. The adverse events leading to discontinuation from the study were increased bone marrow reticulin in 2 patients, vaginal hemorrhage, musculoskeletal pain and headache, deep vein thrombosis, septic thrombophlebitis, and monoclonal gammopathy of undetermined significance in 1 patient each. “Other” reasons for discontinuation were lack of response to romiplostim (2), possible ITP remission/cure (2), patient request (1), and unsteady platelet count (1).
Disposition of patients with chronic ITP treated with romiplostim for up to 156 weeks. One patient did not receive romiplostim because he withdrew consent. The adverse events leading to discontinuation from the study were increased bone marrow reticulin in 2 patients, vaginal hemorrhage, musculoskeletal pain and headache, deep vein thrombosis, septic thrombophlebitis, and monoclonal gammopathy of undetermined significance in 1 patient each. “Other” reasons for discontinuation were lack of response to romiplostim (2), possible ITP remission/cure (2), patient request (1), and unsteady platelet count (1).
Of the 142 patients who received romiplostim, 18 (13%) never achieved a study-defined platelet response. Of these 18 patients, 10 patients continue on study. The median (range) time on study for these 10 patients was 433 (1-998) days. The other 8 nonresponders discontinued the study: 2 required an alternative therapy, 2 withdrew consent, 2 withdrew because of the investigator's decision that the patient was not responding and/or not receiving benefit from the drug, 1 had an adverse event (mild headache), and 1 died (myocardial infarction). The median (range) time on study for these 8 patients was 130 (1-861) days.
Ninety patients who received the same romiplostim dose for greater than or equal to 3 consecutive weeks initiated self-administration. Of these 90, 3 discontinued self-administration, 1 because of noncompliance and 2 because of the investigator's decision; these patients resumed romiplostim injections at weekly visits to the study center.
Patient demographics and clinical characteristics
Sixty-seven percent of patients were women, 83% were white, with 8% Latino, the median age was 53, and 60% (86/143) had undergone splenectomy. Twenty-two percent of patients (32/143) were receiving concurrent treatment for ITP at study entry (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Efficacy evaluation
Median platelet counts increased sharply during the first 4 weeks of treatment and then more gradually through week 16 (Figure 2A). Subsequently, median platelet counts remained in the range of 61 to 149 × 109/L through week 144.
Platelet counts and platelet responses by study week. (A) Median (25th [Q1] and 75th [Q3] percentiles) platelet counts by study week. Median platelet counts increased sharply during the first 4 weeks of treatment and then more gradually through week 16. (B) Percentage of patients with a platelet response over time. A platelet response was defined as a platelet count of 50 × 109/L or more that was at least double the platelet count at baseline in the absence of rescue medication within the preceding 8 weeks. After 1 dose, 30% of patients achieved a platelet response, and after 3 doses, 51% achieved a response. Over the course of the study, a platelet response was observed in 87% of all patients.
Platelet counts and platelet responses by study week. (A) Median (25th [Q1] and 75th [Q3] percentiles) platelet counts by study week. Median platelet counts increased sharply during the first 4 weeks of treatment and then more gradually through week 16. (B) Percentage of patients with a platelet response over time. A platelet response was defined as a platelet count of 50 × 109/L or more that was at least double the platelet count at baseline in the absence of rescue medication within the preceding 8 weeks. After 1 dose, 30% of patients achieved a platelet response, and after 3 doses, 51% achieved a response. Over the course of the study, a platelet response was observed in 87% of all patients.
The percentage of patients with a platelet response (platelet count ≥ 50 × 109/L and double baseline in the absence of rescue medication within the preceding 8 weeks) over time is shown in Figure 2B. A platelet response had been achieved by 30% of patients after the first dose and by 51% of patients after the third dose. Thereafter, up to week 144, between 47% and 74% of patients demonstrated a response. Over the course of the study, a platelet response was observed at least once in 87% of patients (124/142). On average, patients who responded at any point during the study had a platelet response during 67% of the weeks on study (median, 78%; range, 2%-100% of weeks).
Comparison of the effects of romiplostim by the treatment assigned in the previous study (placebo or romiplostim) demonstrated that median platelet counts in patients previously treated with placebo were generally similar to those of patients previously treated with romiplostim at weeks 4, 16, and 52 (Table S2). The time to achieve a platelet response was several weeks longer in the patients who had previously received placebo, because they initiated treatment at 1 μg/kg, which was less than the average initial dose in patients who had previously received romiplostim. The magnitude of the response and the proportion of patients responding were lower in splenectomized patients (Table S2); however, the time course of the platelet response was not affected by splenectomy status in responders. After week 12 and through week 84, median platelet counts in splenectomized patients ranged from 58 to 106 × 109/L and were consistently lower than those in patients who had not undergone splenectomy, which ranged from 96 to 209 × 109/L.
