Abstract
Human interferon (IFN)–α is the standard therapy for chronic hepatitis C to prevent its progression to liver cirrhosis and hepatocellular carcinoma. Thrombocytopenia is one of the major adverse effects of IFN-α and often leads to dose reduction or treatment discontinuation. However, there is little information on how IFN-α inhibits human megakaryopoiesis. In this study, we demonstrated that IFN-α did not inhibit colony formation of megakaryocytes from human CD34+ hematopoietic stem cells. IFN-α did not inhibit endomitosis but did inhibit cytoplasmic maturation of megakaryocytes and platelet production in vitro. IFN-α suppressed the expression of transcription factors regulating late-stage megakaryopoiesis, such as GATA-1, p45NF-E2, MafG. IFN-α also significantly reduced the number of human platelets but not megakaryocytes, and did not inhibit endomitosis of human megakaryocytes in immunodeficient NOD/Shi-scid/IL-2Rγnull (NOG) mice transplanted with human CD34+ cells (hu-NOG). We also demonstrated that a novel thrombopoietin mimetic, NIP-004, was effective for treating IFN-α–induced thrombocytopenia in hu-NOG mice. From ultrastructural study, IFN-α inhibited the maturation of demarcation membranes in megakaryocytes, although NIP-004 prevented the inhibitory effects of IFN-α. These results defined the pathogenesis of IFN-α–induced thrombocytopenia and suggested possible future clinical applications for thrombopoietin mimetics.
Introduction
Human interferon (IFN)-α is the standard treatment for patients with chronic hepatitis C.1,2 It is well known that chronic hepatitis C can lead to liver cirrhosis and hepatocellular carcinoma; the risk of progression to cirrhosis within 5 years is approximately 40%.3 Among patients with compensated liver cirrhosis, the probability of its transition to decompensated cirrhosis and hepatocellular carcinoma within 5 years is approximately 20% and 10%, respectively.4,5 Thus, it is important for chronic hepatitis C patients to receive IFN-α treatment to prevent malignant transformation by eradicating hepatitis C virus.6-11 However, IFN-α–induced thrombocytopenia often leads to dose reduction or discontinuation of IFN-α therapy. In particular, it is difficult to treat patients with advanced cirrhosis by IFN-α because often these patients also have severe thrombocytopenia.
Several studies have suggested that IFN-α induces thrombocytopenia by inhibiting the proliferation of human megakaryocytes, as the number of colony-forming units of megakaryocytes (CFU-MK) is reduced by relatively high concentrations of IFN-α in vitro.12-15 In clinical studies, however, the administration of human IFN-α to patients with chronic hepatitis, solid tumors, and myeloproliferative disorders does not affect the number of megakaryocytes in bone marrow.16-19 We speculate that this discrepancy is caused by differences in the dose of IFN-α and hematopoietic microenvironment between CFU-MK in vitro assays and in vivo studies. Clinical studies have also proposed autoimmune reaction and capillary sequestration as causes of IFN-α–induced thrombocytopenia.18,20-22 Megakaryocytes differentiate from hematopoietic stem cells and undergo endomitosis followed by cytoplasmic maturation.23,24 In endomitosis, megakaryocytes repeat DNA replication without cytokin-esis to develop polyploidy. At the stage of cytoplasmic maturation in megakaryocytes, both the demarcation membrane system and granules develop, which enlarges the cell body. At the final stage of megakaryopoiesis, mature megakaryocytes extend thin protrusions called proplatelets, and their tips are released into the circulation as platelets. The lack of a suitable experimental animal model of human megakaryopoiesis has hampered the study of the mechanism of IFN-α–induced thrombocytopenia. We recently developed a new experimental animal model of human megakaryopoiesis using immunodeficient nonobese diabetic (NOD)/Shi-scid/IL-2Rγnull (NOG) mice transplanted with human CD34+ cells (hu-NOG mice).25 This prompted us to undertake this study, which investigates the mechanism of human IFN-α–induced thrombocytopenia.
Thrombopoietin (TPO) is a lineage-specific cytokine that regulates both proliferation and differentiation of megakaryocytes.26 The receptor for TPO is c-Mpl and c-mpl–deficient mice demonstrated severe thrombocytopenia, with an 85% reduction in the number of platelets and megakaryocytes.27 Although several clinical trials of TPO have demonstrated that recombinant human (rh) TPO and pegylated recombinant human megakaryocyte growth and development factor (PEG-rhMGDF) are effective for treating thrombocytopenia associated with nonmyeloablative chemotherapy, some individuals treated with PEG-rhMGDF have demonstrated thrombocytopenia because of the development of neutralizing antibodies to endogenous TPO,28,29 which led to a clinical trial of rhTPO and PEG-rhMGDF being aborted.
Recently, second-generation of thrombopoietic growth factors such as TPO peptide (AMG 531) and TPO nonpeptide mimetics (eltrombopag, AKR501) have been shown to increase platelet counts in healthy volunteers and patients with chronic idiopathic thrombocytopenic purpura.30-33 We recently created a novel TPO receptor activator, NIP-004.25 NIP-004 is a nonpeptidyl mimetic of TPO and induces colony formation of megakaryocytes from human CD34+ hematopoietic cells by activating the human TPO receptor. Interestingly, NIP-004 displays strict species specificity for the human TPO receptor, as the histidine residue in the transmembrane domain of human TPO receptor is critical for NIP-004 to induce intracellular signaling.25
In this study, we use human primary megakaryocytes to demonstrate that IFN-α inhibits maturation of demarcation membranes and platelet production, but not endomitosis. We also confirm, in a hu-NOG mouse model, that NIP-004 is effective for the treatment of IFN-α–induced thrombocytopenia.
Methods
Reagents and cells
Cytokines, including recombinant human IFN-α2b (Imgenex, San Diego, CA), human PEG-IFN-α2b (PEG-Intron; Schering Plough, Kenilworth, NJ), and recombinant human TPO (R&D Systems, Minneapolis, MN) were obtained as indicated. NIP-004 was chemically synthesized at Nissan Chemical Industries (Chiba, Japan).25 Human bone marrow– and cord blood–derived CD34+ cells were purchased from Lonza Walkersville (Walkersville, MD).
CFU assay
CFU-MK assay was performed using a MegaCult-C (StemCell Technologies, Vancouver, BC). A total of 5 × 103 human bone marrow–derived CD34+ cells were cultured in collagen-based medium containing rhTPO in combination with rhIFN-α2b, in a CO2 incubator for 10 days. After fixation, megakaryocytes were visualized with antihuman CD41a antibody using alkaline phosphatase staining. Nuclei were counterstained with Evans blue. Stained colonies were counted under a microscope (BX51; Olympus, Tokyo, Japan), and colonies, including one or more CD41a-positive megakaryocytes more than 100 μm in diameter were counted separately.
