Abstract
Clonal T-cell expansion in patients with T-large-granular lymphocyte (LGL) leukemia occurs by an undefined mechanism that may be related to Fas apoptosis resistance. Here, we demonstrate polarized expansion of CD8+ terminal-memory differentiation in such patients, as demonstrated by CD45RA expression and absence of CD62L expression, suggesting repeated stimulation by antigen in vivo. Elimination of antigen-stimulated T cells normally occurs through Fas-mediated apoptosis. We show that cells from LGL leukemia patients express increased levels of c-FLIP and display resistance to Fas-mediated apoptosis and abridged recruitment of proteins that comprise the death-inducing signaling complex (DISC), including the Fas-associated protein with death-domain (FADD) and caspase-8. Exposure to interleukin-2 (IL-2) for only 24 hours sensitized leukemic LGL to Fas-mediated apoptosis with enhanced formation of the DISC, and increased caspase-8 and caspase-3 activities. We observed dysregulation of c-FLIP by IL-2 in leukemic LGL, suggesting a role in Fas resistance. Our results demonstrate that expanded T cells in patients with LGL leukemia display both functional and phenotypic characteristics of prior antigen activation in vivo and display reduced capacity for Fas-mediated DISC formation.
Introduction
T-cell large granular lymphocyte (LGL) leukemia is a clonal disorder of CD3+, CD8+, CD57+ T cells that is associated with autoimmunity and chronic cytopenias. In these patients and in murine models of lymphoproliferation, the T-cell populations that are expanded share many properties with antigen-activated cytotoxic T lymphocytes,1-6 such as constitutively expressing perforin and Fas Ligand (FasL, CD178).7,8 The etiology of LGL leukemia is undefined, but chronic activation by a putative virus with structural relationship to the human T-cell leukemia/lymphoma virus (HTLV)-family has been proposed as a potential etiologic event that contributes to the initial expansion of a population of T cells.9 In support of a viral etiology, our previous studies have shown that sera from approximately 50% of patients with LGL leukemia react to a recombinant HTLV-I transmembrane envelope protein, p21e.10 We hypothesize that the specific target region of the p21e protein, known as BA21, or a cross-reacting cellular protein may persistently stimulate leukemic LGL, providing a survival-promoting stimulus that contributes to the pathogenesis of this disease.11
Under physiologic conditions, Fas-mediated apoptosis limits the survival of antigen-stimulated T lymphocytes and maintains T-cell homeostasis.6,12 The Fas receptor (FasR, CD95) protein is a member of the tumor necrosis factor-receptor super-family that can trigger apoptosis through the induction of a death-inducing signaling complex (DISC) that forms through FasL cross-linking of the FasR.13,14 In general, the DISC is assembled through aggregation of the FasR, an adaptor protein known as the Fas-associated death domain (FADD) protein, and the initiator caspase protein caspase-8. Fas-mediated apoptosis through DISC formation limits the expansion of potentially harmful activated, cytotoxic effector T cells after a successful immune response. We previously reported that cells from patients with LGL leukemia are resistant to Fas-mediated apoptosis despite high surface expression of FasR (CD95) and abundant constitutive expression of FasL, and absence of mutations in the FasR gene.7 Furthermore, our in vitro studies showed that treatment with phytohemagglutinin (PHA) and interleukin-2 (IL-2) or treatment with ceramide reversed Fas resistance, suggesting that the apoptotic machinery is intact in these cells and that alternative signaling defects limit Fas-mediated apoptosis. In several leukemic cell lines, FasR surface expression does not necessarily render cells susceptible to FasL-induced apoptosis.15-18 Fas resistance can occur in cells with overexpression of the DISC inhibitory protein known as cellular FADD-like IL-1-converting enzyme (FLICE)-inhibitory protein (c-FLIP).19-21 c-FLIP is the cellular homologue of a virus-encoded apoptosis inhibitory molecule, designated viral FLICE-inhibitory protein (v-FLIP).19,22-26 c-FLIP-short (c-FLIP-S) and c-FLIP-long (c-FLIP-L) represent 2 distinct isoforms of this protein that are both capable of inhibiting Fas signaling. In this study, we focus on the ability of cells in patients with LGL leukemia to form the DISC after cross-linking of the Fas receptor.
Methods
Chemicals and antibodies
All chemicals were purchased from Sigma Chemicals (St Louis, MO) unless otherwise specified. Antibodies were obtained from the following sources and used at the dilutions recommended by the manufacturer: anti-Fas antibody (CH11 clone; Upstate Cell Signaling Solutions, Lake Placid, NY), anti-Fas antibody (Apo-1 clone; Kamiya Biomedical, Seattle, WA), anti-FADD monoclonal antibody and mouse IgG3 isotype control antibody (BD Biosciences, San Diego, CA), monoclonal anti-caspase-8 antibody (clone 12F5), monoclonal anti-caspase-3 antibody, anti-c-FLIP monoclonal antibody (clone NF6; Alexis Biochemicals, San Diego, CA), anti-mouse IgG2b (γ2b chain specific), IgG1 (Southern Biotech, Birmingham, AL), β-actin monoclonal antibody (Sigma, St. Louis, MO), and mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody (Chemicon International, Temecula, CA).