During this study, individual patients' romiplostim doses were adjusted on the basis of their platelet counts (Table 1). The mean dose of romiplostim increased over the first 24 weeks and then remained relatively constant (Figure 3A). The mean (± SD) of the average weekly dose was 5.9 (± 3.9) μg/kg (range, 1-17 μg/kg), and the mean (± SD) duration of treatment was 68.5 (± 39.4) weeks (range, 1-156 weeks). As anticipated, doses for patients previously treated with placebo were increased frequently during the first weeks of the study to achieve a platelet response. Early dose increases were less frequent in patients who previously had received romiplostim, as their doses had already been adjusted during the prior study. After the first 12 weeks of treatment, for 77% of patients, their romiplostim doses were within 2 μg/kg of their most frequent weekly romiplostim dose greater than or equal to 90% of the time (Figure 3B).
Romiplostim dose and variability in doses received. (A) Mean (SE) doses of romiplostim (μg/kg) by study week. Over the course of the study, the mean (±SD) total number of doses of romisplostim was 63.2 (±37.1), the mean (±SD) duration of treatment was 69 (±39.4) weeks, and the mean (±SD) average weekly dose was 5.9 (±3.9) μg/kg. The overall mean dose of romiplostim increased over the first 24 weeks and then remained relatively constant. (B) Variability in doses received. Percentage of weeks during which patients' doses changed by greater than 2 μg/kg from the patient's most frequent dose (excluding weeks 1-12; n = 133). After the first 12 weeks of treatment, in 77% of patients (103/133), romiplostim doses were within 2 μg/kg of the patient's most frequently administered weekly dose greater than 90% of the time.
Romiplostim dose and variability in doses received. (A) Mean (SE) doses of romiplostim (μg/kg) by study week. Over the course of the study, the mean (±SD) total number of doses of romisplostim was 63.2 (±37.1), the mean (±SD) duration of treatment was 69 (±39.4) weeks, and the mean (±SD) average weekly dose was 5.9 (±3.9) μg/kg. The overall mean dose of romiplostim increased over the first 24 weeks and then remained relatively constant. (B) Variability in doses received. Percentage of weeks during which patients' doses changed by greater than 2 μg/kg from the patient's most frequent dose (excluding weeks 1-12; n = 133). After the first 12 weeks of treatment, in 77% of patients (103/133), romiplostim doses were within 2 μg/kg of the patient's most frequently administered weekly dose greater than 90% of the time.
Overall, 36% of patients (51/142) used rescue medications at some time during the study. The percentage of patients who used rescue medications at least once in 12-week periods decreased from 23% of the patients (33/142) treated during weeks 1 through 12 to 15% of the patients (18/124) treated during weeks 24 through 36. Thereafter, the percentages of patients who used rescue medications during each 12-week period ranged from 12% to 18%, except for weeks 132 through 144, when 24% of the patients (4/17) used rescue medication.
Thirty-two patients were receiving concurrent treatment for ITP at baseline. At the cutoff time for this report, 50% (16/32) had discontinued this medication, and a further 34% (11/32) had reduced the dose by at least 25%.
Safety evaluation
Ninety-five percent of patients (135/142) reported at least one adverse event. Headache was the most commonly reported adverse event, followed by nasopharyngitis, contusion, and fatigue (Table S3). In most patients, adverse events were rated as mild or moderate in severity. When adverse event rates in this study were calculated on the basis of romiplostim exposure, rates were low; the only events with frequencies of greater than or equal to one event per 100 patient-weeks were headache (1.8), contusion (1.1), and epistaxis (1.0).
Serious adverse events were reported in 31% of patients (44/142). Serious adverse events reported in 3 or more patients each were thrombocytopenia in 10 patients, increased bone marrow reticulin in 5, and congestive cardiac failure in 3. Three other cases of increased bone marrow reticulin were reported; 1 was not considered by the investigator to be serious, and the other 2 were noted in the patient records but not listed as an adverse event. Nineteen serious adverse events considered related to treatment were reported in 13 (9.2%) patients (Table 2). Of these 19 events, 2 cases of increased bone marrow reticulin and one each of vaginal hemorrhage, deep vein thrombosis, and monoclonal gammopathy of undetermined significance led to discontinuation from the study. Treatment-related adverse events that were severe (grade 3) but not serious (not leading to hospitalization or life-threatening), were reported in 6 patients (Table 2).