Proplatelet formation assay
Human bone-marrow-derived CD34+ cells were cultured at 105 cells/mL in StemSpan serum-free expansion medium (StemCell Technologies) supplemented with 40 μg/mL low density lipoprotein and 10 ng/mL TPO at 37°C with 5% CO2 for 7 days. Megakaryocytes were enriched by velocity sedimentation, as described by Choi et al.34 Briefly, cultured cells were suspended in CATCH buffer (phosphate-buffered saline with 13.6 mM sodium citrate, 2.2 μM prostaglandin E1, 1 mM theophylline, and 1 mM glucose) at 5 × 105 cells/mL and were subjected to a 2-step bovine serum albumin (BSA) gradient, 2.41% (2.5 mL) and 4.83% (5.0 mL), at 1 g for 1.5 hours at room temperature. Large megakaryocytes were collected from the bottom layer and cultured with 10 ng/mL of TPO in combination with 0 to 10 ng/mL of IFN-α2b in 96-well plates for 4 to 6 days. Proplatelet-displaying megakaryocytes were defined as cells that exhibited one or more filament-like protrusions with tips, and were counted under an inverted microscope at a magnification of ×200. We counted 500 cells and calculated the percentage of megakaryocytes with proplatelets. Three independent experiments were performed using 3 different donor cells.
Fluorescent images
Human CD34+ bone marrow–derived cells were cultured with 10 ng/mL TPO for 7 days. Large megakaryocytes were collected by BSA velocity sedimentation and cultured with 10 ng/mL TPO in combination with 0 or 10 ng/mL IFN-α2b on poly-D-lysine culture slide (BD Biosciences, San Jose, CA) for 5 days. Culture-derived cells were fixed with 1% paraformaldehyde for 15 minutes at room temperature and then permeabilized with 0.2% Triton X-100 for 5 minutes at room temperature. Cells were blocked with 2% fetal bovine serum in phosphate-buffered saline for 1 hour and stained with Alexa Fluor 555-conjugated β-tubulin (9F3) antibody (Cell Signaling Technology, Danvers, MA) overnight at 4°C. Images were captured by confocal laser and fluorescence microscopy. Single-photon confocal fluorescence images were collected using a Zeiss LSM510 (Carl Zeiss, Welwyn Garden City, United Kingdom) coupled to an inverted microscope (Zeiss Axiovert 100) equipped with a 63×/1.4 numeric aperture oil objective. Alexa Fluor 555 was excited at 535 nm and emissions were collected above 560 nm.
Real-time quantitative reverse-transcriptase polymerase chain reaction
Total RNA was extracted using a RNeasy Mini Extraction Kit (Qiagen, Hilden, Germany), and 0.5 μg of RNA was subjected to reverse transcription using a SuperScript First-Strand Synthesis System for reverse-transcriptase polymerase chain reaction (RT-PCR; Invitrogen, Carlsbad, CA). TaqMan Gene Expression Assays were used for RT-PCR following the manufacturer's instructions. The plate was run on an Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, Foster, CA). All reactions were normalized with the respective hypoxanthine ribosyl transferase (HPRT) mRNA levels, and experiments were performed in triplicate. HPRT was chosen as an endogenous control from the results using a TaqMan Human Endogenous Control Plate (Applied Biosystems).
In vivo assay
Immunodeficient NOG mice were created at the Central Institute for Experimental Animals (Kawasaki, Kanagawa, Japan) and were maintained under specific pathogen-free conditions.25 NOG mice were provided with sterile water containing prophylactic neomycin sulfate (Invitrogen). After 2.4 Gy irradiation, 105 human umbilical cord blood–derived CD34+ cells were intravenously injected into NOG mice. Three months later, PEG-IFN-α2b was subcutaneously administered into the NOG mice 3 times weekly for 3 weeks. After we confirmed PEG-IFN-α2b–induced thrombocytopenia, NIP-004 was administered in combination with 30 μg/kg PEG-IFN-α2b for an additional 4 weeks. The number of human platelets was measured by flow cytometry using species-specific antibodies against human CD41 and Flow-Count Fluorospheres (Beckman Coulter, Fullerton, CA). For measuring the life span of human platelets in hu-NOG mice, in vivo biotinylation was performed as previously described.35 Briefly, 3 mg sulfo-NHS-LC-biotin (Pierce Chemical, Rockford, IL) was dissolved in 300 μL saline, and 150 μL of the solution was injected intravenously. Blood from biotinylated mice was stained by antibodies against human CD41a and CD42b and phycoerythrin Texas red (ECD)–labeled streptavidin (BD Biosciences PharMingen, San Diego, CA). Samples were analyzed by flow cytometry to obtain the number of biotinylated human platelets. From plots of the number of biotinylated human platelets vs time, an estimate of the life span was obtained by linear extrapolation. Animal experiments were conducted according to the guidelines for animal experiments36 of the Japanese Association for Laboratory Animal Science. All experimental protocols were approved by the ethics review committees for animal experiments of Keio University and Nissan Chemical Industries.
Flow cytometry
Multicolor flow cytometry was performed using an EPICS-XL flow cytometer (Beckman Coulter) as previously described.25 Antibodies used in this study were as follows: antihuman CD33-fluorescein isothiocyanate (FITC), CD3-FITC, CD41a-FITC, CD71-FITC, CD42a-FITC, CD34-phycoerythrin (PE), CD41a-PE, glycophorin A (GPA)-PE, CD42b-PE, CD19-PE, CD45-ECD, CD41a-PE 5 succinimidylester (PC5), CD38-PC5, antimurine CD45-FITC, and CD41-FITC. Antibodies against antihuman CD45-ECD were purchased from Immunotech (Marseille, France). Other antibodies were purchased from BD Biosciences PharMingen. The actual number of platelets and bone marrow cells in hu-NOG mice and culture-derived platelets was measured by flow cytometry using the indicated species-specific antibodies and Flow-Count Fluorospheres. To analyze human megakaryocyte ploidy, culture-derived cells and bone marrow cells from hu-NOG mice were stained with antihuman CD41a-PE antibody and fixed with 1% paraformaldehyde. Fixed cells were treated with Tween 20 and 7-amino-actinomycin D (7-AAD) dye (Immunotech) followed by 2-color cytometric analysis.
Electron microscopy
Human bone marrow–derived CD34+ cells were cultured with 10 ng/mL of TPO for 7 days. Large megakaryocytes were collected by BSA velocity sedimentation and cultured with 10 ng/mL TPO in combination with 0 or 10 ng/mL IFN-α2b and 0 or 1 μg/mL NIP-004 for 5 days. Culture-derived cells were fixed in 2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 60 minutes at 4°C. The samples were washed, then fixed with 1% osmium tetroxide in 0.1 M phosphate buffer for 60 minutes at 4°C, dehydrated with a graded ethanol series, and embedded in Epon (TAAB Laboratories, Aldermaston, United Kingdom), as described previously.37 Ultrathin sections were prepared, stained with uranyl acetate and lead citrate, and then examined with a JEM1200EX transmission electron microscope (JEOL, Tokyo, Japan) at an accelerating voltage of 80 kV. To evaluate the differentiation of megakaryocytes, we divided megakaryocytes into 3 types according to their developmental stage. We defined immature megakaryocytes, with a large round nucleus but no demarcation membrane system, as type 1 megakaryocytes. Type 2 megakaryocytes were the intermediate stage between types 1 and 3 megakaryocytes. Type 3 were fully matured megakaryocytes, in which the nucleus was pushed to the side of the cell, cytoplasm was abundant, and the area of the demarcation membrane system was more than 20% of the cell body. We counted more than 100 megakaryocytes by electron microscopy and calculated the percentage of each type.