Patient characteristics and preparation of peripheral blood mononuclear cells
All patients met the clinical criteria of T-LGL leukemia with increased numbers of CD3+, CD8+, or CD57+ lymphocytes in the peripheral blood. Patients were clinically stable and received no treatment at the time of sample acquisition. Peripheral blood specimens from 10 T-LGL leukemia patients were obtained according to a protocol approved by the Institutional Review Board of Penn State Cancer Institute (Hershey, PA) and H. Lee Moffit Cancer Center and Research Institute (Tampa, FL). Informed consent was obtained in accordance with the Declaration of Helsinki. Not all of the investigational studies were performed in each patient, as outlined below. Buffy coats from age- and sex-matched normal donors were also obtained from the blood bank of Milton S. Hershey Medical Center at the College of Medicine, Penn State University. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque gradient separation, as described previously.7 Cell viability was determined by trypan blue exclusion assay with more than 95% viability in all the samples.
Cell culture and medium
Culture of all cell lines and PBMCs were carried out using RPMI-1640 medium supplemented with 10% fetal calf serum (both from Invitrogen), glutamine (200 μmol/L/mL) and penicillin (100 units/mL)/streptomycin (100 μg/mL) and grown in 5% CO2 at 37°C.
DISC formation and immunoprecipitation
Induction of the DISC was carried out in 5 paired normal and patient PBMCs, as described elsewhere.27 In brief, freshly isolated PBMCs (3 × 107 cells/sample) were pretreated with or without recombinant human IL-2 (500 IU/mL; Promega, Madison, WI) for 24 hours followed by the addition of either anti-Fas antibody (Apo-1, 1 μg/mL) or IgG3 isotype control antibody (1 μg/mL) and then further incubated for 45 minutes or 6 hours. As an additional negative control, Apo-1 antibody was added into the lysates collected from IgG3 antibody-treated cells for an additional 30 minutes to test for spontaneous formation of the DISC. CD4 positive human leukemia cell line H9 (American Type Culture Collection, Manassas, VA) cells were used as a positive control for the induction of the DISC components. After anti-Fas incubation, unbound antibodies were removed through repeated washes with ice-cold phosphate-buffered saline. Coimmunoprecipitation (IP) of DISC components was performed using cells lysed with 1 mL of modified IP lysis buffer (30 mM Tris/HCl, pH 7.5, 1% Triton X-100, 10% glycerol, 150 mM/L NaCl, and 1 μg/mL of leupeptin, 10 μg/mL of aprotinin, 1.5 μmol/L pepstatin, 1 mmol/L sodium orthovanadate, and 1 mmol/L phenylmethylsulfonyl fluoride), as described previously.27 Cell lysates were then incubated with 100 μL of protein A/G Sepharose beads (Immunoprecipitation Starter Pack; GE Healthcare, Chalfont St Giles, UK) at a 1:1 ratio overnight at 4°C, then washed with ice-cold IP lysis buffer; proteins were separated by gel electrophoresis. Western immunoblotting assays were performed for caspase-8 and FADD using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Western blot analysis
Western blot analyses of caspase-8, caspase-3, FADD, and c-FLIP were performed using mouse monoclonal antibodies on whole-cell lysates 8 from patients and the matched healthy donors. Blots were washed and developed with enhanced chemiluminescence (ECL-plus; GE Healthcare) following the manufacturer's instructions. Semiquantitative densitometry analysis was performed by used ChemDox XR (a gel documentary system; Bio-Rad Laboratories, Hercules, CA) to determine the volume as well as the densities of the protein bands on the Western blot membranes.
Apoptosis assay
Apoptosis was determined in samples from 8 patients with LGL by 2-color flow cytometry with annexin-V (5 μL per sample; BD Pharmingen, San Diego, CA) and 7-amino-actinomycin D (7-AAD; 10 μL per sample) staining using 5 × 105 cells per sample. Cell viability was also confirmed by trypan blue exclusion. The percentage specific apoptosis was calculated using the following formula: Apoptosis (%) = (% Annexin-V-fluorescein isothiocyanate [FITC]-positive in assay well − % Annexin-V-FITC positive in the control well) × 100/(100 − % Annexin-V-FITC positive in the control well).
