The terminal complement inhibitor eculizumab was recently shown to be effective and well tolerated in patients with paroxysmal nocturnal hemoglobinuria (PNH). Here, we extended these observations with results from an open-label, non–placebo-controlled, 52-week, phase 3 clinical safety and efficacy study evaluating eculizumab in a broader PNH patient population. Eculizumab was administered by intravenous infusion at 600 mg every 7 ± 2 days for 4 weeks; 900 mg 7 ± 2 days later; followed by 900 mg every 14 ± 2 days for a total treatment period of 52 weeks. Ninety-seven patients at 33 international sites were enrolled. Patients treated with eculizumab responded with an 87% reduction in hemolysis, as measured by lactate dehydrogenase levels (P < .001). Baseline fatigue scores in the FACIT-Fatigue instrument improved by 12.2 ± 1.1 points (P < .001). Eculizumab treatment led to an improvement in anemia. The increase in hemoglobin level occurred despite a reduction in transfusion requirements from a median of 8.0 units of packed red cells per patient before treatment to 0.0 units per patient during the study (P < .001). Overall, transfusions were reduced 52% from a mean of 12.3 to 5.9 units of packed red cells per patient. Forty-nine patients (51%) achieved transfusion independence for the entire 52-week period. Improvements in hemolysis, fatigue, and transfusion requirements with eculizumab were independent of baseline levels of hemolysis and degree of thrombocytopenia. Quality of life measures were also broadly improved with eculizumab treatment. This study demonstrates that the beneficial effects of eculizumab treatment in patients with PNH are applicable to a broader population of PNH patients than previously studied. This trial is registered at http://clinicaltrials.gov as NCT00130000.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired clonal disorder caused by a somatic mutation of the phosphatidylinositol glycan-complementation class A(PIG-A) gene in hematopoietic stem cells. The disorder results in a deficiency of glycosylphosphatidylinositol (GPI), which serves as an anchor for several cell surface proteins including the terminal complement regulator, CD59. The absence of CD59 from the surface of the affected PNH red blood cells (RBCs) renders them susceptible to terminal complement-mediated lysis. The subsequent chronic hemolysis is the primary clinical manifestation of the disease and leads to disabling morbidities that include anemia, fatigue, thrombosis, pain, and impaired quality of life (QoL).1,,–4 Lactate dehydrogenase (LDH) is released during RBC destruction and grossly elevated serum LDH is a common finding in patients with PNH.3,5
Prior to eculizumab approval by the FDA and the European Commission, there were no approved therapeutic options for patients with PNH. Treatments were generally supportive in nature. Transfusions of packed RBCs were given in an attempt to alleviate anemia, but not to treat the ongoing hemolysis and its related symptoms. In some cases, insufficient erythropoiesis contributed to the anemia. In addition, folate supplements were administered to help support erythropoiesis.3 Attempts to increase RBC production, however, would have only increased hemolysis-related symptoms as PNH RBCs that are formed are lysed by the unopposed action of terminal complement. Although corticosteroid and androgen therapy may have been used in PNH in an attempt to help treat the anemia, no controlled data exist to suggest clinical efficacy or whether any potential benefit outweighs the established risks of such therapies.3
Eculizumab (Soliris; Alexion Pharmaceuticals, Cheshire, CT) is a humanized monoclonal antibody that blocks the activation of terminal complement at C5 and prevents the formation of C5a and the terminal complement complex, C5b-9.6 In a previous randomized, placebo-controlled, phase 3 study (TRIUMPH), eculizumab treatment markedly reduced hemolysis and transfusion requirements and improved anemia, fatigue, and broad measures of quality of life in transfusion-dependent PNH patients with good bone marrow reserve.7 Here, we examined the safety and efficacy of eculizumab in a broader, more diverse population of PNH patients by relaxing the inclusion criteria to allow patients with minimal transfusion requirements and evidence of thrombocytopenia to enter the trial. Results from this phase 3 study, Safety and Efficacy of the Terminal Complement Inhibitor Eculizumab in Patients with Paroxysmal Nocturnal Hemoglobinuria (SHEPHERD), are presented.
