Patients with imatinib-resistant or -intolerant accelerated-phase chronic myelogenous leukemia (CML-AP) have very limited therapeutic options. Nilotinib is a highly selective BCR-ABL tyrosine kinase inhibitor. This phase 2 trial was designed to characterize the efficacy and safety of nilotinib (400 mg twice daily) in this patient population with hematologic response (HR) as primary efficacy endpoint. A total of 119 patients were enrolled and had a median duration of treatment of 202 days (range, 2–611 days). An HR was observed in 56 patients (47%; 95% confidence interval [CI], 38%-56%). Major cytogenetic response (MCyR) was observed in 35 patients (29%; 95% CI, 21%-39%). The median duration of HR has not been reached. Overall survival rate among the 119 patients after 12 months of follow-up was 79% (95% CI, 70%-87%). Nonhematologic adverse events were mostly mild to moderate. Severe peripheral edema and pleural effusions were not observed. The most common grade 3 or higher hematologic adverse events were thrombocytopenia (35%) and neutropenia (21%). Grade 3 or higher bilirubin and lipase elevations occurred in 9% and 18% of patients, respectively, resulting in treatment discontinuation in one patient. In conclusion, nilotinib is an effective and well-tolerated treatment in imatinib-resistant and -intolerant CML-AP. This trial is registered at www.clinicaltrials.gov as NCT00384228.
Introduction
Chronic myelogenous leukemia (CML) is a myeloproliferative disorder resulting from a chromosomal rearrangement leading to the formation of a novel fusion gene, BCR-ABL, which encodes the BCR-ABL protein that has constitutive protein tyrosine kinase activity. The oncogene results from a reciprocal translocation between the long arms of chromosomes 9 and 22, t(9;22)(q34;q11), forming the Philadelphia chromosome (Ph), which can be detected by cytogenetic analysis.1,2
In the absence of treatment, CML progresses within several years from a chronic phase to an accelerated phase, and eventually culminates in blastic phase and death.3 The treatment of chronic-phase CML has been transformed by the introduction of imatinib, an inhibitor of the BCR-ABL tyrosine kinase. Newly diagnosed patients with chronic-phase CML treated with imatinib (Gleevec/Glivec; Novartis, Basel, Switzerland) in the International Randomized trial of Interferon-α plus cytarabine versus STI571 (IRIS) achieved complete cytogenetic response (CCyR) rates in 87% of patients and had an estimated 5-year survival of 89%. Only 6% of patients progress to an accelerated phase or blastic phase during this time.4,5 In a large phase 2 trial, 20% of 454 patients in late chronic-phase CML who failed prior interferon therapy, treated with imatinib, progressed to accelerated phase or blastic phase within the first 3 years of therapy.6 Fewer than 15% of patients with CML are in accelerated phase at the time of first diagnosis of CML.7 Therefore, most patients diagnosed today with CML-AP are expected to have failed prior imatinib therapy given either in the chronic or accelerated phases of their leukemia. Before the introduction of imatinib, the median survival of patients with accelerated phase CML was less than 18 months.7 In a long-term follow-up of the large imatinib phase 2 study in patients with accelerated phase, the 3-year survival rates of patients with and without a major cytogenetic response (MCyR) were 85% and 52%, respectively.8 At the time the present study was initiated (May 2005), few therapeutic options existed for the management of patients with CML in accelerated phase who failed prior imatinib therapy. These included bone marrow transplantation if a suitable donor is available, or chemotherapy.
