Abstract
Resistance to apoptosis in CLL B cells is associated with overexpression of Bcl-2 family antiapoptotic proteins. Their expression is endogenous, but is also induced by signals from the microenvironment resulting in intrinsic and extrinsic drug resistance. Because AT-101 binds to the BH3 motif of all Bcl-2–family antiapoptotic proteins, we hypothesized that this molecule could overcome resistance. AT-101 treatment (20 μM for 24 hours) resulted in a median 72% apoptosis in CLL cells (patients; n = 32, P < .001). Stromal cells protected CLL B cells from spontaneous and fludarabine-induced apoptosis (P = .003) by increasing the Mcl-1 protein levels. However, AT-101 induced similar extent of down-regulation of Mcl-1 and apoptosis in CLL lymphocytes cultured in suspension or on stroma (P = .999). Stromal cells expressed undetectable levels of antiapoptotic but high levels of activated ERK and AKT proteins and had low or no apoptosis with AT-101. Collectively, these data demonstrate that AT-101 induces apoptosis in CLL B cells and overcomes microenvironment-mediated resistance while sparing normal stromal cells.
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by relentless accumulation of mature resting B cells in the peripheral blood, bone marrow and lymphatic tissue. This accumulation is due primarily to defective apoptosis rather than increased proliferation.1,2 Malignant B cells express high levels of the antiapoptotic protein Bcl-2, either as a result of epigenetic alterations in Bcl-2 gene regulation3 or because of loss of miR-15a and miR-16-1.4 Another Bcl-2 family member, Mcl-1, is also overexpressed in CLL B cells5 ; its expression is associated with survival, chemoresistance, and B-cell receptor signaling.6
Signals from the microenvironment also amplify antiapoptotic pathways in normal and malignant B cells.1 Increasing evidence suggests that the interactions between CLL B cells and nonmalignant accessory cells in the bone marrow and lymphatic tissue are pivotal to the maintenance of CLL clone.7-10
Accessory cells such as bone marrow stromal cells,7 monocyte-derived nurse-like cells,11,12 and follicular dendritic cells13 can induce the expression of prosurvival proteins such as Mcl-111,13 and Bcl-2.13,14 In vivo, residual leukemic cells, which may contribute to disease relapse, are harbored in association with stromal cells in the bone marrow/lymphatic tissue.14 Collectively, this inherent and acquired expression of Bcl-2 family antiapoptotic proteins protects CLL cells from spontaneous and drug-induced apoptosis. Therefore, therapeutic approaches that target these prosurvival proteins within the tissue microenvironment are needed to overcome stromal cell–mediated resistance and improve treatment outcome.
Recently, small molecule antagonists are being developed to target prosurvival proteins. These include gossypol,15 ABT-737,16,17 and AT-101.18,19 AT-101 is an enantiomer [R(−)-gossypol] or λ-isomer of gossypol that is being tested in phase 1 clinical trials as a single agent for prostate cancer20 or in combination with rituximab for CLL.19,21,22 Importantly, AT-101 binds to the BH3 motif of all major antiapoptotic proteins, with high affinity (eg, 230 nM, 570 nM, and 130 nM for Bcl-2, Bcl-xl, and Mcl-1, respectively).22 With these inhibitory properties, we hypothesized that AT-101 could target all major Bcl-2 family antiapoptotic proteins in CLL lymphocytes inducing cell death when grown alone or in presence of microenvironment. To test this postulate, we used a coculture of CLL cells and stromal cells that protect CLL lymphocytes; stromal cell–leukemic cell cocultures are better models for studying the potential of in vivo interactions to perpetuate CLL B-cell survival.
Methods
All patients provided written informed consent in accordance with the Declaration of Helsinki, and the laboratory protocol was approved by the institutional review board at the University of Texas M. D. Anderson Cancer Center. Isolated lymphocytes were cultured either in suspension or with confluent layers of bone marrow stromal cells (M210B4; ATCC, Manassas, VA) at a ratio of 100:1. After incubation, CLL B cells (which were free-floating) were carefully removed, leaving the adherent stromal layer undisturbed. AT-101 was obtained from Ascenta Therapeutics (San Diego, CA) and F-ara-A fludarabine nucleoside was from Berlex Biosciences (Richmond, CA).
Apoptosis measured by annexin binding assay, protein expression by immunoblots as described previously23 using antibodies for Mcl-1 (sc-819), Bcl-xl (Sc-20 067), Bcl-2 (sc-509), poly ADP-ribose polymerase (PARP; BD Pharmingen, San Diego, CA), total and pAKT (Ser 473) and total and pERK (Cell Signaling Technology, Danvers, MA).