Caspase-8 and caspase3/7 assay for enzymatic activity
Caspase-8 and caspase-3/7 activity was determined in samples from 5 patients with LGL using the Caspse-8/FLICE fluorometric protease kit (MBL Co., LTD, Woburn, MA) and APO-ONE homogeneous caspase-3/7 assay kit (Promega, Madison, WI), respectively. Assays were performed in triplicate, and each experiment was repeated 3 times to determine the reproducibility of these assays.
Statistics
All data are expressed as mean (± SEM). Paired Student t test (2-tail paired 2;1) was used to determine the statistical significance, and P values no greater than .05 were considered statistically significant. Nonparametric tests Wilcoxon W and matched-pairs Wilcoxon test were also used for freedom from variant problems, and Z score either more than 2.0 or less than −2.0 was considered significantly different.
Results
Leukemic LGL display a CD8+ terminal-effector memory phenotype
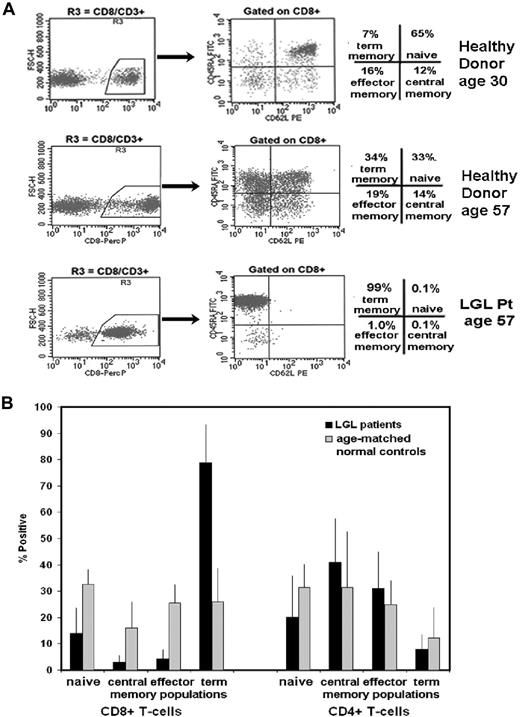
CD45RA and CD62L expression define 4 distinct subpopulations of naive and memory T cells with diverse functions in lymph node homing, cytotoxicity, antigen activation, survival, homeostatic proliferation, and cytokine secretion.28-31 These 4 naive and memory subpopulations consist of (1) naive CD8+ T cells that coexpress CD62L and CD45RA, (2) central memory cells that express CD62L but are CD45RA negative, (3) effector memory cells that lack expression of both molecules, and (4) terminal-effector memory cells that are CD45RA positive and CD62L negative. T cells originate from the thymic export of naive cells, and then repeated in vivo antigen stimulation is required for terminal-effector memory differentiation.28,31,32 As shown in Figure 1A, the percentage of naive CD8+ T cells decreases with age. This has been shown previously to correlate with an age-dependent decline in thymic function.33,34 In studying PBMCs from 6 patients with LGL leukemia and 6 healthy age-matched control subjects, we found that patients had a dramatic increase in the CD8+ terminal-effector memory T-cell subpopulation and reduced percentages of CD8+ T cells with naive, central memory, and effector memory phenotypes (Figure 1B). This altered distribution pattern was restricted to CD8+ T cells because a difference in subpopulation distribution was not detected in CD4+ T cells. These results suggest that CD8+ T cells accumulate in LGL leukemia with a polarized differentiation pattern, which suggests that the CD8+ cells have undergone repeated in vivo antigen stimulation.
Naive and memory phenotyp13.6pe distribution in CD8+ and CD4+ T cells from patients with LGL leukemia compared with healthy control subjects using coexpression patterns of CD45RA and CD62L. (A) Flow cytometry was performed on cells stained with CD3, CD8, CD62L, and CD45RA. A gate was set on CD3+ cells and a second gate set on CD8+ (R3) and CD8− (CD4+) T cells. CD62L (x-axis) and CD45RA (y- axis) were used to distinguish specific T-cell subsets that included naive (top right quadrant), central memory (bottom right quadrant), effector memory (bottom left quadrant), and terminal-effector memory (top left quadrant) T cells. Representative results from healthy donors aged 30 and 57 years and a patient with LGL leukemia aged 57 years are shown. (B) Graphic representation of the mean percentage positive of these 4 distinct T-cell subsets in patients with LGL leukemia (n = 6) compared with age-matched healthy control subjects (n = 6). Standard deviation (SD) is indicated, and statistical significance was determined by a Wilcoxon sum rank test.