Bone marrow samples were taken from 16 patients during this study. Nine patients participated in a supplemental prospective study designed to evaluate the effect of romiplostim treatment on bone marrow morphology in patients with ITP. Baseline biopsies were evaluable in 8 patients, and reticulin staining was absent at baseline in all but 1 case, in which reticulin staining was mild. Six patients had evaluable biopsies at both baseline and follow-up. Reticulin staining after romiplostim treatment remained absent in 5 of 6 cases, and in 1 patient treated with romiplostim for 3 months changed from none to mild. Trichrome staining, tested in 8 baseline and 7 follow-up samples, was absent in all cases. The presence of or an increase in bone marrow reticulin was spontaneously reported in samples from 8 patients (Table 3), 1 of whom (patient no. 3231) also participated in the prospective study. Two patients discontinued the study because of these bone marrow findings. Trichrome staining, done in only 4 patients, was negative for type I collagen formation. Immunophenotyping, performed in only 3 patients, and cytogenetics, performed in only 5 patients, did not reveal any clonal abnormalities. No clinical symptoms related to the observed bone marrow reticulin were reported. In general, the presence of bone marrow reticulin was associated with prior splenectomy, exposure to multiple prior ITP therapies, relatively high doses of romiplostim, minimal platelet response, and possibly the presence of nucleated red blood cells (Table 3). Other variables such as age, sex, and concomitant steroid treatment did not appear to be associated with bone marrow reticulin. Follow-up bone marrow examinations were performed after discontinuation of romiplostim in only 2 patients and showed improvement in 1 patient and no change in the other.
In a post-hoc analysis, the proportion of patients experiencing bleeding events decreased from 42% (60/142) in the first 24 weeks to 29% (37/126) in the following 24 weeks, declining to 23% (22/97) and 20% (13/65) of patients during weeks 48 to 72 and weeks 72 to 96, respectively. Fourteen severe bleeding events were reported in 8.5% of patients (12/142) (Table 4); no life-threatening or fatal bleeding events were reported. Severe epistaxis was reported in 2 patients, and all other severe bleeding events were reported in one patient each. All but one bleeding event (vaginal hemorrhage) were considered by the investigator to be unrelated to romiplostim. Eleven of the 14 events occurred within the first 24 weeks of treatment. Platelet counts taken at or near the time of occurrence of the bleeding event were less than or equal to 30 × 109/L for all but one event (conjunctival hemorrhage).
Twelve thrombotic or thromboembolic events were reported in 7 (4.9%) patients. Five of these were seen in 4 patients and are described in Table 2. The other events were not deemed related to treatment. They include deep vein thrombosis, a myocardial infarction, coronary artery occlusion, septic thrombophlebitis, and transient ischemic attack. Most thrombotic events (8/12) occurred in the absence of a platelet count above 400 × 109/L in the 2 weeks before or after the event. In 6 of 7 patients, the events were serious; however, all 6 of these patients were known to have one or more preexisting risk factors for thrombosis before romiplostim treatment was initiated in the prior treatment study.
A blood sample from one patient was transiently positive for anti-romiplostim neutralizing antibodies but always negative for anti-TPO antibodies. This patient withdrew consent and discontinued the study at week 79, before the results of the antibody tests were available. The blood sample was obtained at the time of discontinuation. The patient's platelet count had been consistently between approximately 50 and 200 × 109/L but decreased to 37 × 109/L at week 79, the week after his romiplostim dose was tapered off. No thrombocytopenia related to the presence of antibody was observed. The patient returned to provide a follow-up sample approximately 4 months later. The follow-up sample was negative for anti-romiplostim neutralizing antibodies and negative for anti-TPO antibodies. The maximum dose of romiplostim the patient received was 3 μg/kg, and platelet counts were maintained above the baseline level throughout the study.
Three patients died during this study. One patient developed renal failure and subsequently experienced a myocardial infarction and congestive cardiac failure, followed by cardiac arrest. One patient died from acute cardiorespiratory failure secondary to overwhelming postsplenectomy sepsis due to streptococcus pneumonia. The third died as a result of hepatic and renal failure secondary to underlying liver cirrhosis and hepatocellular carcinoma. None of the deaths was considered by the investigators to be treatment-related.
With the exception of platelet counts, no clinically significant treatment-related changes were observed in vital signs, physical examination findings, or hematology or serum chemistry values.