Statistical analyses were done using GraphPad Prism (GraphPad Software, San Diego, CA) and the 2-tailed paired t test.
Results and discussion
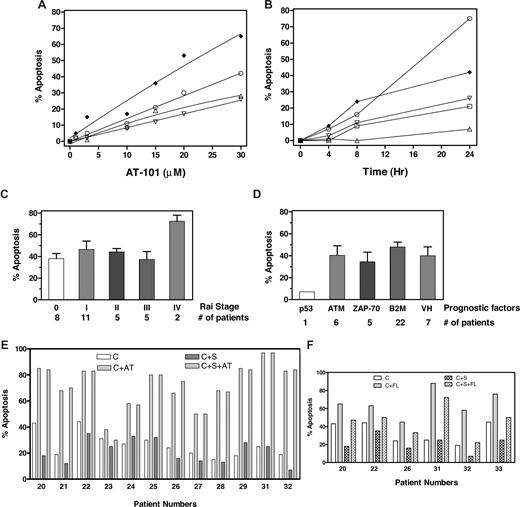
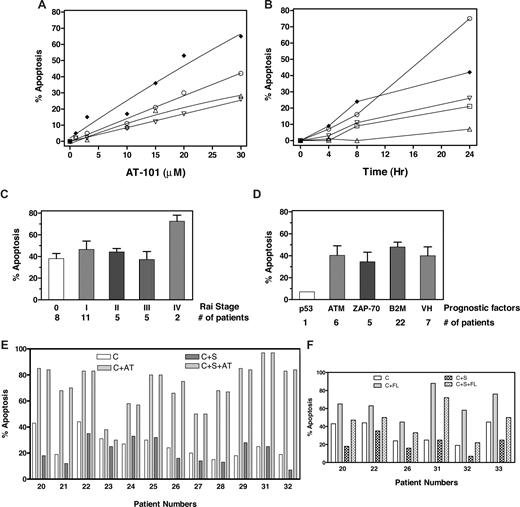
AT-101 induces cytotoxicity in CLL B cells in a dose- (Figure 1A) and time- (Figure 1B) dependent manner with heterogeneity among samples. Some samples did not show any apoptosis until 24 hours; however, most samples responded (> 20% apoptosis) to 24-hour incubation with 20 μM AT-101. This was significantly different from spontaneous apoptosis after 24 hours and increased to a median-72% total apoptosis (range, 12%-97%; n = 32; Table S1 [available on the Blood website; see the Supplemental Materials link at the top of the online article]; P < .001). The apoptotic response was not related to Rai stage (Figure 1C) or other prognostic markers such as ATM mutation, ZAP-70 expression, β2M level, or IgVH gene mutation (Figure 1D). There was only 1 sample for 17p deletion.
AT-101 induced cell death of CLL lymphocytes in both suspension culture as well as stromal coculture. (A,B) Dose and time response to AT-101. CLL lymphocytes in suspension culture were incubated with AT-101 at different concentrations (1, 3, 10, 15, 20, 30 μM; 1A; patients 2 ♦, 18 ○, 4 ▵, and 19 ▿) for 24 hours or with 20 μM AT-101 at 4, 8 and 24 hours; 1B; (patients 2 ♦, 16 ○, 19 ▿, 14 □, and 15 ▵) and the induction of apoptosis was measured by annexin-binding assay. (C,D) AT-101–induced apoptosis is independent of Rai stage or other prognostic factors. CLL lymphocytes in suspension culture were incubated with 20 μM AT-101 for 24 hours and assayed for annexin positivity (“% Apoptosis”). p53 indicates 17p deletion; ATM, 11q deletion; ZAP-70, > 20% ZAP-70 positivity; B2M, β2 microglobulin level higher than 2; and VH, IgVH gene mutation based on less than 96% nucleic acid sequence homology. (E) AT-101–induced apoptosis in CLL B cells was not abolished by stromal cells. CLL lymphocytes from patients (n = 12) were cultured either in suspension medium (C) in suspension medium with 20 μM AT-101 (C + AT), or with stromal cells in the absence (C + S) or presence of AT-101 (C + S + AT) and the apoptosis was measured after 24 hours (72 hours for samples from patients 31 and 32) by annexin-binding assay. The numbers below the abscissa indicate patient numbers, which coincide with the numbers given in Table S1. (F) Fludarabine-induced apoptosis in CLL B cells was reduced by stromal cell cocultures. CLL lymphocytes from patients (n = 6) were either cultured in suspension medium (C), in suspension medium with 10 μM fludarabine (C + FL) or with stromal cells in the absence (C + S) or presence (C + S + FL) of fludarabine, and the apoptosis was measured after 24 hours (72 hours for samples from patients 31 and 32) by annexin-binding assay. Numbers below the abscissa are patient identification numbers, which coincide with the numbers given in Table S1.