Resistance to imatinib has been associated with a heterogeneous array of mechanisms that range from nonspecific multidrug resistance to inherent genetic alterations in BCR-ABL. The most frequently identified mechanism of acquired imatinib resistance is point mutations in the BCR-ABL kinase domain. These mutations, which are more frequently observed in patients with advanced stages of the disease, are linked with impaired binding of imatinib to BCR-ABL. In a cohort of 21 patients with accelerated phase and imatinib resistance (described as primary and secondary resistance in 5 and 16 patients, respectively), 52% had point mutations at the time of resistance.9 In another retrospective analysis of 34 patients with accelerated phase and imatinib failure, mutations were identified in 17 patients (50%).10
Nilotinib (Tasigna [nilotinib], formerly known as AMN107; Novartis) is a novel oral aminopyrimidine derivative that has been rationally designed to be more selective against the Bcr-Abl tyrosine kinase than imatinib.11,,–14 Like imatinib, it acts through competitive inhibition of the ATP binding site of BCR-ABL. In vitro nilotinib is 30 times more potent than imatinib against imatinib-sensitive cell lines and 3 to 7 times more potent against imatinib-resistant cell lines. In addition, nilotinib is effective in inhibiting the growth of 32 of 33 cell lines bearing mutations causing imatinib resistance, the exception being the T315I mutant.12,–14 Results from a phase 1 dose escalation study performed in imatinib-resistant patients with CML and Ph+ acute lymphoblastic leukemia indicated that nilotinib produced significant hematologic and cytogenetic responses in all phases of CML.15 Side effects potentially related to nilotinib included grade 3 or 4 hematologic toxicities as well as transient indirect hyperbilirubinemia and skin rash.
The current single-arm, open-label phase 2 study was undertaken to evaluate the efficacy and safety of nilotinib in a larger cohort of patients with imatinib-resistant and/or intolerant CML in accelerated phase.
Methods
Study design and patient population
This study was conducted as a multicenter, international, single-arm, open-label trial examining the efficacy and safety of nilotinib in patients with hematologic malignancies. Patients at least 18 years of age and with imatinib-resistant or -intolerant CML in accelerated phase were eligible.
CML in accelerated phase was defined by one or more of the following characteristics present within 4 weeks of the start of treatment, and in the absence of previous transformation to blastic phase: 15% or more but less than 30% of blasts in blood or bone marrow, 30% or more blasts plus promyelocytes in peripheral blood or bone marrow (providing that less than 30% of blasts were present in the bone marrow), peripheral blood basophils of 20% or more, and/or thrombocytopenia of less than 100 × 109/L unrelated to the administration of therapy.16 Patients with a clonal evolution without any of these hematologic criteria were not eligible.
Imatinib resistance was defined by one of the following criteria during treatment with at least 600 mg per day of imatinib: (1) disease progression from chronic phase to accelerated phase occurring during imatinib therapy; (2) disease progression defined as at least a 50% increase in peripheral white blood cell count, blast count, basophils, or platelets during imatinib therapy for accelerated phase; or (3) lack of hematologic response (HR) in the bone marrow following a minimum of 4 weeks of imatinib therapy for accelerated phase. In addition, patients receiving less than 600 mg per day of imatinib were eligible for participation if BCR-ABL mutations were found present at any one of the following amino acids by sequencing: L248, G250, Q252, Y253, E255, T315, F317, and H396.
Imatinib intolerance was defined as the discontinuation of imatinib therapy due to any of the following: grade 3 or 4 adverse events that persisted in spite of optimal supportive care measures, or grade 2 adverse events related to imatinib therapy in spite of optimal supportive care measures that persisted for at least 1 month or that recurred more than 3 times whether the dose was reduced or discontinued. In addition, the protocol definition of imatinib intolerance required the lack of an MCyR with imatinib.
Patients were also required to have a World Health Organization (WHO) Performance Status score of 2 or lower and normal serum electrolytes as well as hepatic, renal, and pancreatic function. Serum potassium and magnesium were required to be within the normal range and calcium (adjusted for serum albumin) and phosphate had to be either within the normal range or correctable to within normal range with supplements. Serum hepatic transaminase levels were required to be less than or equal to 2.5 times the upper limit of normal (ULN) in the absence of suspected disease involvement or less than or equal to 5 times ULN when related to disease. Serum creatinine, amylase, and lipase were all required to be less than 1.5 times ULN.