Discussion
In this study of long-term management of patients with chronic ITP, weekly subcutaneous administration of romiplostim was an effective and apparently safe means to increase the platelet count in both splenectomized and nonsplenectomized patients over up to 156 weeks of treatment. A platelet response was achieved by 87% of all patients at one or more points during the study, without evidence of tachyphylaxis. Almost all (84%) patients taking concurrent treatment for ITP at the time of study entry were able to reduce or completely discontinue it, and rescue medication was required in only one-third of patients.
The decrease in bleeding events observed over time and the small number of severe bleeding events reported during this long-term study provide evidence that platelet count increases produce clinical benefit in patients with ITP. This is consistent with the generally agreed upon hypothesis that bleeding is often correlated with platelet counts. When platelet counts were available at or near the time of a severe, life-threatening, or fatal bleeding event, the counts were less than 30 × 109/L in all but one case. These findings support the 30 × 109/L platelet count threshold for treatment recommended in international practice guidelines4,38,39 and the greater than or equal to 50 × 109/L treatment goal for romiplostim. The results of this study extend those from the two 24-week phase 3 studies in splenectomized and nonsplenectomized patients.36
As in other studies of ITP,32,36 variability was noted in the responses at given doses of romiplostim, with week-to-week fluctuations in platelet counts, which were substantial in a small number of patients. Given the inter- and intrapatient variability in response, it appears that patients initially require monitoring at weekly intervals to adjust doses and then no less than once per month during treatment. That having been said, most patients in this study had their romiplostim dose almost always remain within 2 μg/kg of their most frequently administered dose and therefore met the stability criteria that allowed them to initiate and continue self-administration of romiplostim. Their experience suggests that self-administration at home may be a convenient option for many patients.
In the phase 3 studies, the mean of each patient's average weekly dose of romiplostim was 2.5 μg/kg,36 whereas in this long-term study, the mean of each patient's average weekly dose of romiplostim was 5.9 μg/kg. There are several possible reasons for the higher dose used in this study, including different platelet ceilings leading to dose adjustments, allowing concurrent medication adjustment in only the first 12 weeks of the 24-week studies, and enrolling patients in this study from the phase 1 and 2 studies who were allowed to dose up to 15 to 30 μg/kg.
Long-term treatment with romiplostim had an acceptable safety profile consistent with that observed in the 24-week phase 3 placebo-controlled studies.36 In this long-term study with treatment up to 156 weeks, adverse events in most patients were mild or moderate in severity. There was no placebo control group in this study; however, for 23 of the 26 most common adverse events, the exposure-adjusted frequency was less than 1 per 100 patient-weeks (Table S3). For patients who were self-injecting, adverse events were recorded at the clinic visits every 4 weeks. Thus, accuracy of the adverse event reports for these patients depended upon the reliability of their memory of events that may have occurred several weeks previously. Given the long time-period of conducting the study, almost all patients reported adverse events at some time during the study, but the great majority were considered unrelated to treatment.
Eight patients were noted to have bone marrow reticulin present or increased without any evidence suggesting progression to a clonal disorder, particularly chronic idiopathic myelofibrosis (CIMF) (Table 3). The number of patients who underwent bone marrow biopsies in this study and had no reticulin is unknown. In one pilot study of serial bone marrows, only 1 of 6 patients who underwent bone marrow biopsies, both before and after treatment with romiplostim, had a mild increase in reticulin. Reticulin is a normal component of the bone marrow, and grade N or grade 1 reticulin40 is found in 69 to 76% and grade 2 reticulin in 4 to 5% of bone marrow biopsy specimens obtained from healthy subjects.40-42 No normal biopsies have grade 3 or 4 reticulin. In a retrospective study of bone marrow biopsy material from ITP patients, Mufti et al found a similar pattern of reticulin staining; no grade 3 or 4 reticulin was found.43 In a prior study in acute myeloid leukemia patients, 8 of 9 patients treated with recombinant human TPO and granulocyte macrophage–colony-stimulating factor (GM-CSF) developed increased bone marrow reticulin, versus only 2 of 6 who received GM-CSF alone.44 Upon discontinuation of the TPO, the reticulin fully reversed within an average of 30 days (range: 13-42). Of the 2 patients in the study reported here in whom follow-up bone marrow biopsies were performed after discontinuation of romiplostim, one showed improvement in the amount of reticulin and the other did not; however, neither had total regression. It is believed that interleukin-11, GM-CSF, and thrombopoietic agents may increase bone marrow reticulin, possibly through local release of transforming growth factor (TGF)–β from (the increased number of) bone marrow megakaryocytes.42 The role of this cytokine in TPO-induced reticulin deposition has also been studied in transgenic mice overexpressing TPO, in which increased bone marrow fibrosis was accompanied by elevated plasma levels of TGF-β.45 In animal models and humans, increased bone marrow reticulin does not seem to be associated with either hematologic abnormalities or progression to clonal myeloproliferative diseases and is reversible.42 Six of the 8 patients with spontaneously reported reticulin in this study had reticulin levels of mild to moderate (grade 2 or less or within the normal range). All patients continue to be monitored for clinical signs of any progressive bone marrow abnormalities. Bone marrow samples taken as part of the supplemental prospective study demonstrated that reticulin was (mildly) increased in only 1 of 6 patients treated with romiplostim. Future studies, including bone marrow biopsies before and after treatment in large numbers of patients with ITP treated with romiplostim, will be required to clarify the incidence and clinical significance of bone marrow reticulin and to determine the degree of regression that occurs upon withdrawal of treatment.