Statistical analysis
Comparisons among groups were performed by Student t test. All tests were 2-sided and P less than .05 was considered significant.
Results
Colony-forming assay of megakaryocytes using human CD34+ hematopoietic stem cells
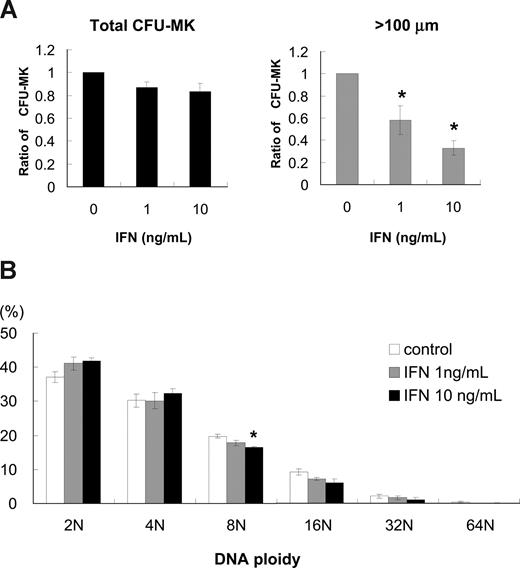
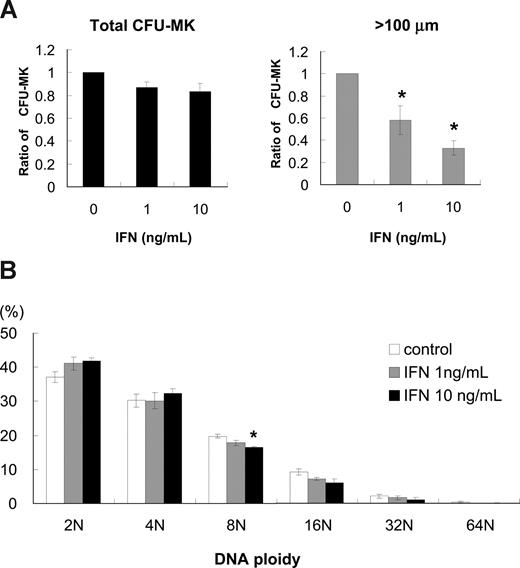
To investigate how IFN-α reduces the number of platelets, we performed colony-forming assays of megakaryocytes using human bone marrow–derived CD34+ cells. IFN-α2b at 1 and 10 ng/mL reduced the number of CFU-MK by 13% and 18%, respectively; however, these decreases were not statistically significant (Figure 1A left panel). The average CFU-MK was calculated from 3 independent experiments. We noticed that the size of megakaryocytes treated with IFN-α was smaller than that without IFN-α; thus, we counted the number of CFU-MK that included one or more megakaryocytes more than 100 μm in diameter. IFN-α2b at 1 and 10 ng/mL significantly reduced the number of CFU-MK that included at least one large megakaryocyte by 42% and 67%, respectively (P < .05, n = 3; Figure 1A right panel).
Effects of IFN-α2b on colony formation and DNA ploidy of primary human megakaryocytes. (A) Human bone marrow–derived CD34+ cells were treated with 10 ng/mL TPO and 0, 1, or 10 ng/mL IFN-α2b for 10 days. (Left graph) Total number of CFU-MK. The average number was calculated from 3 independent experiments. (Right graph) Number of CFU-MK that included one or more megakaryocytes more than 100 μm in diameter. Diameters were measured under a microscope using a microscale within an eyepiece. Data are means plus or minus SEM from the results of 3 independent experiments (*P < .05 vs 0 ng/mL IFN-α2b). (B) Effects of IFN-α2b on DNA ploidy of megakaryocytes in vitro. Human bone marrow–derived CD34+ cells were cultured with 10 ng/mL of TPO in combination with 0, 1, or 10 ng/mL IFN-α2b. Megakaryocytes were stained with antihuman CD41a-PE antibody and 7-AAD dye, and examined by 2-color cytometry. Data are means plus or minus SEM from the results of 3 independent experiments (*P < .05 vs 0 ng/mL IFN-α2b).
Effects of IFN-α2b on colony formation and DNA ploidy of primary human megakaryocytes. (A) Human bone marrow–derived CD34+ cells were treated with 10 ng/mL TPO and 0, 1, or 10 ng/mL IFN-α2b for 10 days. (Left graph) Total number of CFU-MK. The average number was calculated from 3 independent experiments. (Right graph) Number of CFU-MK that included one or more megakaryocytes more than 100 μm in diameter. Diameters were measured under a microscope using a microscale within an eyepiece. Data are means plus or minus SEM from the results of 3 independent experiments (*P < .05 vs 0 ng/mL IFN-α2b). (B) Effects of IFN-α2b on DNA ploidy of megakaryocytes in vitro. Human bone marrow–derived CD34+ cells were cultured with 10 ng/mL of TPO in combination with 0, 1, or 10 ng/mL IFN-α2b. Megakaryocytes were stained with antihuman CD41a-PE antibody and 7-AAD dye, and examined by 2-color cytometry. Data are means plus or minus SEM from the results of 3 independent experiments (*P < .05 vs 0 ng/mL IFN-α2b).
The effects of IFN-α2b on DNA ploidy of human megakaryocytes in vitro
To investigate whether the reduction of megakaryocyte cell size by IFN-α2b was caused by the inhibition of endomitosis, we measured DNA ploidy of megakaryocytes by flow cytometry. We cultured human bone marrow–derived CD34+ cells with 10 ng/mL of TPO in combination with 0, 1, and 10 ng/mL IFN-α2b for 10 days and analyzed DNA ploidy of megakaryocytes. IFN-α2b did not alter DNA ploidy of human megakaryocytes except 8N with 10 ng/mL IFN-α2b (P < .05, n = 3; Figure 1B). Three independent experiments were performed to confirm reproducibility.