Methods
Patients
Patients 18 years or older with a diagnosis of PNH made more than 6 months prior to screening were eligible. Patients were required to have had at least 1 transfusion in the past 2 years for anemia or anemia-related symptoms or personal beliefs that precluded transfusions. A PNH type III RBC proportion of 10% or more as assessed by flow cytometry, platelet counts of 30 ×109/L or higher, and LDH levels of 1.5 times or more the upper limit of the normal range were also required. All patients were vaccinated against Neisseria meningitidis at least 14 days prior to receiving the first dose of eculizumab. Throughout the 52-week study, patients received transfusions with packed RBCs if medically indicated. Patients who had received another investigational drug within 30 days of the first visit or had an absolute neutrophil count of less than .5 × 109/L were excluded. Patients with complement deficiency, active bacterial infection, prior meningococcal disease, or prior bone marrow transplant were also excluded. This study was reviewed and approved by the Institutional Review Board at each center. In accordance with the Declaration of Helsinki, written informed consent was obtained from all patients prior to their entering the study.
Study design and treatment
SHEPHERD was an open-label, phase 3, clinical study designed to investigate the long-term safety and efficacy of eculizumab. The study design consisted of a 2-week screening period and a 52-week treatment period with a prespecified 26-week interim analysis (the online reference for the phase 3 SHEPHERD study can be found at http://www.clinicaltrials.gov/ct2/show/NCT00130000?term = shepherd&rank = 2).
Patients received eculizumab using a 25- to 45-minute intravenous infusion in the following dosing schedule: an induction dose of 600 mg every 7 (± 2) days for 4 doses; then 900 mg 7 (± 2) days later; followed by a maintenance dose of eculizumab 900 mg every 14 (± 2) days for a total of 52 weeks.
Study end points
The primary safety end points were adverse events (AEs), clinical laboratories, electrocardiogram (ECG) data, and vital signs. The primary efficacy end point was hemolysis as assessed by LDH area under the curve (AUC). Secondary efficacy end points included fatigue as measured by the validated Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) instrument8 and LDH change from baseline. Additional end points included quality of life (QoL), as measured by the validated European Organisation for Research and Treatment of Cancer (EORTC QLQ-C30) instrument,9 and thrombosis. Data were also collected to assess the effects of eculizumab on the PNH RBC clone size, hemoglobin levels, and transfusion requirements.
Study assessments
Safety.
Adverse events were defined using MedDRA preferred terms and were recorded as incidence rates by treatment group. Thrombotic events were categorized using predefined major adverse vascular event (MAVE) categories and by location and diagnostic method. Adverse events were recorded at each dosing visit and tabulated by severity and by investigator-assessed relationship to study drug. Adverse events related to the occurrence of any infection were tabulated separately. Adverse event rates during SHEPHERD were analyzed during the first 26 weeks (prespecified interim analysis), during the second 26 weeks, and for the duration of the 52-week study.
Efficacy.
Hemolysis was assessed by measuring levels of LDH at multiple time points during the study. Anemia was assessed throughout the study by measuring hemoglobin levels and the proportions of PNH RBCs. Transfusion requirements 1 year before and during the 52-week study were recorded and compared. Transfusion avoidance at 52 weeks was also assessed. Changes in the proportion of PNH RBCs were measured by flow cytometry. All biochemical and clinical measurements were performed at 1 of 3 central laboratories (in the United States, Europe, or Australia).
Fatigue was assessed using the FACIT-Fatigue and EORTC QLQ-C30 instruments. Total scores on the FACIT-Fatigue instrument range from 0 to 52, with higher scores indicating an improvement in fatigue.10 Various health-related QoL outcomes were assessed using the EORTC QLQ-C30 instrument; scores range from 0 to 100, with an increase in the score for the global health status and functioning scales and a decrease in the scores for the symptom scales and single-item measures indicating improvement.9
Statistical analysis
The study plan allowed enrollment of at least 85 patients with PNH. Data from any patient who received eculizumab were used for the primary safety and efficacy analyses. For subpopulation analyses, patients were categorized into strata based on baseline LDH quartiles and platelet counts higher than and lower than 65 × 109/L. There was a prespecified interim analysis performed at 26 weeks. Final data analysis was performed at 52 weeks.
The AUC of the change in LDH from baseline to 52 weeks, units transfused, and change in FACIT-Fatigue score were analyzed using a signed rank sum test. For those patients with missing values, the last observation carried forward method was used. The changes in LDH and hemoglobin levels from baseline to 52 weeks were analyzed using a mixed-model analysis. Transfusion avoidance was evaluated with a Fisher exact test.