In this study, 12 thrombotic or thromboembolic events were reported in 4.9% of patients (7/142) treated up to 156 weeks. By comparison, incidence rates of 2.4% were observed both in patients with ITP who received romiplostim and in those who received placebo in the two 24-week phase 3 studies; exposure rates were not calculated in these studies.36 As evidence ITP itself may be a prothrombotic disease, in a retrospective series of 186 adults with ITP, thrombotic events were identified by history in 5% of patients.46
During the study, one patient transiently developed neutralizing antibodies against romiplostim but not worsened thrombocytopenia. Romiplostim has no amino acid sequence homology to eTPO, and therefore antiromiplostim antibodies would not be expected to cross-react with eTPO as this one did not.
Conclusions based on the results of this open-label study are limited by the lack of a control group. It is not known how many patients might have improved spontaneously, without treatment over the time of the study; however, given that 60% of patients had failed to respond to splenectomy, it is unlikely that it would have been a substantial number. Similarly, it is hard to judge the incidence of adverse events, but the finding that rates were lower in this study than in the 24-week studies suggests that the longer term usage in this study was not associated with cumulative toxicity. This initial report of increased marrow reticulin, seen in 8 patients in this study, is tempered by the inability to estimate a true incidence of this occurrence.
This study, the longest to date by far of a thrombopoietic agent in the treatment of ITP, shows romiplostim to be an effective and well-tolerated maintenance treatment in patients with chronic ITP for up to 3 years, even in those with severe, refractory disease who had undergone splenectomy. These results extend previous findings that romiplostim induces platelet count increases within 1 to 3 weeks in most patients32 and amplifies the findings of the phase 3 studies,36 which were only of 24 weeks' duration. Additional studies are exploring the ability to use romiplostim as a maintenance treatment with the specific aim of avoiding splenectomy.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the study investigators, coordinators, nurses, and patients and their families for their invaluable contributions to this study. In addition to the authors, the following investigators participated in the study: Louis Aledort, Mount Sinai Hospital (New York, NY); James George, University of Oklahoma Health Sciences Center, Hematology Oncology Section (Oklahoma City, OK); Jeffery Wasser, DeQuattro Community Cancer Center (Manchester, CT); Terry Gernsheimer, Puget Sound Blood Center (Seattle, WA); Alan E. Lichtin, The Cleveland Clinic Foundation, Department of Hematology/Oncology (Cleveland, OH); Jeffrey Andrey, Scripps Clinic (San Diego, CA); Ronald Go, Gundersen Lutheran Health System (La Crosse, WI); M.S. Murali, Central Indiana Cancer Centers-Rama (Indianapolis, IN); Howard Liebman, USC Keck School of Medicine, Division of Hematology (Los Angeles, CA); Robert Redner, University of Pittsburgh Department of Medicine, Hillman Cancer Center (Pittsburgh, PA); Craig Kessler, Georgetown University Medical Center (Washington, DC); Michael Tarantino, Comprehensive Bleeding Disorders Center (Peoria, IL); Gail Macik, University of Virginia Health System (Charlottesville, VA); Solomon Hamburg, Tower Cancer Research Foundation (Beverly Hills, CA); Frank Slovick, Heartland Hematology Oncology Associates (Kansas City, MO); Maria Tirona, Marshall University (Huntington, WV); Gow Arepally, Duke University Medical Center (Durham, NC); Troy Guthrie, Regional Consultants in Hematology and