Naive and memory phenotyp13.6pe distribution in CD8+ and CD4+ T cells from patients with LGL leukemia compared with healthy control subjects using coexpression patterns of CD45RA and CD62L. (A) Flow cytometry was performed on cells stained with CD3, CD8, CD62L, and CD45RA. A gate was set on CD3+ cells and a second gate set on CD8+ (R3) and CD8− (CD4+) T cells. CD62L (x-axis) and CD45RA (y- axis) were used to distinguish specific T-cell subsets that included naive (top right quadrant), central memory (bottom right quadrant), effector memory (bottom left quadrant), and terminal-effector memory (top left quadrant) T cells. Representative results from healthy donors aged 30 and 57 years and a patient with LGL leukemia aged 57 years are shown. (B) Graphic representation of the mean percentage positive of these 4 distinct T-cell subsets in patients with LGL leukemia (n = 6) compared with age-matched healthy control subjects (n = 6). Standard deviation (SD) is indicated, and statistical significance was determined by a Wilcoxon sum rank test.
TCR-independent sensitivity to Fas-mediated apoptosis in PBMC from patients with LGL leukemia
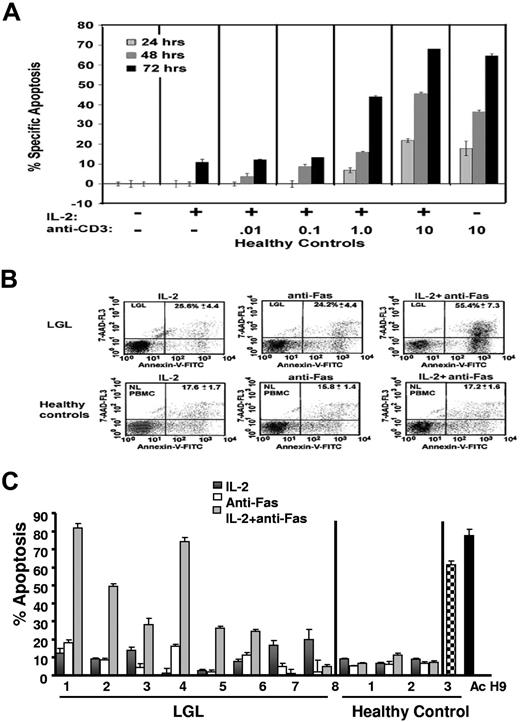
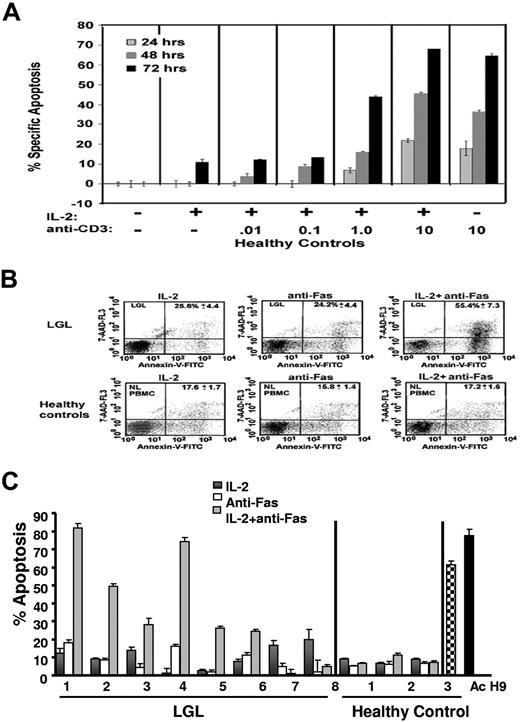
Sensitization of normal T cells to Fas-mediated apoptosis, a process known as activation-induced cell death (AICD), requires exposure to antigen stimulation.35,36 As expected, PBMC from healthy control subjects, in the absence of T cell receptor (TCR) stimulation, failed to exhibit Fas-mediated apoptosis (Figure 2A,B). A time- and dose-dependent increase in Fas-mediated apoptosis occurred in the presence of increasing doses of anti-CD3 antibody cross-linking used to stimulate the TCR (Figure 2A). Next, we examined Fas-mediated apoptosis sensitivity in PBMC from 8 patients with LGL leukemia compared with healthy control subjects. We found that Fas-receptor cross-linking resulted in little or no increase in the percentage of apoptotic cells in PBMC from either healthy control subjects or patients with LGL leukemia consistent with our previous reports.7 However, the addition of IL-2 to the culture medium in the absence of in vitro antigen stimulation sensitized PBMCs from 6 of 8 patients with LGL leukemia, but not those from healthy subjects, to Fas apoptosis (Figure 2B,C). In the healthy control subjects, we show that Fas apoptosis was elicited as expected by the addition of PHA (Figure 2C, healthy control, Ac [hatched bar]). We conclude that leukemic LGL cells are resistant to Fas-mediated apoptosis but immediately respond to IL-2 in the absence of in vitro TCR stimulation, which provides further functional evidence that these cells were exposed to antigen in vivo before isolation. In 2 patients, IL-2 sensitization to Fas-mediated apoptosis was not observed. This might indicate some heterogeneity in molecular survival pathways among patients with LGL leukemia. We had described similar heterogeneity in inhibition of STAT signaling leading to sensitization of Fas-mediated apoptosis.37
TCR-independent sensitivity to Fas-mediated apoptosis in PBMCs from LGL leukemia patients. (A) The percentage of apoptotic cells was determined by annexin-V-FITC/7-AAD staining in healthy control subjects treated in the absence of IL-2 and anti-CD3-antibody (dose 0) or in the presence of IL-2 (500 IU/mL) in combination with increasing doses of plate-bound anti-CD3 antibody (0.01, 0.1, 1.0, and 10 μg/mL). (B) Flow cytometric dot plots of PBMCs from a representative control donor (lower row) and from an LGL leukemia patient (upper row). Cells that stained with both annexin-V-FITC (x axis) and 7-AAD (y axis) plus cells stained with annexin-V-FITC