Fatigue and QoL measures were recorded in accordance with the scoring guidelines using the FACIT-Fatigue and EORTC QLQ-C30 instruments.11,12 Change of total FACIT-Fatigue score and each domain score of the EORTC QLQ-C30 instrument from baseline through 26 and 52 weeks was analyzed using a mixed-model analysis, with baseline scores as covariate, time as fixed effect, and patient as random effect.
Results
Patients
The SHEPHERD study was designed to evaluate the safety and efficacy of eculizumab in PNH patients by relaxing the inclusion criteria of the previous randomized, placebo-controlled TRIUMPH study,7 to allow patients with minimal transfusion support and evidence of thrombocytopenia to participate. Baseline transfusion requirements and platelet counts were statistically different between SHEPHERD and TRIUMPH (P < .001 and P = .009, respectively). A total of 107 patients with PNH entered the SHEPHERD screening phase and 97 patients were enrolled as the intent-to-treat (ITT) population. Demographics and baseline characteristics of patients in the SHEPHERD study are shown in Table 1. The median age was 41 years, and the median duration of PNH was 4.9 years. SHEPHERD patients demonstrated a diverse patient population; platelet counts ranged from 23 to 355 × 109/L, prestudy transfusion requirements ranged from 0 to 66 units in the 12 months before the study, and levels of LDH at baseline ranged from 537 to 5245 U/L. Of the 97 patients enrolled, 96 completed the 52-week study.
Safety
Adverse events.
Treatment-emergent AEs occurring in 10% or more of patients during the 52-week study are shown in Table 2. The most common AEs were headache, nasopharyngitis, and upper respiratory tract infection. The majority (96.4%) of AEs were mild to moderate in intensity. Headaches were mild to moderate in intensity in all but 5 patients. There were no cases of nasopharyngitis or upper respiratory tract infection that were reported as severe. The majority (76.1%) of AEs were deemed unrelated to study medication. Only 2 patients reported AEs that were considered definitely related to study drug (dysgeusia and mild hematoma), which were mild in intensity. Two patients with a history of thrombosis experienced a thrombotic event (a pulmonary thromboembolism and a deep vein thrombosis) during the study. One thrombotic event occurred approximately 1 month following discontinuation of eculizumab (the patient was treated for a total of 4 weeks), and the second event occurred on study day 170. Both events were considered by the respective investigators to be unrelated to study drug. The incidence of AEs during the second 26 weeks of eculizumab treatment was either similar or lower in frequency compared with the first 26 weeks of treatment. In particular, there were substantially fewer headaches during the second 26 weeks of treatment. For patients who experienced headaches, 94% experienced them within the first 48 hours of drug administration and most were restricted to the first 2 weeks of therapy during the induction phase. Headaches were generally reported to be mild or moderate in severity, of short duration, and were adequately managed with nonopioid analgesics. Vital signs, physical examination, and ECG data did not reveal temporally associated AEs. No clinically significant laboratory abnormalities were seen.
Considering that SHEPHERD was an open-label study with no placebo comparator arm, AEs in placebo-treated PNH patients from the companion 26-week double-blind, placebo-controlled study of eculizumab in PNH (TRIUMPH) were included for numeric comparisons of AE rates. Although the TRIUMPH patient population differed from that of SHEPHERD in regard to higher transfusion support and platelet counts of at least 100 × 109/L, these patients provide perspective regarding the event rates in the current study. With the exception of headache, pyrexia, and myalgia, the incidences of AEs during the 2 consecutive 26-week treatment periods of SHEPHERD were similar to that reported for the placebo-treatment arm in the TRIUMPH study. The frequency of headaches with eculizumab during the second 26 weeks was lower than that of the TRIUMPH placebo group.
Serious adverse events.
There were a total of 44 serious adverse events (SAEs) during 52 weeks of eculizumab treatment in SHEPHERD, of which 7 were considered to be possibly related to study drug including pyrexia (2), headache (1), abdominal distension (1), viral infection (1), anxiety (1), and renal impairment (1). No SAE was considered probably or definitely related to eculizumab treatment. Serious adverse events that were considered possibly related to study drug are summarized in Table 3. One patient withdrew from eculizumab treatment due to an AE unrelated to study drug (disc prolapse); the patient was admitted to a nonstudy hospital where eculizumab treatment was discontinued, and the patient later experienced a thrombotic event, which was deemed by the investigator to be unrelated to study drug. This patient subsequently died due to complications that were determined by the investigator to also be unrelated to study drug. There were no new cases of aplastic anemia, myelodysplastic syndrome, or hematologic malignancies during treatment with eculizumab in SHEPHERD.