Patients were excluded if they had evidence of abnormal cardiac function or cardiac conduction, including individuals who had a myocardial infarction within the previous 12 months, individuals with left ventricular ejection fractions of 45% or less by echocardiogram or multiple-gated acquisition scan (MUGA), and individuals with a history of congenital long QT syndrome or a corrected QT interval (QTc) of more than 450 milliseconds on screening ECG using QTcF (QTc according to Frederica formula). Treatment with chemotherapy other than hydroxyurea was not permitted within 1 week of starting therapy with nilotinib.
Study treatment
All patients were initially treated with nilotinib 400 mg orally twice daily (800 mg total daily dose). Patients were instructed to fast for at least 2 hours prior to and 1 hour after taking nilotinib. Dose escalation to 600 mg twice daily was permitted in the absence of toxicity and at the discretion of the investigator, under the following circumstances: failure to achieve return to chronic phase by 1 month, loss of an achieved hematologic or cytogenetic response, or disease progression. Dose reductions to 400 mg daily and subsequently 200 mg daily were permitted for the management of toxicity. If a dose delay of more than 21 days (or more than 42 days for grades 3 and 4 hematologic toxicity) was required, the patient was discontinued from the study. National Cancer Institute Common Terminology Criteria (NCI-CTC) for Adverse Events Version 3.0 were used to grade all adverse events.
Patients who experienced toxicity were evaluated at least once a week following emergence of the toxicity until either resolution or stabilization of the toxicity, or study discontinuation. Patients who experienced QTc intervals of more than 480 milliseconds, grade 3 or higher hepatic toxicity (aspartate aminotransferase [AST; serum glutamic oxaloacetic transaminase], alanine aminotransferase [ALT; serum glutamic pyruvic transaminase], total bilirubin greater than or equal to 2 times ULN), serum creatinine greater than or equal to 2.0 times ULN, and grade 4 neutropenia and/or thrombocytopenia had the study drug withheld until resolution to grade 1 or lower or baseline for hepatic toxicity.
Treatment with nilotinib was continued until the patient experienced disease progression, developed unacceptable toxicity that precluded any further treatment, withdrew consent, and/or if the patient was felt by the investigator to be no longer benefiting.
Efficacy
The primary efficacy variable was the rate of HR confirmed at 2 consecutive visits at least 4 weeks apart, defined as either complete HR (CHR), bone marrow response (otherwise termed “no evidence of leukemia,” or MR/NEL) or a return to chronic phase (RTC). A CHR required that all the following criteria be met: marrow blasts of less than 5%, no blasts in peripheral blood, neutrophil counts of 1.5 × 109/L or more, platelet counts of 100 × 109/L or more, basophils of less than 5% and no extramedullary disease. A response was categorized as MR/NEL if all the following criteria were met: marrow blasts of less than 5%, no blasts in peripheral blood, neutrophil counts of 1.0 × 109/L or more, platelet counts of 20 × 109/L or more, and no extramedullary disease. An RTC required all the following criteria: less than 15% blasts in marrow and peripheral blood, less than 30% blasts plus promyelocytes in marrow and peripheral blood, less than 20% basophils, and no extramedullary disease (with the exception of liver or spleen enlargement). Secondary efficacy variables included time to HR, duration of HR, MCyR, time to and duration of MCyR, and overall survival. Cytogenetic response was based on assessment of at least 20 metaphases. Responses were categorized as follows: CCyR, Ph 0%; partial cytogenetic response (PCyR), Ph 1% to 35%; minor, Ph 36% to 85%; and minimal, Ph 66% to 95%. A MCyR included CCyR plus PCyR. In case of failure of cytogenetic evaluation (because of insufficient number of metaphases [less than 20 metaphases] or failure of cell growth), assessment by fluorescent in situ hybridization (FISH) was permitted. HR was assessed on the basis of (1) complete blood count (CBC) collected weekly for the first 8 weeks and every 2 weeks thereafter; and (2) bone marrow aspirate and/or biopsy performed on days 28, 56, and 84, and every 84 days thereafter.