IFN-α2b inhibited platelet production in vitro
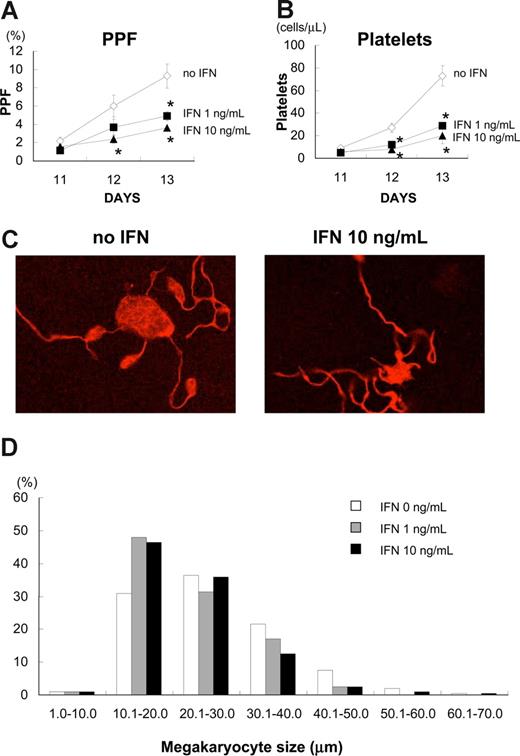
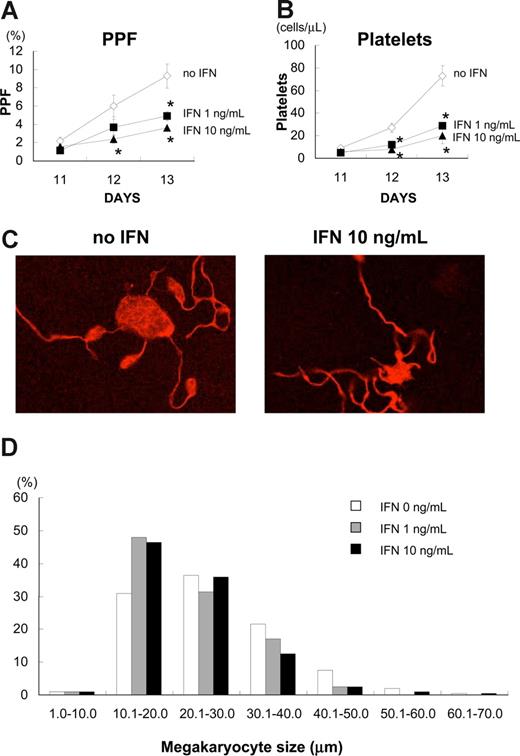
As we found that IFN-α2b did not inhibit endomitosis of megakaryocytes, we investigated the effects of IFN-α2b on proplatelet formation (PPF) and platelet production, using primary human megakaryocytes. We defined PPF as megakaryocytes that displayed at least one filament-like extension with tips, and counted 500 cells in 3 independent experiments (Figure 2). IFN-α2b significantly inhibited PPF in a dose-dependent manner (Figure 2A). IFN-α2b at 1 and 10 ng/mL inhibited PPF by 39% and 60%, respectively, on day 12, and by 47% and 61%, respectively, on day 13 (P < .05, n = 3).
IFN-α2b inhibited proplatelet formation (PPF) and production of platelets from primary human megakaryocytes. Human bone marrow–derived CD34+ cells were cultured with 10 ng/mL TPO for 7 days. After collecting large megakaryocytes using velocity sedimentation, we maintained the cells with 10 ng/mL TPO in combination with 10 ng/mL IFN-α2b in 96-well plates for 6 days. (A) The numbers of megakaryocytes displaying PPF were counted under an inverted microscope at ×200. We counted 500 cells for each sample 3 times. Data are means plus or minus SEM (n = 3). (B) Platelets in the culture supernatant of primary human megakaryocytes were stained with antihuman CD41a and CD42a antibodies and counted by flow cytometry. Data are means plus or minus SEM (n = 3). (C) A representative picture of PPF with TPO and TPO plus IFN-α2b. Megakaryocytes were stained with Alexa Fluor 555-conjugated β-tubulin (9F3) antibody on day 12 and photographed under confocal laser microscopy. (D) IFN-α2b decreased the size of human megakaryocytes. Megakaryocytes were collected on day 13 and spun down on to glass slides. After staining with Wright-Giemsa solution, the diameters of the megakaryocytes were measured with a microscope using a scale within an eyepiece.
IFN-α2b inhibited proplatelet formation (PPF) and production of platelets from primary human megakaryocytes. Human bone marrow–derived CD34+ cells were cultured with 10 ng/mL TPO for 7 days. After collecting large megakaryocytes using velocity sedimentation, we maintained the cells with 10 ng/mL TPO in combination with 10 ng/mL IFN-α2b in 96-well plates for 6 days. (A) The numbers of megakaryocytes displaying PPF were counted under an inverted microscope at ×200. We counted 500 cells for each sample 3 times. Data are means plus or minus SEM (n = 3). (B) Platelets in the culture supernatant of primary human megakaryocytes were stained with antihuman CD41a and CD42a antibodies and counted by flow cytometry. Data are means plus or minus SEM (n = 3). (C) A representative picture of PPF with TPO and TPO plus IFN-α2b. Megakaryocytes were stained with Alexa Fluor 555-conjugated β-tubulin (9F3) antibody on day 12 and photographed under confocal laser microscopy. (D) IFN-α2b decreased the size of human megakaryocytes. Megakaryocytes were collected on day 13 and spun down on to glass slides. After staining with Wright-Giemsa solution, the diameters of the megakaryocytes were measured with a microscope using a scale within an eyepiece.
We also counted the number of culture-derived platelets by flow cytometry. The platelets produced from primary human megakaryocytes in the culture supernatant were collected and stained with antihuman CD41a and CD42a antibodies. IFN-α2b at 1 and 10 ng/mL reduced the number of culture-derived platelets by 60% and 73%, respectively, on day 13 (P < .05, n = 3; Figure 2B). We performed 3 independent experiments using 3 different donors to confirm reproducibility. IFN-α2b did not change the appearance of proplatelets, such as branches, shafts, and tips (Figure 2C), although it significantly inhibited the percentage of megakaryocytes demonstrating PPF and the count of culture-derived platelets (Figure 2A,B). Because PPF and the number of platelets reflect the development of the demarcation membrane system in cytoplasm, we measured the size of each megakaryocyte on day 13; these were then cytospun on to glass slides and stained with Giemsa solution (Figure 2D). IFN-α2b at 1 and 10 ng/mL decreased the mean size of human megakaryocytes from 27.3 (0 ng/mL) to 23.5 and 23.7 μm, respectively.