Infections.
Infection-related AEs occurring during the SHEPHERD study are shown in Table 4. A total of 89 patients experienced at least 1 infection during the 52-week treatment period. A majority of the infections (98.9%) were reported as mild to moderate in intensity. Most infection-related AEs (91.3%) were considered unrelated to study medication, while 8.3% of infections were reported to be possibly related. No infections were reported as definitely related to eculizumab treatment. No patients withdrew from the study due to an infection and 7 infection-related adverse events occurring in 6 patients were considered as SAEs: 3 pyrexia events and a single event each of cholangitis, endometritis, pyelonephritis, and viral infection. The proportion of patients experiencing 1 or more infections during the first 26-week (75.3%) and second 26-week (70.8%) treatment periods in SHEPHERD was similar to that of placebo-treated patients (77.3%) during the 26-week TRIUMPH study.
Immunogenicity.
Immunogenicity, as assessed by the development of antieculizumab antibodies, occurred in 2 patients (2.1%) and at low titers. There was no disruption of complement inhibition and no evidence of reduced efficacy for eculizumab in these patients. In the placebo-controlled TRIUMPH study, one placebo-treated patient (2.3%) and one eculizumab-treated patient (2.3%) were reported to have a transient, low-titer antibody response to eculizumab.7
Efficacy
Levels of eculizumab higher than 35 μg/mL have been shown to completely inhibit serum hemolytic activity.13 Eighty-nine of 97 patients maintained complete inhibition of serum hemolytic activity with every 14-day dosing throughout the duration of the treatment period. Eight patients demonstrated a return of terminal complement activity and hemolysis during the last 1 or 2 days of the 14-day dosing interval. Sustained blockade of complement and reduced hemolysis were achieved in each of these patients for whom the dosing interval was adjusted, per protocol, to 12 days (n = 6).
Every patient treated with eculizumab had a substantial reduction in hemolysis as measured by levels of LDH. The primary efficacy end point of LDH AUC was significantly reduced compared with baseline with a median change of − 632 264 U/L × day (P < .001). Lactate dehydrogenase was reduced from a mean of 2201 ± 105 U/L at baseline to 297 ± 21 U/L at 52 weeks (P < .001, mixed model analysis). As reported previously for the placebo-controlled TRIUMPH study,7 the significant reduction in LDH to near-normal levels (normal range: 103 to 223 U/L) in SHEPHERD was achieved within 1 week following initiation of treatment and at each subsequent study visit (P < .001 at each time point).
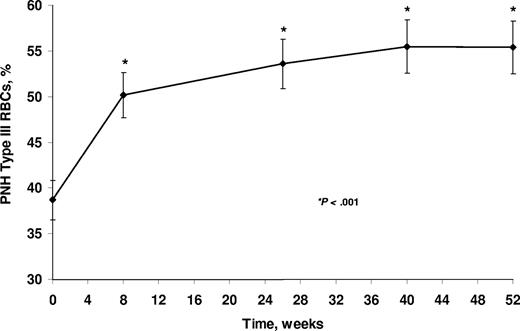
The reduction in hemolysis resulted in an improvement in anemia as evidenced by significant increases in the proportion of PNH type III RBCs from a mean of 38.7% (± 2.17%) to 55.4% (± 2.85%; Figure 1). Median proportions of PNH type III RBCs increased from 33.5% at baseline to 55.7% at week 52 (P < .001). By contrast, the median proportions of PNH granulocytes did not change during the course of the study (96.0% at baseline and 96.6% at 52 weeks). Corresponding to the increase in the proportion of PNH type III RBCs, a significant rise in hemoglobin level was observed from a median of 93 g/L at baseline to a median of 102 g/L at 52 weeks (P < .001 by mixed-model analysis).
PNH RBC proportions (mean ± SE) during eculizumab treatment. Patients received eculizumab for up to 52 weeks. P value compared with baseline is based on signed rank test. RBC indicates red blood cell.
PNH RBC proportions (mean ± SE) during eculizumab treatment. Patients received eculizumab for up to 52 weeks. P value compared with baseline is based on signed rank test. RBC indicates red blood cell.