BCR-ABL mutation assessments
Peripheral blood samples were obtained prior to the first dose of nilotinib. The total blood RNA was reverse transcribed and amplified by nested polymerase chain reaction (PCR) using primers located in the BCR and ABL region of the BCR-ABL gene. The amplicons that extend over the entire BCR-ABL tyrosine kinase domain (ranging from amino acids 230 to 490; GenBank accession no. M14752) and the surrounding regions were then screened for mutations by denaturing high-performance liquid chromatography (D-HPLC) and direct sequencing technology. This BCR-ABL mutational analysis was performed by 5 individual academic laboratories (“Acknowledgments”). All 5 laboratories reported reliable detection of mutant clones present at a frequency of 20% or more.
Safety
Safety assessments included regular monitoring of hematology and blood chemistry values, vital signs, and physical examinations, including weight and WHO performance status, and repeat cardiac assessments, including 12-lead ECGs and cardiac enzymes (cardiac troponin, creatine phosphokinase [CK], and the MB isoenzyme of CK [CK-MB]). Adverse events and serious adverse events were collected continuously throughout the trial.
Statistical analysis
The sample size was estimated based on the Fleming single-stage design to test the null hypothesis P is less than or equal to .10, where P is the HR rate. If the true response rate is a P value of .25 or greater, 64 patients are needed to reject the null hypothesis with a power of 90% and a one-sided level of significance of 2.5%. Enrollment of additional patients was allowed given the high level of efficacy observed in the previous study.15 As prespecified in the protocol, efficacy endpoints were analyzed in all the patients who were enrolled at least 6 months prior to the cut-off date of 23 January 2007 (n = 119). These patients had therefore at least 6 months of follow-up unless they discontinued early for any reason. All patients were included in the analysis according to the intent-to-treat principle. HR was calculated based on raw hematologic parameters entered in the database. Response rates were calculated with 95% confidence intervals (CIs) using Clopper-Pearson limits. Time-to-event variables were summarized and are presented using the Kaplan-Meier method. For patients achieving an HR, time to progression was defined as the time from study start to disease progression or death. Safety analyses were performed on all enrolled 119 patients.
Study conduct
The trial was conducted in accordance with the applicable regulatory requirements. The protocol was reviewed and approved by an appropriate institutional review board or ethics committee at every participating center. Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki prior to participation in this study.
Results
Patients and demographics
A total of 119 patients were enrolled into the study from 36 participating centers in 10 countries. The characteristics of the patient population (N = 119) at baseline are shown in Table 1 and include patients with at least 6 months of follow-up or who were prematurely discontinued from study treatment by the time of data cut-off. The median duration of time since first diagnosis of CML was nearly 6 years, and the median duration of prior imatinib therapy was 976 days (range, 2–2163 days). Most patients had also received prior therapies other than imatinib, including hydroxyurea (92%) and interferon-α (58%). Imatinib-resistant patients constituted 81% of the population, with 49% previously treated with at least 800 mg of imatinib at some time during their course.
The median dose intensity of therapy with nilotinib in all 119 patients was 790 mg/day (range, 180–1149 mg/day). For the 90 patients who did not have dose escalation, this value was 727 mg/day (range, 180–800 mg/day); for the 29 patients who did have dose escalation, it was 919 mg/day (range, 684–1149 mg/day). The median duration of treatment with nilotinib was 202 days (range, 2–611 days).
Efficacy
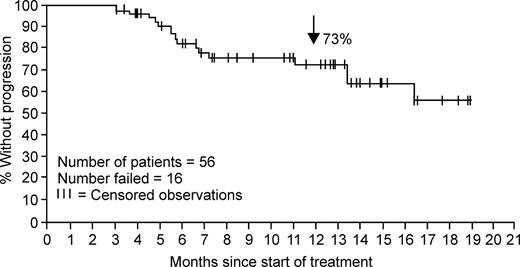
Among the 119 patients analyzed for efficacy (“Methods; Efficacy”), an overall HR rate of 47% (95% CI, 38%-56%) was observed, as noted in Table 2. The median time to first HR in the 56 individuals experiencing an HR was 1 month (range, 0.8–5.5 months). The median duration of response was not reached at the time of this analysis. As shown in Figure 1, of those patients achieving HR, an estimated 70% (95% CI, 57%-83%) remained in response at 12 months. It should be noted, however, that 30% of patients were not assessable for response, since as per protocol no second post-baseline efficacy assessment was available at the time of data collection of this report.