alone were considered apoptotic and the values are shown in the upper right hand corner of each dot plot. (C) The percentage of specific apoptosis was determined in cells from 8 patients with LGL leukemia and from 3 healthy donors. Cells were assigned to one of 3 treatment groups: (1) IL-2 (500 IU/mL) for 48 hours, (2) anti-Fas (1.0 μg/mL) for 24 hours, or (3) IL-2 for 24 hours plus anti-Fas for an additional 24 hours. H9 cells and PHA/IL-2-activated normal PBMCs served as positive control. Results shown represent the mean percentage of specific apoptosis in individual samples using the equation described in “Apoptosis assay.” Standard error of the mean (SEM) is indicated for experiments performed in triplicate.
TCR-independent sensitivity to Fas-mediated apoptosis in PBMCs from LGL leukemia patients. (A) The percentage of apoptotic cells was determined by annexin-V-FITC/7-AAD staining in healthy control subjects treated in the absence of IL-2 and anti-CD3-antibody (dose 0) or in the presence of IL-2 (500 IU/mL) in combination with increasing doses of plate-bound anti-CD3 antibody (0.01, 0.1, 1.0, and 10 μg/mL). (B) Flow cytometric dot plots of PBMCs from a representative control donor (lower row) and from an LGL leukemia patient (upper row). Cells that stained with both annexin-V-FITC (x axis) and 7-AAD (y axis) plus cells stained with annexin-V-FITC alone were considered apoptotic and the values are shown in the upper right hand corner of each dot plot. (C) The percentage of specific apoptosis was determined in cells from 8 patients with LGL leukemia and from 3 healthy donors. Cells were assigned to one of 3 treatment groups: (1) IL-2 (500 IU/mL) for 48 hours, (2) anti-Fas (1.0 μg/mL) for 24 hours, or (3) IL-2 for 24 hours plus anti-Fas for an additional 24 hours. H9 cells and PHA/IL-2-activated normal PBMCs served as positive control. Results shown represent the mean percentage of specific apoptosis in individual samples using the equation described in “Apoptosis assay.” Standard error of the mean (SEM) is indicated for experiments performed in triplicate.
IL-2 facilitates Fas apoptosis in PBMC from patients with LGL leukemia via induction of DISC formation in the absence of TCR stimulation
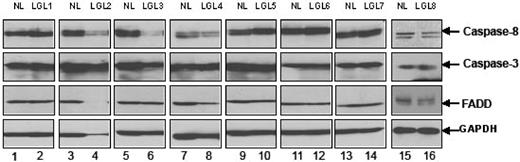
Fas-mediated apoptosis requires the formation of a DISC with recruitment of FasR, FADD, and caspase-8.24,27 We first examined levels of expression of caspase 8, caspase 3, and FADD before activation. We found that PBMCs from patients with LGL leukemia expressed levels of these proteins generally comparable with levels seen in samples from healthy control subjects (Figure 3). Next, we examined DISC formation in PBMCs from patients compared with those from healthy controls by coimmunoprecipation of FADD and caspase-8 after anti-Fas antibody cross-linking, using methods described previously.27
Expression of caspase-8, caspase-3, and FADD protein. The basal protein expression levels of caspase-8, caspase-3, and FADD were determined by Western blot assay using whole-cell lysates from 8 patients with LGL leukemia and from 8 healthy donors. Bands indicate the position of caspase-8, caspase-3, and FADD. Western blot analysis for GAPDH was performed to confirm equal loading of total protein in each lane.
Expression of caspase-8, caspase-3, and FADD protein. The basal protein expression levels of caspase-8, caspase-3, and FADD were determined by Western blot assay using whole-cell lysates from 8 patients with LGL leukemia and from 8 healthy donors. Bands indicate the position of caspase-8, caspase-3, and FADD. Western blot analysis for GAPDH was performed to confirm equal loading of total protein in each lane.