The protection of the PNH RBCs with eculizumab was associated with a significant reduction in the number of packed RBC units transfused compared with pretreatment transfusion requirements (Table 5). The number of packed RBC units transfused was reduced from a median of 8.0 units per patient during the year prior to eculizumab treatment to 0.0 units per patient during 52 weeks of eculizumab treatment (P < .001). The mean number of packed RBC units transfused was reduced 52% from 12.3 units per patient pretreatment to 5.9 units per patient during eculizumab. A total of 49 patients (51%) were transfusion independent for the entire 52 weeks of treatment (P < .001).
Hemolysis (LDH AUC) was significantly reduced across all quartiles of baseline LDH levels (P < .001; Table 5) and among patients with different baseline platelet counts (P < .001). Reductions in packed RBC units transfused were observed in all quartiles of baseline LDH levels with statistically significant reductions in the 3 upper quartiles (P ≤ .033) and in patients with platelet counts higher than and less than 65 × 109/L (P ≤ .047). Transfusion independence was achieved with statistical significance in all quartiles of LDH (P ≤ .021) and in patients with different platelet counts (P < .001). The demonstrated significant improvement in fatigue was also observed regardless of baseline LDH level (P < .001) or platelet counts (P < .001).
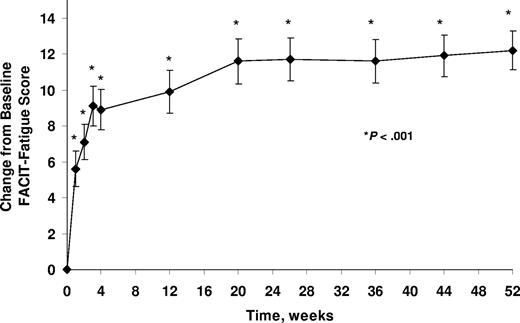
Related to the immediate and sustained improvement in hemolysis, fatigue, as measured by the FACIT-Fatigue instrument, significantly improved within 1 week of eculizumab treatment, and this improvement was maintained throughout the 52-week study period (P < .001; Figure 2). The mean ± SE change from baseline in FACIT-Fatigue score at 52 weeks was 12.2 ± 1.1 (P < .001).
Change from baseline in FACIT-Fatigue scores (mean ± SE) during eculizumab treatment. Patients received eculizumab for up to 52 weeks. P value is based on change from baseline using a signed rank test. (FACIT indicates Functional Assessment of Chronic Illness Therapy.)
Change from baseline in FACIT-Fatigue scores (mean ± SE) during eculizumab treatment. Patients received eculizumab for up to 52 weeks. P value is based on change from baseline using a signed rank test. (FACIT indicates Functional Assessment of Chronic Illness Therapy.)
Assessment of quality of life was also determined using the EORTC QLQ-C30 instrument (Table 6). Similar to results from the FACIT-Fatigue instrument, a significant improvement in fatigue scores was demonstrated on the EORTC QLQ-C30 fatigue scale by study week 1, and this improvement was maintained at each study visit through week 52 (P < .001, mixed model analysis). Significant improvement in scores was shown on the scale for global health status (P < .001), on all 5 scales for functioning (P < .001), on all 3 symptom scales (P ≤ .002), and on 4 of 6 single-item measures (P < .001).
Discussion
In the SHEPHERD study, we evaluated safety and efficacy of eculizumab in a broader PNH patient population than was studied in the previous phase 3 TRIUMPH trial.7 A total of 69 patients (71%) in the SHEPHERD study would not have met the inclusion criteria for entry into TRIUMPH, including 35 patients with a platelet count of less than 100 × 109/L and 46 patients receiving fewer than 4 transfusion episodes in the year prior to the study(12 of the 69 patients failed to meet both of these inclusion criteria). Mild to moderate thrombocytopenia is common in patients with PNH,14 and transfusion requirements vary greatly among patients. Therefore, SHEPHERD patients represent a broad PNH patient population. A total of 96 (99%) of 97 patients completed the SHEPHERD study and 92 chose to continue eculizumab therapy in an extension study.