AT-101 induced cell death of CLL lymphocytes in both suspension culture as well as stromal coculture. (A,B) Dose and time response to AT-101. CLL lymphocytes in suspension culture were incubated with AT-101 at different concentrations (1, 3, 10, 15, 20, 30 μM; 1A; patients 2 ♦, 18 ○, 4 ▵, and 19 ▿) for 24 hours or with 20 μM AT-101 at 4, 8 and 24 hours; 1B; (patients 2 ♦, 16 ○, 19 ▿, 14 □, and 15 ▵) and the induction of apoptosis was measured by annexin-binding assay. (C,D) AT-101–induced apoptosis is independent of Rai stage or other prognostic factors. CLL lymphocytes in suspension culture were incubated with 20 μM AT-101 for 24 hours and assayed for annexin positivity (“% Apoptosis”). p53 indicates 17p deletion; ATM, 11q deletion; ZAP-70, > 20% ZAP-70 positivity; B2M, β2 microglobulin level higher than 2; and VH, IgVH gene mutation based on less than 96% nucleic acid sequence homology. (E) AT-101–induced apoptosis in CLL B cells was not abolished by stromal cells. CLL lymphocytes from patients (n = 12) were cultured either in suspension medium (C) in suspension medium with 20 μM AT-101 (C + AT), or with stromal cells in the absence (C + S) or presence of AT-101 (C + S + AT) and the apoptosis was measured after 24 hours (72 hours for samples from patients 31 and 32) by annexin-binding assay. The numbers below the abscissa indicate patient numbers, which coincide with the numbers given in Table S1. (F) Fludarabine-induced apoptosis in CLL B cells was reduced by stromal cell cocultures. CLL lymphocytes from patients (n = 6) were either cultured in suspension medium (C), in suspension medium with 10 μM fludarabine (C + FL) or with stromal cells in the absence (C + S) or presence (C + S + FL) of fludarabine, and the apoptosis was measured after 24 hours (72 hours for samples from patients 31 and 32) by annexin-binding assay. Numbers below the abscissa are patient identification numbers, which coincide with the numbers given in Table S1.
Stromal cells have been found to guard CLL B cells from spontaneous and drug-induced apoptosis6-8 ; this protection has been related to the expression of antiapoptotic proteins. To evaluate AT-101 activity, CLL B cells from 12 patients were cultured in suspension or cocultured with M210B4 in presence or absence of 20 μM AT-101 for 24 hours (Figure 1E); 10 μM fludarabine was used as a positive control (Figure 1F). Stromal cells partially protected CLL B cells from spontaneous apoptosis (median 25%; range, 15%-44% without and median 17%; range, 7%-35% with stromal cells; n = 12). AT-101–induced cytotoxicity was similar in both culture conditions (median 77% with stroma and 76% without stroma; range, 30%-97%; n = 12; P = .999), demonstrating that AT-101 is able to overcome stromal cell–mediated protection (Figure 1E). As reported previously,7 total apoptosis induced by fludarabine in CLL B cells (median 64%; range, 45%-76%) was abrogated (median 48%; range, 22%-72%) by stromal cells (n = 6, P = .003; Figure 1F).