Kaplan-Meier plot of time to progression for the 56 patients with accelerated-phase CML who had an HR with nilotinib (40 censored observations).
Kaplan-Meier plot of time to progression for the 56 patients with accelerated-phase CML who had an HR with nilotinib (40 censored observations).
An MCyR was achieved in 35 patients (29%; 95% CI, 21%-39%). CCyRs occurred in 19 patients (16%). One patient with a CCyR and 4 patients with a PCyR at baseline were entered in to the study. In addition, 35 patients (29%) entered with additional chromosomal abnormalities at baseline. The median time to MCyR for the 35 individuals experiencing MCyR was 2 months (range, 0.9 to 8.5 months). The median duration of response was 15.4 months (range, 0.6–17.9 months). The HR rates in patients with and without additional chromosomal abnormalities (ACAs) were similar. Of 35 patients with ACAs, 21 (60%) responded, compared with 23 (55%) of 42 patients with no ACAs. Data on ACAs were not available for 42 patients.
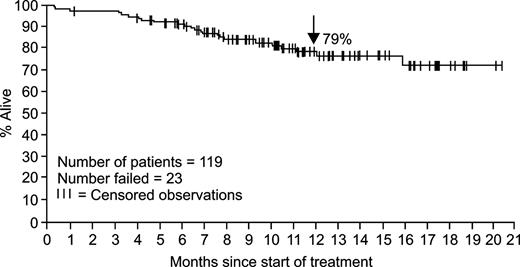
The estimated rate of overall survival in all 119 study patients at 12 months was 79% (95% CI, 70%-87%; Figure 2).
Kaplan-Meier plot of overall survival for the 119 patients with accelerated-phase CML (96 censored observations).
Kaplan-Meier plot of overall survival for the 119 patients with accelerated-phase CML (96 censored observations).
Baseline BCR-ABL mutational assessment data were available in 51 patients at the time of analysis: 17 different BCR-ABL mutations involving 14 amino acids were detected in 57% (29 of 51) of the patients, 10% (5 of 51) of whom showed more than one mutation. After 6 months of therapy, HR was achieved in 48% and MCyR in 21% of patients with baseline mutations, compared with 45% and 36% of patients without baseline mutations, respectively (Table 3). HR and/or MCyR were observed across a variety of BCR-ABL mutant genotypes. Based on the preclinical data, the T315I mutation is the only imatinib-resistant mutation among other 32 tested that showed in vitro insensitivity against nilotinib. A total of 2 patients had the T315I mutation at baseline. One T315I+ patient was not assessed for both hematologic and cytogenetic responses at the time of analysis. The other patient achieved a return to chronic phase and minimal cytogenetic response at day 29 after the first dosing and maintained the same response status until he showed continued return to chronic phase but no cytogenetic response after 141 days of nilotinib therapy. The fraction of T315I mutant clone found in this patient, estimated at 50%, was copresent with G250E at baseline. Postbaseline mutational assessment was not available for this patient at the time of analysis, and the frequency of the T315I clone was unknown at day 141. The response seen in this patient may reflect the sensitivity of the G250E clone and other BCR/ABL unmutated clones to nilotinib.