IFN-α2b suppressed mRNA expression of transcription factors regulating late-stage megakaryopoiesis
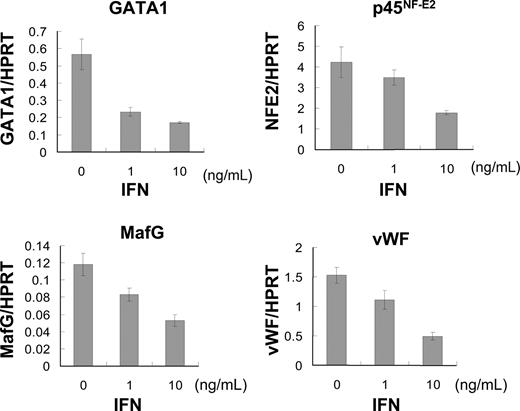
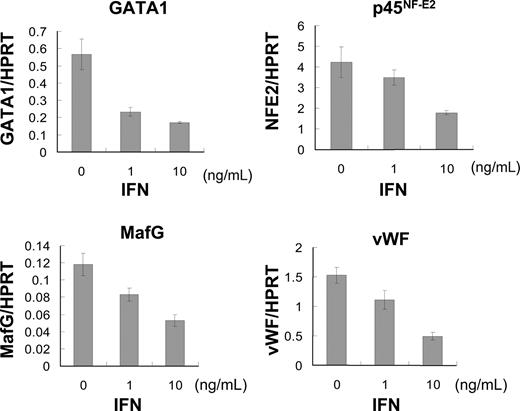
To clarify the molecular mechanism of how IFN-α2b inhibits cytoplasmic maturation and platelet production in megakaryocytes, we analyzed mRNA expression of GATA-1, p45NF-E2, MafG, and VWF using quantitative PCR. The transcription factors GATA-1, p45NF-E2, and MafG are reported to be involved in late-stage megakaropoiesis.38-40 VWF in α-granules is a marker of cytoplasmic maturation of megakaryocytes.41 We extracted total RNA from human megakaryocytes that were cultured with 10 ng/mL TPO and 0, 1, and 10 ng/mL IFN-α2b for 5 days after BSA-based velocity sedimentation. The mRNA expression of these transcription factors was inhibited by IFN-α2b in a dose-dependent manner (Figure 3). IFN-α2b at 10 ng/mL suppressed the mRNA expression levels of GATA-1, p45NF-E2, MafG, and VWF by 70%, 58%, 55%, and 68%, respectively (Figure 3).
IFN-α2b suppressed expression of transcription factors regulating late-stage megakaryopoiesis. Human bone marrow–derived CD34+ cells were incubated with 10 ng/mL TPO for 7 days. Large megakaryocytes were enriched by velocity sedimentation, followed by 6 days of incubation with 10 ng/mL TPO and 0, 1, or 10 ng/mL IFN-α2b. Total RNA was extracted from these megakaryocytes. TaqMan Gene Expression Assay was used for real-time PCR. All data were standardized with respective HPRT mRNA levels. Data are means plus or minus SEM of 3 samples.
IFN-α2b suppressed expression of transcription factors regulating late-stage megakaryopoiesis. Human bone marrow–derived CD34+ cells were incubated with 10 ng/mL TPO for 7 days. Large megakaryocytes were enriched by velocity sedimentation, followed by 6 days of incubation with 10 ng/mL TPO and 0, 1, or 10 ng/mL IFN-α2b. Total RNA was extracted from these megakaryocytes. TaqMan Gene Expression Assay was used for real-time PCR. All data were standardized with respective HPRT mRNA levels. Data are means plus or minus SEM of 3 samples.
IFN-α2b decreased the number of human platelets without affecting the number and DNA ploidy of human megakaryocytes in hu-NOG mice
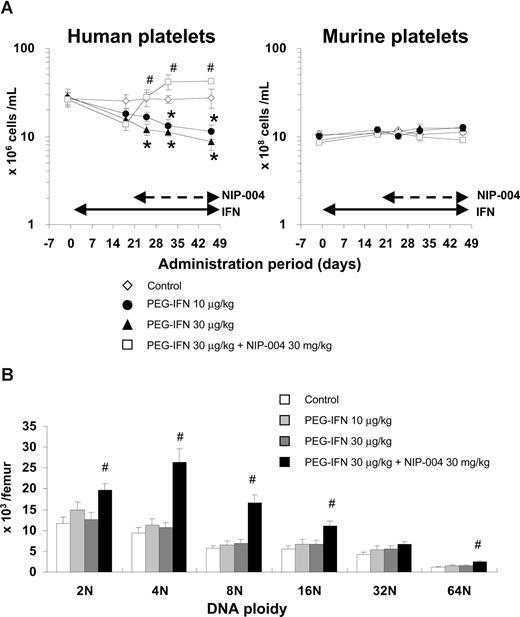
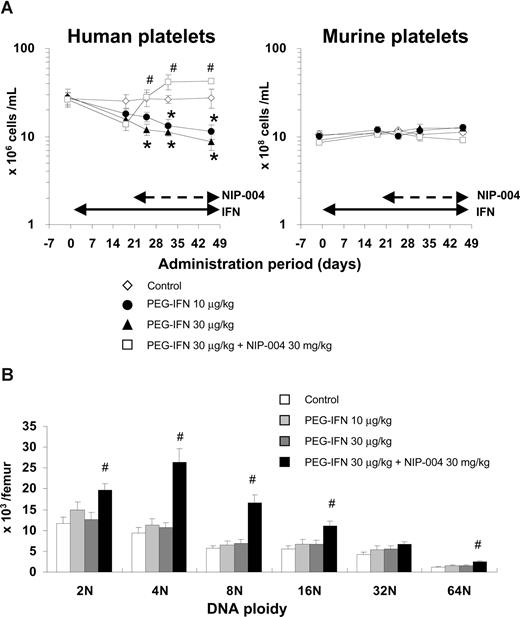
To evaluate the effects of human IFN-α2b on human megakaryopoiesis in vivo, we transplanted human umbilical cord blood–derived CD34+ cells into NOG mice. We administered PEG-IFN-α2b at 10 and 30 μg/kg to hu-NOG mice 3 times weekly for 7 weeks and found that IFN-α2b significantly reduced the number of human platelets by 59% and 68%, respectively, compared with that in the control mice (Figure 4A left panel). The number of murine platelets was not changed by PEG-IFN-α2b because human IFN-α2b does not affect murine cells (Figure 4A right panel). To precisely analyze the effects of PEG-IFN-α2b on human hematopoiesis, we counted the number in each lineage of human hematopoietic cells in bone marrow of hu-NOG mice, using flow cytometry (Table 1). PEG-IFN-α2b at 10 or 30 μg/kg 3 times weekly for 7 weeks did not reduce the number of human CD41+ megakaryocytes. By contrast, PEG-IFN-α2b significantly reduced the number of human CD45+CD33+ myeloid cells and CD45−CD71+GPA+ erythroblasts in the bone marrow of hu-NOG mice (Table 1). None of the lineages of murine hematopoietic cells was changed by PEG-IFN-α2b (data not shown) because of species specificity. As PEG-IFN-α2b decreased the number of human platelets in hu-NOG mice, we evaluated the effects of PEG-IFN-α2b on endomitosis of human megakaryocytes by flow cytometry. PEG-IFN-α2b had no effects on DNA ploidy of human megakaryocytes in hu-NOG mice (Figure 4B), which was consistent with the data from in vitro experiments.