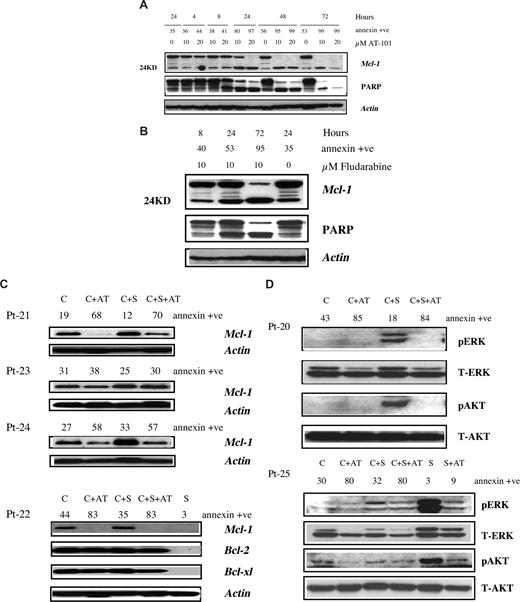
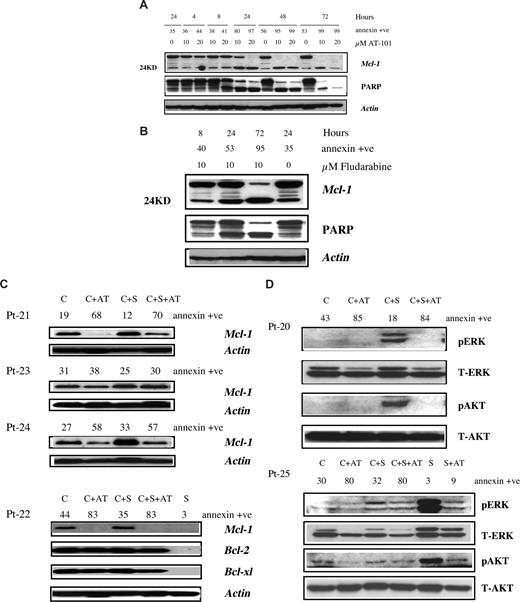
The expression of antiapoptotic proteins in CLL B cells, and influence of AT-101 in presence and absence of microenvironment was determined by immunoblotting. AT-101 treatment resulted in cleavage of Mcl-1 (24KD) in a time- and dose-dependent fashion (Figure 2A). The decrease in full-length Mcl-1 correlated well with annexin positivity and PARP cleavage. In contrast to Mcl-1, AT-101 did not decrease Bcl-2 or Bcl-xl levels in CLL cells (Figure S1A). In fact, it appears that the endogenous Bcl-2 and Bcl-xl levels increased with time suggesting the activation of survival pathway in CLL cells and perhaps its resistance to chemotherapy. Despite high Bcl-2 or Bcl-xl levels, the cells still underwent apoptosis suggesting that Mcl-1 is the critical survival protein for CLL.24 Parallel experiments with fludarabine showed decline in Mcl-1 (Figure 2B), without much effect on Bcl-2 or Bcl-xl (Figure S1B).
Effect of AT-101 and fludarabine on survival proteins in CLL cells in suspension culture or on stromal coculture. (A) Effect of AT-101 on Mcl-1 and PARP cleavage in CLL cells (patient 30) growing in suspension culture. CLL lymphocytes were incubated without or with 10 and 20 μM AT-101 for different time periods and the cleaved and uncleaved Mcl-1 and PARP were measured by immunoblotting. Annexin positivity for each sample is given above the immunoblots. (B) Effect of fludarabine on Mcl-1 and PARP cleavage in CLL cells (patient 30) growing in suspension culture. CLL lymphocytes were incubated without or with 10 μM fludarabine for 8, 24, and 72 hours and cleaved and uncleaved Mcl-1 and PARP were measured by immunoblotting. The annexin positivity for each sample is given above the immunoblots. (C) Antiapoptotic protein expression in CLL cells and influence of stromal microenvironment. CLL lymphocytes from patients were either cultured in suspension (C), in suspension with 20 μM AT-101 (C + AT), or with stromal cells in the absence (C + S) or presence of AT-101 (C + S + AT) for 24 hours. Antiapoptotic protein expression (Mcl-1; Bcl-2; Bcl-xl) was analyzed by immunoblotting. Actin was used as a loading control. S denotes stroma alone. Sample numbers are patient identification (Pt) numbers; see Table S1. (D) Effect of stroma and AT-101 on expression level of ERK and AKT proteins in CLL and stromal cells. CLL lymphocytes from patients were either cultured in suspension (C), in suspension with 20 μM AT-101 (C + AT), or with stromal cells in the absence (C + S) or presence of AT-101 (C + S + AT) for 24 hours. Stroma cells were cultured alone (S) or with AT-101 (S + AT) for 24 hours. pERK and pAKT were measured by immunoblotting, and the T-AKT and T-ERK were used for equal loading. Sample numbers are patient identification (Pt) numbers.