Eculizumab was well tolerated throughout the 52-week treatment period with a majority of AEs reported as mild to moderate in intensity and as unrelated to study drug. The incidence of AEs reported during the second 26-week treatment period during SHEPHERD was similar to that reported during the first 26-weektreatment period except for headache. During the second 26-week treatment period in SHEPHERD, only pyrexia appeared to be increased over that reported for placebo-treated patients in TRIUMPH. There were no clinically significant adverse findings associated with eculizumab in vital signs, physical examination, ECG, and laboratory data. No SAEs were considered probably or definitely related to eculizumab treatment, and the incidence of SAEs was similar to that reported for placebo-treated patients in TRIUMPH. There was 1 death in the study due to thrombosis, which was deemed unrelated to study drug. Few infections were severe and none was deemed definitely related to eculizumab. The incidence of infections during either the first or second 26-week period of eculizumab treatment in SHEPHERD was similar to that reported in patients receiving placebo during the TRIUMPH study. Due to the known risk of meningococcal infections in individuals with terminal complement deficiencies, all patients received an N meningitidis vaccine at least 2 weeks prior to commencement of eculizumab. Patients should be monitored for early signs of meningococcal infections; no cases of meningococcal infection were reported during the SHEPHERD study.
In the previous placebo-controlled TRIUMPH study, headache was the most common AE, and the frequency of headaches that occurred was similar in placebo- and eculizumab-treated patients after the first 2 weeks of therapy.7 These results are in agreement with the SHEPHERD study in which headache was also the most frequent AE. The incidence of headache in the second 26 weeks of treatment (14.6%) was markedly lower than that during the first 26 weeks (48.5%). During SHEPHERD, most headaches occurred within 24 hours of study drug administration. The initial increase in headaches during the induction period may be related to a sudden increase in the potent vasodilator nitric oxide. The immediate reduction in hemolysis with eculizumab may lead to a transient surge in plasma levels of nitric oxide as the depletion of nitric oxide by cell-free plasma hemoglobin stops. A rapid increase in nitric oxide has been shown to result in the transient induction of headache,15 and the use of the nitric oxide donor nitroglycerin has been associated with headache.16,17 Similarly, the decrease in headache after the first 2 doses of eculizumab may represent a physiologic restoration to steady-state levels of nitric oxide.
Hemolysis is common in patients with PNH and is central to the clinical morbidities of the disease.1,4 Eculizumab treatment resulted in a rapid, sustained reduction in hemolysis (as assessed by levels of LDH). Statistically significant reduction in hemolysis and improvement in fatigue were observed in eculizumab-treated patients regardless of baseline LDH levels or platelet counts. Improvement in fatigue with eculizumab appeared to be greater in patients with higher baseline levels of hemolysis. Reductions in transfusion with eculizumab were observed in patients with baseline platelet counts higher than or lower than 65 ×109/L and in patients in all baseline LDH quartiles (although transfusion reduction was not significant in the lowest quartile). The proportion of patients achieving transfusion independence with eculizumab was statistically significant regardless of baseline levels of hemolysis (all LDH quartiles). The reduction in hemolysis and increase in the proportion of PNH RBCs was associated with a significant improvement in anemia as evidenced by a rise in hemoglobin levels from a median of 93 g/L at baseline to 102 g/L at 52 weeks. The improvement in anemia was achieved despite a concomitant reduction in the number of packed RBC units transfused. Importantly, a small subgroup of 12 patients with both lower levels of hemolysis (< 2050 U/L LDH) and lower platelet counts (< 65 ×109/L) experienced significant improvements in hemolysis and fatigue (data not shown). Although not statistically significant in this subgroup, transfusion requirement was reduced from a median of 11.5 to 5.0 units of packed RBCs, and 50% of these patients remained transfusion independent during the one-year treatment period. Patients with a platelet count lower than 65 ×109/L are of particular interest as thrombocytopenia has been proposed as a defining feature of bone marrow insufficiency in PNH,13 and the data presented here suggest that patients with lower platelet counts and lower levels of hemolysis will also experience a benefit from eculizumab.
Because the proportion of PNH type III RBCs increases during eculizumab treatment, the possibility of serious hemolysis (rebound hemolysis greater than pretreatment levels with adverse clinical outcomes) on drug withdrawal should be considered. There was one withdrawal from eculizumab during this study, without evidence of serious hemolysis. A total of 195 PNH patients have been treated with eculizumab in various clinical trials, of whom 19 have discontinued therapy; withdrawal from eculizumab was not associated with serious hemolysis in these patients.