Effects of IFN-α2b and NIP-004 on human platelets and megakaryocytes in hu-NOG mice. Human cord blood–derived CD34+ cells were transplanted into immunodeficient NOG mice (hu-NOG). Three months after transplantation, human PEG-IFN-α2b was administered to hu-NOG mice 3 times weekly for 7 weeks. NIP-004 was administered daily to hu-NOG mice for 4 weeks, 3 weeks after initial treatment with PEG-IFN-α2b. (A) The number of human platelets was decreased by PEG-IFN-α2b in a dose-dependent manner. NIP-004 reversed the decrease in human platelets. By contrast, the number of murine platelets in hu-NOG mice was not changed because of the species specificity of human PEG-IFN-α2b and NIP-004. (B) DNA ploidy of human megakaryocytes in hu-NOG mice. Bone marrow cells collected from hu-NOG mice were stained with antihuman CD41a antibody and 7-AAD dye. The number of each ploidy in human megakaryocytes was measured by flow cytometry using Flow-Count Fluorospheres. Data are means plus or minus SEM from 5 mice (*P < .05 vs control; #P < .05 vs PEG-IFN-α2b 30 μg/kg).
Effects of IFN-α2b and NIP-004 on human platelets and megakaryocytes in hu-NOG mice. Human cord blood–derived CD34+ cells were transplanted into immunodeficient NOG mice (hu-NOG). Three months after transplantation, human PEG-IFN-α2b was administered to hu-NOG mice 3 times weekly for 7 weeks. NIP-004 was administered daily to hu-NOG mice for 4 weeks, 3 weeks after initial treatment with PEG-IFN-α2b. (A) The number of human platelets was decreased by PEG-IFN-α2b in a dose-dependent manner. NIP-004 reversed the decrease in human platelets. By contrast, the number of murine platelets in hu-NOG mice was not changed because of the species specificity of human PEG-IFN-α2b and NIP-004. (B) DNA ploidy of human megakaryocytes in hu-NOG mice. Bone marrow cells collected from hu-NOG mice were stained with antihuman CD41a antibody and 7-AAD dye. The number of each ploidy in human megakaryocytes was measured by flow cytometry using Flow-Count Fluorospheres. Data are means plus or minus SEM from 5 mice (*P < .05 vs control; #P < .05 vs PEG-IFN-α2b 30 μg/kg).
IFN-α did not promote the clearance of human platelets in hu-NOG mice
To exclude the possibility that IFN-α may facilitate the clearance of human platelets in hu-NOG mice by activating human platelets or macrophages, we measured the life span of human platelets in hu-NOG mice by labeling them with biotin. When hu-NOG mice were treated with PEG-IFN-α2b 30 μg/kg 3 times weekly for 3 weeks, the number of human platelets was decreased by 47%, and the life span of human platelets was 6.1 (± 0.2) days (mean ± SEM, n = 5). In control hu-NOG mice treated with vehicle, the life span of human platelets was 6.0 (± 0.2) days (n = 4). Because the life span of human platelets was not changed by IFN-α2b treatment, we concluded that IFN-α2b decreased human platelet count by inhibiting platelet production, but not by promoting their clearance.
NIP-004 was effective for treating IFN-α–induced thrombocytopenia in hu-NOG mice
We recently developed a novel nonpeptidyl Mpl activator, NIP-004, that can induce formation of CFU-MK and increase human platelets in hu-NOG mice.25 To evaluate the effects of NIP-004 on IFN-α–induced thrombocytopenia, we administered NIP-004 30 mg/kg per day to hu-NOG mice after 3 weeks of treatment with PEG-IFN-α2b. NIP-004 restored human platelet counts in hu-NOG mice undergoing treatment with PEG-IFN-α2b 30 μg/kg (Figure 4A). NIP-004 30 mg/kg per day significantly increased the number of human megakaryocytes in all DNA ploidy classes but the 32N class with statistically significant differences in hu-NOG mice (Figure 4B; Table 1). NIP-004 did not affect murine megakaryopoiesis because it does not work for murine cells, as previously described (Figure 4A right panel, and data not shown).25
IFN-α2b inhibited the development of the demarcation membrane system in human megakaryocytes
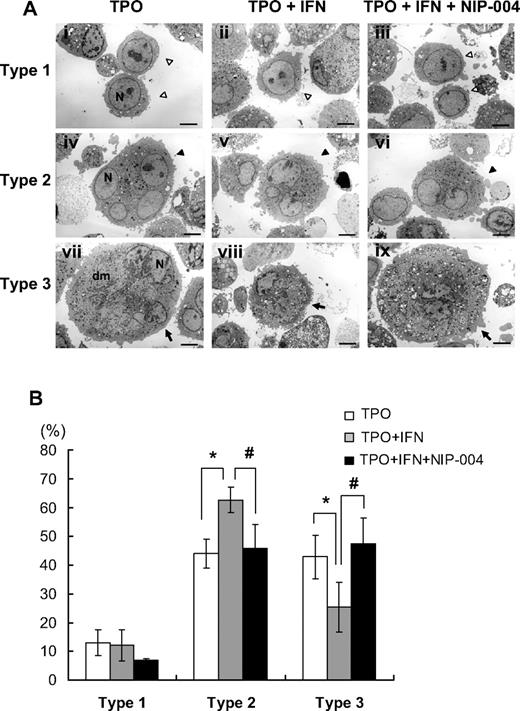
To analyze the effects of IFN-α2b on cytoplasmic maturation of human megakaryocytes, ultrastructural study using electron microscopy was performed. When human megakaryocytes were cultured with TPO, almost half the cells had a lobulated nucleus and abundant cytoplasm with a mature demarcation membrane system (Figure 5Avii). The percentages of each type of megakaryocyte were 13% for type 1 (immature; Figure 5Ai), 44% for type 2 (intermediate; Figure 5Aiv), and 43% for type 3 (mature; Figure 5Avii). When megakaryocytes were cultured with TPO and IFN-α2b, the percentages of each type were 12% for type 1 (Figure 5Aii), 63% for type 2 (Figure 5Av), and 25% for type 3 (Figure 5Aviii). The percentage of type 3 megakaryocytes was reduced by 42% and type 2 was increased by 43% with IFN-α2b compared with controls (Figure 5B). For each type, we observed no obvious effects of IFN-α2b on the number of granules or the appearance of mitochondria and nuclei. We performed 4 experiments separately to confirm reproducibility and to calculate the average and standard deviation (Figure 5B). These results indicated that IFN-α2b inhibited the maturation of the demarcation membrane system in primary human megakaryocytes. Next, we tested whether NIP-004 inhibited the effects of IFN-α2b on the maturation of the demarcation membrane system. When megakaryocytes were cultured with TPO plus IFN-α2b plus NIP-004, the percentages of each type were 7% for type 1 (Figure 5Aiii), 46% for type 2 (Figure 5Avi), and 47% for type 3 (Figure 5Aix). These results indicated that NIP-004 prevented the inhibitory effects of IFN-α2b on the maturation of the demarcation membrane system in primary human megakaryocytes (Figure 5B). We performed 2 experiments separately to confirm reproducibility (P < .05).