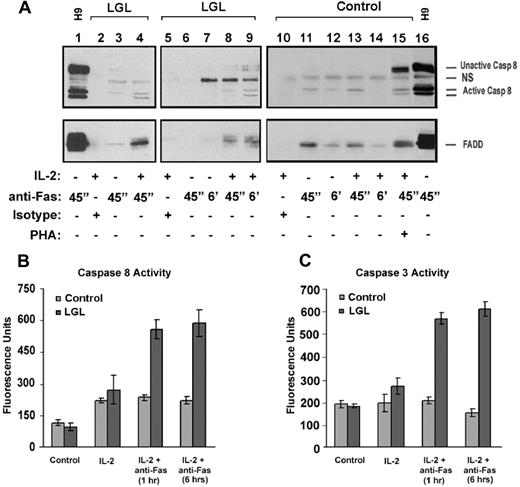
As shown in Figure 4A, there was little or no coprecipitation of FADD and caspase-8 in either healthy controls or patients with leukemic LGL in the absence of IL-2 and TCR stimulation. In contrast, IL-2 treatment for 24 hours enhanced the coimmunoprecipitation of both FADD (Figure 3 bottom panel lanes 4, 8, 9) and caspase-8 (Figure 3 top panel lanes 4, 8, 9) in leukemic LGL cells. These coprecipitated proteins were present after anti-Fas antibody cross-linking for 45 minutes and increased further after 6 hours. Protein interactions were specific to FasR cross-linking, because coprecipitated proteins were not present after incubation with an isotype control antibody (IgG3) (Figure 4 lanes 2, 5, and 10). In normal PBMC samples not stimulated through the T cell receptor, we observed coimmunoprecipitation of some FADD after IL-2 treatment, but without evidence of caspase 8 activation (Figure 4 lanes 13, 14). Treatment with IL-2 and TCR stimulation in vitro with PHA was required to induce DISC formation in normal PBMC after anti-Fas antibody cross-linking (Figure 3A lane 15). Results shown in Figure 4 are representative of 5 patients and 3 normal donors examined in total.
DISC formation in PBMC from LGL leukemia patients and from healthy donors. (A) Fas-mediated DISC formation was determined after 45 minutes and after 6 hours of cross-linking with (500 ng/mL) anti-Fas (APO-1, IgG3) antibody (lanes 1, 3, 4, 6-9, 11-14) in samples from 5 patients with LGL leukemia. Cells were cultured for 24 hours with medium in the absence (−, lanes 1, 3, 6, 7, and 11) and presence of 500 IU/mL IL-2 (+, lanes 2, 4, 5, 8-10, 12, 13) for 24 hours, or PHA (1 μg/mL) plus IL-2 (500 IU/mL) for 5 to 7 days (indicated as PHA in lane 14). IgG3 isotype control antibody (500 ng/mL) was added to demonstrate specificity of protein interactions with the FasR (lanes 2, 5, and 10). After immunoprecipitation and gel electrophoresis, Western blot analysis was performed to detect caspase-8 (top panel) and FADD (bottom panel) in these FasR immunoprecipitates. Immunoprecipitation after anti-Fas antibody cross-linking of H9 cells (T-cell leukemia cell line) was used as a positive control. (B) Whole-cell lysates were prepared from a fraction of cells studied in these immunoprecipitation experiments after 1 and 6 hours of cross-linking with anti-Fas (Apo-1) antibody and isotype control (IgG3). Results shown represent the means (± SEM) of caspase-8, and -3/7 activities that were determined by a fluorometric enzyme activity assay. Protein bands for full-length caspase-8 (casp-8), FADD, and an activated cleaved product of caspase-8 (Ac casp-8) are indicated. NS = nonspecific band observed with the anti-caspase-8 antibody.
DISC formation in PBMC from LGL leukemia patients and from healthy donors. (A) Fas-mediated DISC formation was determined after 45 minutes and after 6 hours of cross-linking with (500 ng/mL) anti-Fas (APO-1, IgG3) antibody (lanes 1, 3, 4, 6-9, 11-14) in samples from 5 patients with LGL leukemia. Cells were cultured for 24 hours with medium in the absence (−, lanes 1, 3, 6, 7, and 11) and presence of 500 IU/mL IL-2 (+, lanes 2, 4, 5, 8-10, 12, 13) for 24 hours, or PHA (1 μg/mL) plus IL-2 (500 IU/mL) for 5 to 7 days (indicated as PHA in lane 14). IgG3 isotype control antibody (500 ng/mL) was added to demonstrate specificity of protein interactions with the FasR (lanes 2, 5, and 10). After immunoprecipitation and gel electrophoresis, Western blot analysis was performed to detect caspase-8 (top panel) and FADD (bottom panel) in these FasR immunoprecipitates. Immunoprecipitation after anti-Fas antibody cross-linking of H9 cells (T-cell leukemia cell line) was used as a positive control. (B) Whole-cell lysates were prepared from a fraction of cells studied in these immunoprecipitation experiments after 1 and 6 hours of cross-linking with anti-Fas (Apo-1) antibody and isotype control (IgG3). Results shown represent the means (± SEM) of caspase-8, and -3/7 activities that were determined by a fluorometric enzyme activity assay. Protein bands for full-length caspase-8 (casp-8), FADD, and an activated cleaved product of caspase-8 (Ac casp-8) are indicated. NS = nonspecific band observed with the anti-caspase-8 antibody.