The most serious complication in patients with PNH is thrombosis. Approximately 40% of patients with PNH experience a thrombotic event,18 and thrombosis is the leading cause of mortality in these patients.18,19 In the 52-week SHEPHERD study, 2 patients experienced a thrombotic event during eculizumab treatment. While the SHEPHERD study was not designed to establish the effect of eculizumab on thrombosis, in a prespecified analysis, data from 3 combined patient cohorts (including SHEPHERD) have demonstrated a significant 92% reduction in the thromboembolic event rate during eculizumab treatment compared with the same period of time prior to treatment.20
PNH is a disabling disease and many patients with PNH experience poor quality of life including severe fatigue, poor global health status, impaired functioning, pain, and dyspnea.21 Impairment in these QoL measures has been attributed, at least in part, to downstream effects of the ongoing hemolysis in these patients.1,22 In particular, fatigue in patients with PNH is not due only to the anemia but also is closely linked to hemolysis.1,22 We have previously shown that reduction in hemolysis in patients treated with eculizumab was associated with improvement in fatigue and several additional health-related QoL measures over a 26-week treatment period.7 In the current long-term SHEPHERD study, patients also responded to eculizumab treatment with a sustained reduction in hemolysis, and significant improvements in the same QoL measures were observed using 2 validated QoL instruments, FACIT-Fatigue and EORTC QLQ-C30.9,23 Levels of fatigue showed the largest improvement, and this improvement was observed using both instruments. Scores in the FACIT-Fatigue were significantly improved within 1 week of treatment (a median increase of 3 points), and the effect was sustained throughout 52 weeks (an overall median increase of 10 points). A change of 3 or more points on this instrument represents a clinically important difference.24 Significant improvement in fatigue was observed regardless of pre-eculizumab transfusion requirements or levels of hemolysis prior to treatment. Other quality of life measures were also broadly improved with eculizumab treatment, including global health status, patient functioning, and several disease-related symptoms.
In the SHEPHERD trial, eculizumab appeared safe and well tolerated. Eculizumab treatment was associated with a substantial reduction in hemolysis in all patients leading to improvements in anemia, the need for transfusions, fatigue, and QoL. This study demonstrates that the beneficial effects of eculizumab treatment in patients with PNH are applicable to a broad population of patients with various levels of hemolysis and thrombocytopenia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are indebted to Christopher Mojcik, MD, PhD, Michael Bombara, BS, and Jing Jing Wang, MD, MS (Alexion Pharmaceuticals) for trial oversight; to Jason Chan, PhD (Alexion Pharmaceuticals) for the statistical analysis; to Kerry Quinn-Senger, PhD (Alexion Pharmaceuticals) for assistance with technical writing; and to Leonard Bell, MD for critical review of the paper.
This work was supported by Alexion Pharmaceuticals.
Authorship
Contribution: R.A.B. performed the research, collected, analyzed, and interpreted the data, and drafted the paper; N.S.Y. designed the trial, performed the research, collected the data, and reviewed the paper; E.A., A.G., L.C., C.C., and C.-L.F. performed the research and collected the data; A.M.R. and J.S. performed the research, collected, analyzed, and interpreted the data, and reviewed the paper; H.S. performed the research and reviewed the paper; J.P.M. performed the research, collected the data, and reviewed the paper; M.B. performed the research, collected the data, and drafted the paper; H.-A.K. performed trial oversight and reviewed the paper; R.P.R. designed the trial, analyzed and interpreted the data, and drafted the paper; P.H. designed the trial, performed the research, collected, analyzed, and interpreted the data, and drafted the paper.
Please see Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article) for other investigators and institutions who participated in the clinical study in addition to the authors.
Conflict-of-interest disclosure: J.S., M.B., and P.H. have served as consultants for Alexion Pharmaceuticals. P.H. has received grant support from Alexion Pharmaceuticals. R.A.B., H.S., J.S., C.C., J.P.M., and P.H. have received lecture fees from Alexion Pharmaceuticals. J.S., C.C., and J.P.M. have served on Advisory Committees for Alexion Pharmaceuticals. H.-A.K. and R.P.R. are employees of and have equity ownership in Alexion Pharmaceuticals. R.P.R. has assigned to Alexion Pharmaceuticals his inventions made as an employee and has received no royalties from the company for these inventions. The remaining authors declare no conflicting financial interests.
Correspondence: Robert A. Brodsky, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross Bldg, Rm 1025, Baltimore, MD 21205; e-mail: brodsro@jhmi.edu.