Ultrastructure of megakaryocytes cultured with or without IFN-α2b. (A) Ultrastructures of representative human megakaryocytes cultured with 10 ng/mL TPO, 10 ng/mL TPO plus 10 ng/mL IFN-α2b, and 10 ng/mL TPO plus 10 ng/mL IFN-α2b plus 1 μg/mL NIP-004. ◁ represent type 1 immature megakaryocytes (i-iii); ◀, type 2 intermediate megakaryocytes (iv-vi);  , type 3 fully matured megakaryocytes (vii-ix). The definition of each type is in “Electron microscopy.” Original magnification, ×3000. Scale bar represents 4 μm. N indicates nucleus; Dm, demarcation membranes. (B) The percentage of each type of megakaryocytes. IFN-α2b decreased type 3 megakaryocytes and increased type 2 compared with control (TPO) with statistically significant differences, indicating that IFN-α2b inhibits the development of the demarcation membrane system of megakaryocytes. Data are means plus or minus SD from the results of 4 independent experiments (*P < .05 vs TPO). NIP-004 prevented the inhibitory effects of IFN-α2b on the development of the demarcation membrane system of megakaryocytes. Data are means plus or minus SD from the results of 2 independent experiments (#P < .05 vs TPO + IFN).
, type 3 fully matured megakaryocytes (vii-ix). The definition of each type is in “Electron microscopy.” Original magnification, ×3000. Scale bar represents 4 μm. N indicates nucleus; Dm, demarcation membranes. (B) The percentage of each type of megakaryocytes. IFN-α2b decreased type 3 megakaryocytes and increased type 2 compared with control (TPO) with statistically significant differences, indicating that IFN-α2b inhibits the development of the demarcation membrane system of megakaryocytes. Data are means plus or minus SD from the results of 4 independent experiments (*P < .05 vs TPO). NIP-004 prevented the inhibitory effects of IFN-α2b on the development of the demarcation membrane system of megakaryocytes. Data are means plus or minus SD from the results of 2 independent experiments (#P < .05 vs TPO + IFN).
Ultrastructure of megakaryocytes cultured with or without IFN-α2b. (A) Ultrastructures of representative human megakaryocytes cultured with 10 ng/mL TPO, 10 ng/mL TPO plus 10 ng/mL IFN-α2b, and 10 ng/mL TPO plus 10 ng/mL IFN-α2b plus 1 μg/mL NIP-004. ◁ represent type 1 immature megakaryocytes (i-iii); ◀, type 2 intermediate megakaryocytes (iv-vi);  , type 3 fully matured megakaryocytes (vii-ix). The definition of each type is in “Electron microscopy.” Original magnification, ×3000. Scale bar represents 4 μm. N indicates nucleus; Dm, demarcation membranes. (B) The percentage of each type of megakaryocytes. IFN-α2b decreased type 3 megakaryocytes and increased type 2 compared with control (TPO) with statistically significant differences, indicating that IFN-α2b inhibits the development of the demarcation membrane system of megakaryocytes. Data are means plus or minus SD from the results of 4 independent experiments (*P < .05 vs TPO). NIP-004 prevented the inhibitory effects of IFN-α2b on the development of the demarcation membrane system of megakaryocytes. Data are means plus or minus SD from the results of 2 independent experiments (#P < .05 vs TPO + IFN).
, type 3 fully matured megakaryocytes (vii-ix). The definition of each type is in “Electron microscopy.” Original magnification, ×3000. Scale bar represents 4 μm. N indicates nucleus; Dm, demarcation membranes. (B) The percentage of each type of megakaryocytes. IFN-α2b decreased type 3 megakaryocytes and increased type 2 compared with control (TPO) with statistically significant differences, indicating that IFN-α2b inhibits the development of the demarcation membrane system of megakaryocytes. Data are means plus or minus SD from the results of 4 independent experiments (*P < .05 vs TPO). NIP-004 prevented the inhibitory effects of IFN-α2b on the development of the demarcation membrane system of megakaryocytes. Data are means plus or minus SD from the results of 2 independent experiments (#P < .05 vs TPO + IFN).
Discussion
IFN-α has been proposed to induce thrombocytopenia mainly by inhibiting proliferation and maturation of megakaryocytes.12-15 Autoimmune-based destruction of platelets20,21 and capillary sequestration have also been proposed as causes of IFN-α–induced thrombocytopenia.18,20-22 In this study, we used primary human megakaryocytes to successfully demonstrate that IFN-α2b inhibited cytoplasmic maturation and platelet production of megakaryocytes, but not proliferation and endomitosis. Leukemia cell lines do not demonstrate PPF or produce platelets; thus, in this study, we used human primary megakaryocytes for in vitro assays and in vivo models to analyze the mechanism of IFN-induced thrombocytopenia.
Because IFN-α2b did not decrease the number of CFU-MK using human CD34+ cells, we speculate that IFN-α2b does not inhibit the commitment of CD34+ cells to the megakaryocytic lineage and proliferation of immature megakaryocytes, but it does inhibit late-stage megakaryopoiesis. PPF and platelet production represent late-stage megakaryopoiesis; therefore, we used human primary megakaryocytes to analyze the effects of IFN-α2b on PPF and platelet production. As expected, IFN-α2b significantly inhibited PPF and platelet production in vitro. We also evaluated cytoplasmic maturation of megakaryocytes by electron microscopy. IFN-α2b reduced by half the percentage of type 3 megakaryocytes with a fully matured demarcation membrane system. This result suggested that IFN-α2b inhibited maturation of the demarcation membrane system in megakaryocytes. Demarcation membranes are thought to be the membrane reservoir for formation of proplatelets. The precise molecular mechanisms of how IFN-α2b inhibits the maturation of demarcation membranes and platelet formation remain to be elucidated. Inhibition of maturation of the demarcation membrane system by IFN-α2b may be involved in the impairment of PPF. This might affect the inhibition of PPF and platelet production.
IFN-α2b did not reduce the number of human megakaryocytes, although it significantly reduced the number of human platelets in hu-NOG mice. As well, IFN-α2b did not alter DNA ploidy of human megakaryocytes in the bone marrow of hu-NOG mice, indicating that IFN-α2b inhibited late-stage megakaryopoiesis. Previous clinical studies have demonstrated that IFN-α reduces the number of platelets without decreasing the number of megakaryocytes in patients with chronic hepatitis, solid tumors, and myeloproliferative disorders,16-19 which is consistent with our results. IFN-α has been reported to significantly decrease the size of megakaryocytes in patients with essential thrombocythemia and polycythemia vera.17,19 These clinical observations also support our conclusion that IFN-α inhibits cytoplasmic maturation of megakaryocytes but not endomitosis.