To achieve the full proapoptotic function of caspase-8, a transcatalytic cleavage process is necessary to generate 18- and 10-kDa cleavage products from the parent protein (ie, p18 and p10 subunits, respectively).20,36,38 As demonstrated by Western blot analysis in Figure 4A, caspase-8 was cleaved after anti-Fas antibody cross-linking in the presence of IL-2 but not without IL-2. Cell lysates from these experiments were also prepared at 1 and 6 hours to confirm that the enzymatically functional proteins were generated. Basal activities of both caspase-8 and caspase-3 were similar in samples from patients with LGL leukemia and healthy donors. Consistent with our findings by Western blot in Figure 4A, cross-linking with the anti-Fas antibody increased caspase-8 enzymatic activity by 6-fold at the 1-hour and 6-hour time-points in IL-2-treated cells from patients with LGL leukemia but not in PBMC from healthy donors. In these same samples, caspase-3 activity was also enhanced by 3-fold after 1 hour and 6 hours (Figure 4B,C). PHA and IL-2 treatment of normal PBMC was required for the activation of these enzymes, as predicted by the Western blot data (data not shown).
c-FLIP expression is up-regulated in samples from patients with LGL leukemia patients and is differentially modulated by IL-2
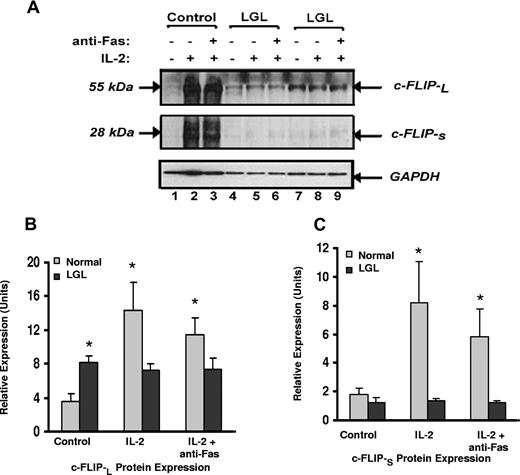
Our results suggest that leukemic LGL are exposed to antigen in vivo but are resistant to Fas-mediated apoptosis by survival-promoting factors that protect cells from DISC formation. This resistance is reversed by IL-2 treatment in the absence of antigen stimulation. Because of the known inhibitory effect of c-FLIP on DISC formation,19-21,39 we determined levels of expression of c-FLIP-L and c-FLIP-S by Western blot analysis with a monoclonal antibody that detects both isoforms. Representative results are shown in Figure 5A and summarized in Figure 5B,C. We found that the basal expression of c-FLIP-L protein in samples from patients from LGL leukemia was significantly higher than in samples from healthy donors (P < .05). Similar results were observed using real-time PCR (data not shown). Only low levels of c-FLIP-s protein were detected, which did not differ in samples from patients with LGL leukemia compared with those from healthy donors. Culture with IL-2 alone, in the absence of antigen stimulation, induced a dramatic increase in both c-FLIP-L and c-FLIP-S proteins in normal PBMC (Figure 5B,C), which correlated with apoptosis resistance, as shown in Figure 2B,C. Levels of either c-FLIP-L and c-FLIP-S in samples from patients LGL leukemia did not change with IL-2 treatment.
Expression of c-FLIP proteins in samples from patients with LGL leukemia and healthy control subjects. (A) c-FLIP Western blot analysis of whole cell lysates from a representative normal donor (control) and from 2 representative patients with LGL leukemia treated with medium alone, IL-2 (500 IU/mL), or IL-2 plus anti-Fas (APO-1) 1.0 μg/mL for 24 hours before lysis. Western blot analysis for GAPDH was performed to confirm equal loading of total protein in each lane. (B) Densitometry was performed on Western blot results from patients 8 LGL leukemia and 6 healthy control subjects to determine the average level of c-FLIP-L and c-FLIP-S protein expression in medium control (control), IL-2, and IL-2 plus anti-Fas (APO-1)-cultured cells. Indicated on the gel are c-FLIP-L and c-FLIP-S of molecular masses 55 and 28 kDa, respectively. *P ≤ .05.