Effect of AT-101 and fludarabine on survival proteins in CLL cells in suspension culture or on stromal coculture. (A) Effect of AT-101 on Mcl-1 and PARP cleavage in CLL cells (patient 30) growing in suspension culture. CLL lymphocytes were incubated without or with 10 and 20 μM AT-101 for different time periods and the cleaved and uncleaved Mcl-1 and PARP were measured by immunoblotting. Annexin positivity for each sample is given above the immunoblots. (B) Effect of fludarabine on Mcl-1 and PARP cleavage in CLL cells (patient 30) growing in suspension culture. CLL lymphocytes were incubated without or with 10 μM fludarabine for 8, 24, and 72 hours and cleaved and uncleaved Mcl-1 and PARP were measured by immunoblotting. The annexin positivity for each sample is given above the immunoblots. (C) Antiapoptotic protein expression in CLL cells and influence of stromal microenvironment. CLL lymphocytes from patients were either cultured in suspension (C), in suspension with 20 μM AT-101 (C + AT), or with stromal cells in the absence (C + S) or presence of AT-101 (C + S + AT) for 24 hours. Antiapoptotic protein expression (Mcl-1; Bcl-2; Bcl-xl) was analyzed by immunoblotting. Actin was used as a loading control. S denotes stroma alone. Sample numbers are patient identification (Pt) numbers; see Table S1. (D) Effect of stroma and AT-101 on expression level of ERK and AKT proteins in CLL and stromal cells. CLL lymphocytes from patients were either cultured in suspension (C), in suspension with 20 μM AT-101 (C + AT), or with stromal cells in the absence (C + S) or presence of AT-101 (C + S + AT) for 24 hours. Stroma cells were cultured alone (S) or with AT-101 (S + AT) for 24 hours. pERK and pAKT were measured by immunoblotting, and the T-AKT and T-ERK were used for equal loading. Sample numbers are patient identification (Pt) numbers.
Mcl-1 protein up-regulation was observed in CLL B cells grown with stromal cells in all 9 patient samples studied, suggesting that bone marrow stromal cells may support CLL B cells via induction of Mcl-1 (Figure 2C). However, AT-101 down-regulated endogenous as well as stroma-induced Mcl-1 levels in CLL cells and the decrease was associated with apoptosis (patient 23 did not show a significant reduction in Mcl-1 and had low apoptosis; Figure 2C). Collectively, these data demonstrate that the stromal cell microenvironment does not abrogate AT-101–mediated decrease of antiapoptotic proteins. In contrast to Mcl-1, there was a minor or no change in Bcl-2 and Bcl-xl (Figure 2C), suggesting that Mcl-1 is induced mostly by the stromal cell support of CLL B cells.11,13
Although abundant in CLL lymphocytes, the Bcl-2 family antiapoptotic proteins were below the level of detection in stromal cells (Figure 2C). In concordance, AT-101 (20 μM for 24 hours) had a minor effect on stromal cell apoptosis (median 12%; n = 7, data not shown). Even longer incubations (72 hours) with AT-101 did not increase apoptosis in stroma cells. These results suggest that absence of target antiapoptotic proteins result in lack of apoptosis. To evaluate differences in response between AT-101 and fludarabine, immunoblots were compared in the same patient sample after a 72-hour treatment for PARP cleavage and decrease in Mcl-1 protein. Stromal cells were able to reduce Mcl-1 decline, PARP cleavage, and apoptosis induced by fludarabine, but this was not the case with AT-101 (Figure S2A,B).
Previous studies25 have demonstrated that PI3K is an essential survival pathway for the CLL B cells and stromal-derived factor 1 (SDF-1) induces phosphorylation of ERK1/2 and AKT in CLL B cells.11,12 Our data showed that 6 of 6 and 3 of 6 samples had an increase in phospho-ERK and phospho-AKT, respectively, in CLL cells in presence of stroma. AT-101 had modest or significant decrease in both these proteins (Figure 2D). This was similar with fludarabine (Figure S3). In contrast to CLL cells, stroma cells showed high levels of phospho-ERK and phospho-AKT proteins, which were decreased by AT-101 treatment (Figure 2D).
In summary, AT-101 induces apoptosis in CLL B cells and overcomes microenvironment-mediated resistance while sparing normal stromal cells. AT-101 acts differently from the current chemotherapeutic agents, providing rationale for testing Bcl-2 antagonists for CLL patients with minimal residual disease and in combination strategies for chemoresistant CLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by Cancer Center Support grants CA57629, CA81534, and P30-16672 from the National Cancer Institute, Department of Health and Human Services (Bethesda, MD); the US/European Alliance of CLL Global Research Foundation (Houston, TX; V.G. and J.A.B.); and an ASCO (Alexandria, VA) Career Development Award (J.A.B.).
National Institutes of Health
Authorship
Contribution: K.B. designed and performed all experiments, analyzed data, and wrote the manuscript; J.A.B. provided expertise in stromal cell coculturing and participated in manuscript writing; W.G.W. identified CLL patients for inclusion in the study and is principal investigator of the laboratory protocol to obtain blood samples; and V.G. conceptualized the research, directed K.B. in experiment design and data analysis, and finalized the text of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Varsha Gandhi, Department of Experimental Therapeutics, Unit 71, University of Texas M. D. Anderson Cancer Center, Houston, TX 77030; e-mail: vgandhi@mdanderson.org.