Because it is possible that IFN-α promotes the consumption of platelets in hu-NOG mice, we measured the life span of human platelets in hu-NOG mice and found that IFN-α2b did not shorten the life span. Therefore, we concluded that IFN-α2b induced thrombocytopenia by inhibiting platelet production, but not by facilitating the consumption of platelets. GATA-1 and NF-E2 are essential transcription factors in late-stage megakaryopoiesis. Lineage-selective GATA-1 knockout mice have abnormal megakaryocytes with a small cytoplasm and few granules.38 GATA-1 knockdown megakaryocytes are small, with low ploidy.42 NF-E2 is composed of 2 basic leucine zipper subunits of p45NF-E2 and p18 and is one of the small Maf proteins that includes MafG.43 p45NF-E2 knockout megakaryocytes do not have platelet formation and granules, but their DNA ploidy is increased in compensation.40 MafG-null mutant mice exhibit thrombocytopenia and impairment of PPF.39,44 We demonstrated that IFN-α2b suppressed mRNA expression levels of GATA-1, p45NF-E2, and MafG in primary human megakaryocytes using quantitative PCR. Down-regulation of these transcription factors might be involved in inhibition of the cytoplasmic maturation of human megakaryocytes by IFN-α. Ishida et al used murine megakaryocytes and reported that limitin and IFN-α induce the expression of Daxx protein and phosphorylation of Crk adaptor protein.45 IFN-α–induced growth inhibition is also mediated by Crk in myeloid and erythroid progenitor cells, and by Daxx in B lymphoid progenitors.46,47 Wang et al demonstrated that IFN-α induces the expression of SOCS-1 transcription factor, which could blunt TPO-induced intracellular signaling in murine primary megakaryocytes.12 IFN-α activates JAK1 and TYK2 tyrosine kinses, which phosphorylate STAT1 and STAT2 transcription factors. Phosphorylated STAT1 and STAT2 form a complex with IFN regulatory factor (IRF)-9, named IFN-stimulating gene factor 3. IFN-stimulating gene factor 3 migrates into the nucleus where it binds to the IFN-stimulated response element in the promoter region of hundreds of genes. STAT1/STAT1 homodimer mediates the alternative pathway by binding to IFN-γ activated sequence. To the best of our knowledge, no other study has demonstrated how IFN-α decreases the expression of GATA-1, p45NF-E2, and MafG. Further investigation will be needed to elucidate this mechanism.
NIP-004 is a novel TPO mimetic and can increase the number of human megakaryocytes and induce their maturation, accompanied by an increase in human platelets in hu-NOG mice.25 We confirmed the effects of NIP-004 on IFN-α–induced thrombocytopenia using hu-NOG mice in this study. NIP-004 increased the number of human megakaryocytes in hu-NOG mice treated with IFN-α2b. The dose of PEG-IFN-α2b in this study was higher than that used in clinical settings. Based on our preliminary data related to the pharmacokinetics of PEG-IFN-α2b in NOG mice, we found that clearance of PEG-IFN-α2b was 3 times faster than that in humans (data not shown). Therefore, we needed to inject PEG-IFN-α2b at higher doses to retain an effective concentration in NOG mice.
In conclusion, we demonstrated that IFN-α2b directly inhibited cytoplasmic maturation and platelet production, but not proliferation and endomitosis in human primary megakaryocytes. TPO mimetics might be useful for treating IFN-induced thrombocytopenia, and their clinical application might encourage physicians and patients to continue IFN therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ms Asako Ikejima for technical assistance, Dr Kenneth Kaushansky for encouragement, and Dr Eri Matsuki for discussions.
Authorship
Contribution: A.Y. and T.N. performed the experiments, analyzed results, and compiled the figures; H.S. performed electron microscopy; M.I. and Y.O. contributed live mice; Y.I. controlled the data; and A.Y., T.N., and Y.M. designed the research and wrote the paper.
Conflict-of-interest disclosure: T.N. is an employee of Nissan Chemical Industries. Y.M. and Y.O. received research grants from Nissan Chemical Industries. The remaining authors declare no competing financial interests.
Correspondence: Yoshitaka Miyakawa, Division of Hematology, Department of Internal Medicine, Keio University School of Medicine, 35 Shinanomachi, Shinjuku, Tokyo 160-8582, Japan; e-mail: yoshi@sc.itc.keio.ac.jp.
References
Author notes
*A.Y. and T.N. contributed equally to this study and should be regarded as co–first authors.


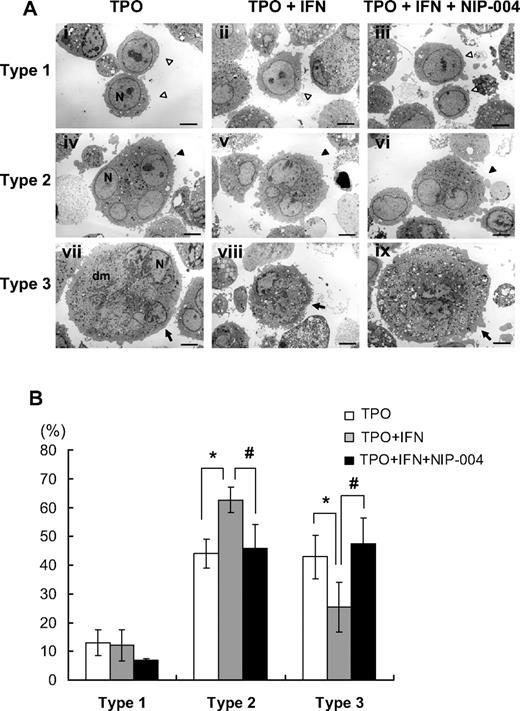
 , type 3 fully matured megakaryocytes (vii-ix). The definition of each type is in “Electron microscopy.” Original magnification, ×3000. Scale bar represents 4 μm. N indicates nucleus; Dm, demarcation membranes. (B) The percentage of each type of megakaryocytes. IFN-α2b decreased type 3 megakaryocytes and increased type 2 compared with control (TPO) with statistically significant differences, indicating that IFN-α2b inhibits the development of the demarcation membrane system of megakaryocytes. Data are means plus or minus SD from the results of 4 independent experiments (*P < .05 vs TPO). NIP-004 prevented the inhibitory effects of IFN-α2b on the development of the demarcation membrane system of megakaryocytes. Data are means plus or minus SD from the results of 2 independent experiments (#P < .05 vs TPO + IFN).
, type 3 fully matured megakaryocytes (vii-ix). The definition of each type is in “Electron microscopy.” Original magnification, ×3000. Scale bar represents 4 μm. N indicates nucleus; Dm, demarcation membranes. (B) The percentage of each type of megakaryocytes. IFN-α2b decreased type 3 megakaryocytes and increased type 2 compared with control (TPO) with statistically significant differences, indicating that IFN-α2b inhibits the development of the demarcation membrane system of megakaryocytes. Data are means plus or minus SD from the results of 4 independent experiments (*P < .05 vs TPO). NIP-004 prevented the inhibitory effects of IFN-α2b on the development of the demarcation membrane system of megakaryocytes. Data are means plus or minus SD from the results of 2 independent experiments (#P < .05 vs TPO + IFN).