Safety
Nilotinib was well tolerated as indicated by the administration of a median dose intensity that was 99% of that initially prescribed by the protocol. Adverse events with a suspected relationship to nilotinib are summarized in Table 4. The most commonly reported nonhematologic events considered possibly related to nilotinib, and of any grade severity, were rash (22%), pruritis (20%), constipation (11%), headache, fatigue, nausea (10% each), diarrhea, and muscle spasm (9%). Gastrointestinal and central nervous system (CNS) hemorrhage of grade 3 or 4 severity were reported in less than 1% and 2% of patients, respectively. The most commonly reported hematologic adverse events of grade 3 or higher severity were thrombocytopenia (35%) and neutropenia (21%; Table 5). Neutropenia and thrombocytopenia were generally manageable with dose interruptions and/or reductions, which occurred in 16% and 24% of patients, respectively. A total of 28% of patients required support with hematopoietic growth factors or platelet transfusions. The majority of serum biochemistry abnormalities observed with nilotinib were mild to moderate in severity, resolved spontaneously with continued dosing of nilotinib, and rarely led to discontinuation of nilotinib. Grade 3 or 4 elevations in AST and ALT occurred in 1% and 2% of patients, respectively, and were manageable with dose interruption and/or reduction. Grade 3 or 4 elevations of total bilirubin occurred in 9% of patients. No patients discontinued therapy because of an elevated transaminase or bilirubin. Grade 3 or 4 lipase elevations occurred in 18% of patients, mostly with single isolated elevated values. Grade 3 or 4 elevations of serum amylase occurred in 2% of patients. One patient discontinued from therapy as a result of pancreatitis.
There were 10 deaths, either occurring on study or within 28 days following discontinuation of nilotinib. Of these 10, 5 patients died of progressive CML, 1 of sepsis, 1 due to intracranial hemorrhage, 1 due to pulmonary infection, 1 due to metastatic melanoma (patient entered 6 months after melanoma was removed by wide surgical excision considered curative and expired due to metastasis, not considered related to nilotinib). One patient died as a result of cardiac failure. The patient had a past medical history at baseline of chronic obstructive pulmonary disease (COPD). The patient had normal left ventricular function at baseline. On study day 117, the patient was admitted to hospital because of pneumonia and pulmonary edema. The patient was in cardiac failure and died 5 days later. A preclinical signal indicating that nilotinib could potentially prolong the QT interval resulted in frequent electrocardiograms being performed during this study and analyzed centrally. No patient experienced a prolongation in the QTcF interval to greater than 500 milliseconds. Increases in the QTcF interval from baseline of more than 60 milliseconds were observed in 5 patients (4%). No episodes of torsades de pointes were observed.
Discussion
Imatinib, the first Bcr-Abl tyrosine kinase inhibitor introduced in clinical practice, has been shown to be remarkably efficacious in patients with CML in accelerated phase.6,8 However, in a large phase 2 study, the estimated rate of patients with disease progression after 1 year of therapy was 41%.6 Therefore, resistance develops in a number of patients, and therapeutic options for patients who are resistant to or intolerant of imatinib have previously been limited.17 These have included chemotherapy or hematopoietic allogeneic stem cell transplantation.
In comparison with imatinib, nilotinib was developed as a more potent and more selective inhibitor of the Bcr-Abl protein tyrosine kinase. Nilotinib inhibits the cellular autophosphorylation of BCR-ABL (p210 BCR-ABL–transfected 32D cells) with a lower mean IC50 value of 20 plus or minus 1 nM compared with 194 plus or minus 7 nM with imatinib. In contrast, the mean IC50 for the inhibition of the phosphorylation of PDGFRα/β (A31 cells) was similar for the 2 drugs (71 ± 7 nM and 74 ± 11 nM), and for the inhibition of c-KIT (GIST882 cells) was higher with nilotinib (200 ± 13 nM) than with imatinib (96 ± 12 nM).13 These data indicate a higher selectivity of nilotinib against BCR-ABL. One of the most important characteristics of nilotinib is its potency against the majority of known BCR-ABL mutations associated with imatinib resistance, with one exception being the T315I mutation.13
The results of this study demonstrate the high level of activity of nilotinib in patients with CML-AP treatment failure with imatinib. Nilotinib therapy resulted in a rate of confirmed HRs of 47%, which were durable, with an estimated 70% rate of patients in continuing response at 12 months. Importantly, 29% of patients also achieved MCyR, which was complete in 16% of patients. The estimated rate of overall survival at 12 months was 79%. In the subset of patients with mutational assessment available, both patients with or without a BCR-ABL mutation at baseline responded to therapy.