Oncology, Baptist Cancer Institute (Jacksonville, FL); Lawrence Rice, Baylor College of Medicine (Houston, TX); Frank M. Senecal, Northwest Medical Specialties (Tacoma, WA); Barry Firstenberg, Arlington Cancer Center (Arlington, TX); Howard Terebelo, Newland Medical Associates (Southfield, MI); Usha Sunkara, Alta Bates Comprehensive Cancer Center (Berkeley, CA); Emmanuelle Bourgeois, Service des Maladies du Sang, CHRU Claude Huriet (Lille, France); Francois Lefrere, Service d'Hématologie Adulte, Hopital Necker (Paris, France); MC Kappers-Klunne, Erasmus MC (Rotterdam, The Netherlands); Adrian Newland, Department of Hematology, The Royal London Hospital (London, United Kingdom); Martin R. Schipperus, Ziekenhuis Leyenburg, Hematology (Den Haag, The Netherlands); Edo Vellenga, Universitair Medisch centrum Groningen (Groningen, The Netherlands); Carlos Grande, Hospital 12 de Octubre (Madrid, Spain); Miguel Sanz, Hospital La Fe (Valencia, Spain); Jean-Francois Viallard, Médecine Interne–Maladies Infectieuses, Hôpital Haut-Lévêque (Pessac, France); Jose Tongol, Phoebe Cancer Center (Albany, GA); and Robert Hermann, Northwest Georgia Oncology Centers PC (Marietta, GA).
This study was supported in part by research funding from Amgen. James O'Kelly, an employee of Amgen, and Mary Royer, a paid consultant to Amgen, assisted with preparation of the manuscript.
Authorship
Contribution: J.B.B., D.J.K., and V.P. performed the research, interpreted the data, and wrote the paper; R.M.L. performed the research and reviewed the paper; M.G. collected, analyzed, and interpreted the data and edited the paper; and J.L.N. designed the trial, analyzed and interpreted the data, and wrote the paper.
Conflict-of-interest disclosure: J.B.B. and D.J.K. have received research support from Amgen and have served on scientific advisory boards for Ligand; J.B.B. has received research support from Biogen Idec, Cangene, Genentech, GlaxoSmithKline, Genzyme, MGI Pharma, and Sysmex; J.B.B. holds stock in Amgen and GlaxoSmithKline; J.B.B. and V.P. are members of speakers bureau for Amgen; J.B.B., D.J.K., and R.M.L. served on scientific advisory boards for Amgen; J.B.B. served on scientific advisory boards for GlaxoSmithKline and Baxter; R.M.L. has acted as a consultant to Amgen; M.G. is an employee of Amgen, and J.L.N. was an employee of Amgen at the time of this study.
Correspondence: James B. Bussel, Weill Medical College of Cornell University, P609, 525 East 68th Street, New York, NY 10065; e-mail: jbussel@med.cornell.edu.

![Figure 2. Platelet counts and platelet responses by study week. (A) Median (25th [Q1] and 75th [Q3] percentiles) platelet counts by study week. Median platelet counts increased sharply during the first 4 weeks of treatment and then more gradually through week 16. (B) Percentage of patients with a platelet response over time. A platelet response was defined as a platelet count of 50 × 109/L or more that was at least double the platelet count at baseline in the absence of rescue medication within the preceding 8 weeks. After 1 dose, 30% of patients achieved a platelet response, and after 3 doses, 51% achieved a response. Over the course of the study, a platelet response was observed in 87% of all patients.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/10/10.1182_blood-2008-04-150078/4/m_zh80040930030002.jpeg?Expires=1767755940&Signature=O32on8dzJbbP9PlmbNs8J2k73a9SlVYtWxZSt5Vy3iXihGyzYpphDxvtxMQiCU8fc0o3mlHqN8ysHGYX9blYw-76ZPjyi1M7nkJ9sitFlgQspr6Z6~2o45bwEr4iC4x18WlfstxLjhl8VT7bLtFP2aMemn8DC4mvKBUgCh7oOsLWcw3weC7Ucir4aUUYfNKKoOkf3oYK11aKRYVAY~wLcl1ju4M9BRvRqtbkAJGyb~imHDl7QM9Hz7ZzMZLS5rWLf7dMswBgNSRUFpKMpIjE2pXRj7c8~TN6UkPWzCxM5pZw2h-ykjtw4usH2HsyAPxjhAVhRPuNKaYcNrni~e-LYw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