The most frequent adverse events were skin rash, pruritus, and constipation. However, grade 3 or 4 events were uncommon. Interestingly, consistent with an increased selectivity toward inhibition of BCR-ABL versus PDGFR, severe edema or fluid retention syndromes or pleural effusions (thought to be related to inhibition of PDGFR) were not observed in this trial. Specifically, there was only one patient who developed grade 3 or 4 pulmonary edema. This patient also had pre-existing COPD and died of cardiac failure (the case is discussed in the previous section). Importantly, grade 3 or 4 peripheral edema, pericardial effusion, or pleural effusion were not reported in any patients. Grade 3 or 4 neutropenia or thrombocytopenia reported as adverse events occurred in occurred in 21% and 35% of patients, respectively. Cytopenia was manageable with dose interruption or reduction. It must be noted, however, that patients in this trial were in late-phase CML, had a median time from initial diagnosis of CML of nearly 6 years and, consequently, were highly susceptible to developing cytopenias.7 Laboratory abnormalities included grade 3 or 4 elevation of serum lipase and bilirubin in 18% and 9% of patients, respectively. These abnormalities are manageable since the majority resolved despite continued therapy with or without dose reduction, and only one patient discontinued treatment as a result of pancreatitis. The mechanism underlying these observations is unknown.
Recently, the results of clinical trials with dasatinib, an inhibitor of BCR-ABL, KIT, and PDGFR, as well as the Ephrin receptor and SRC-family kinases, have been reported.18,19 In a phase 2 study in patients with imatinib-intolerant or -resistant CML in accelerated phase, the level of efficacy reported with dasatinib was similar to the present study with nilotinib, with a rate of MCyR of 33%.19 However, the safety profile of dasatinib was different. The main safety findings included pleural effusion in 23% of patients (3% of grade 3 or 4), grade 3 or 4 gastrointestinal hemorrhage in 12% of patients, and grade 3 or 4 neutropenia and thrombopenia in 76% and 82% of patients, respectively. The reasons for these differences are not clear and may be related to the inhibition of tyrosine kinases other than BCR-ABL.
In summary, nilotinib produced significant hematologic and cytogenetic responses in imatinib-resistant and -intolerant patients with CML in accelerated phase. Similar rates of HRs were observed in patients with and without BCR-ABL tyrosine kinase mutations. Nilotinib was generally well tolerated. Nilotinib thus provides an important alternative for the treatment of imatinib-resistant and -intolerant patients with CML in accelerated phase.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to acknowledge the contribution of Aaron Weitzman and Peter Marks in the conduct of the study, the analysis of the data, and the writing of the manuscript; of Ariful Haque in the statistical analysis of the data; and of Renaud Capdeville in the writing of the manuscript. In addition to those investigators listed as authors, a list of other individuals who participated as investigators in this trial can be found in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
This study was supported by research funding from Novartis.
Authorship
Contribution: P.l.C. performed research and analyzed data; O.O., N.G., J.A., R.A.L., E.A., S.G.O'B., J.R., K.K., F.X.M. performed research, D.-W.K., G.S., and T.H. performed research and conducted mutation analysis, F.G. designed and performed research; A.H. performed research and conducted mutation analysis and quantitative PCR; J.C., M.G., Y.-L.K., M.B., and G.M. performed research; M.Z. analyzed the data; Y.S. conducted mutational study and analyzed mutation data; and H.K. designed and performed research and analyzed data.
Conflict-of-interest disclosure: M.Z. and Y.S. are currently employees of Novartis; P.l.C. received a research grant and honoraria from Novartis; O.O. and K.K. received research support from Novartis; J.C. received a research grant from Novartis; M.B. received research funding and honoraria from Novartis, T.H. received research funding and honoraria from Novartis and Bristol-Myers Squibb (BMS); N.G. received research support from Novartis; F.G., H.K., and A.H. received research support from Novartis and BMS; R.A.L. received research funding from Novartis and BMS; S.G.O'B. received research funding from Novartis, BMS, and Roche; and F.X.M. received research support and honoraria from Novartis.
Correspondence: Philipp le Coutre, Campus Virchow Klinikum, Charité, Humboldtuniversität, Berlin, Germany; e-mail: philipp.lecoutre@charite.de.