Expression of c-FLIP proteins in samples from patients with LGL leukemia and healthy control subjects. (A) c-FLIP Western blot analysis of whole cell lysates from a representative normal donor (control) and from 2 representative patients with LGL leukemia treated with medium alone, IL-2 (500 IU/mL), or IL-2 plus anti-Fas (APO-1) 1.0 μg/mL for 24 hours before lysis. Western blot analysis for GAPDH was performed to confirm equal loading of total protein in each lane. (B) Densitometry was performed on Western blot results from patients 8 LGL leukemia and 6 healthy control subjects to determine the average level of c-FLIP-L and c-FLIP-S protein expression in medium control (control), IL-2, and IL-2 plus anti-Fas (APO-1)-cultured cells. Indicated on the gel are c-FLIP-L and c-FLIP-S of molecular masses 55 and 28 kDa, respectively. *P ≤ .05.
Discussion
The etiology of LGL leukemia is unknown, but antigen activation of T cells has been proposed as the initiating event that leads to the clonal expansion.40 Fas-mediated apoptosis plays a crucial role in the homeostasis of T-cell survival by eliminating antigen-activated effector lymphocytes. During T-cell activation, IL-2 plays an initial role to promote survival and proliferation but then helps to augment Fas sensitivity after a strong TCR stimulus (activation-induced cell death). Control of activation-induced cell death under physiologic situations when the TCR is chronically stimulated, suboptimally stimulated, or stimulated with autologous antigens has not been studied, and the mechanism for apoptosis resistance in LGL leukemia is unknown.
Leukemic LGL were shown to display several activation-specific phenotypic markers, such as constitutive perforin expression, FasL expression, expression of CD57, and diminished expression of CD5 and CD7.7,8,40 In this report, we demonstrate that patients with LGL leukemia have a striking accumulation of CD8+ terminal-effector memory T cells. This particular subpopulation of T cells may accumulate because of repeated antigen stimulation in vivo.32 Mechanisms that control the survival of terminal-effector memory T cells and the mechanisms responsible for expansion of these cells in a normal setting have not been determined.
The assembly of the DISC that is initiated by Fas re-ceptor cross-linking mediates elimination of antigen-activated T cells. We found that cells from patients with LGL leukemia were resistant to Fas-mediated apoptosis and that the initial events of the Fas-apoptosis pathway (ie, FADD and caspase-8 recruitment) failed to occur after cross-linking. We have shown that apoptotic resistance in LGL leukemia is not related to mutations of the FasR gene.7 Impaired DISC formation can be mediated by c-FLIP-L protein blockade or antiapoptotic signaling molecules such as the Bcl-2 family. We have shown that leukemic LGL express the antiapoptotic Bcl-2-family protein Mcl-1, variably express Bcl-2, but entirely fail to express Bcl-xL.40 These proteins generally function downstream of DISC assembly.
The fact that the DISC fails to form after anti-Fas cross-linking, suggests that proximal signaling events may be disrupted.41 Control of the DISC assembly and caspase-8 cleavage is controlled by the protein c-FLIP.42 c-FLIP is composed of 2 death-effector domains (DEDs), which are structurally related to the N-terminal half of caspase-8.20 Through DED interactions between c-FLIP and the FasR, FADD, and caspase-8 proteins fail to be recruited.22 Expression of c-FLIP-L has been reported to confer resistance to Fas-mediated apoptosis in several tumor cell lines.43-45 Persistent in vitro exposure to antigen, in a model of long-term activation of normal T cells, contributed to apoptosis resistance.32 In this model, continuous repetitive antigen stimulation reduced DISC assembly and induced higher basal expression of the c-FLIP-L protein, which contributed to apoptosis resistance. We show similar characteristics in cells from patients with LGL leukemia, including constitutive expression of c-FLIP-L, as shown by Western blot analysis and quantitative real-time PCR. This level of c-FLIP was significantly higher than that in control subjects, and it may be sufficient to prevent clustering of the FasR in vivo, prevent DISC formation, and thus prevent Fas-mediated apoptosis.
Cells from patients with LGL leukemia displayed enhanced sensitivity to Fas-apoptosis and DISC formation after culture in IL-2 in the absence of in vitro antigen treatment. Because DISC formation in normal activated PBMCs required, in addition to Fas triggering, both IL-2 activation and T-cell receptor stimulation by PHA, these findings further suggest that leukemic LGL have already been activated through the T-cell receptor in vivo, by exposure to antigen. How IL-2 promotes DISC formation and Fas sensitivity in leukemic LGL is not known. In healthy control subjects, IL-2 treatment in the absence of antigen strongly induced the expression of c-FLIP-L and correlated with apoptosis resistance. However, IL-2 stimulation had no effect on c-FLIP expression in LGL leukemia samples. This might indicate that apoptosis sensitivity to IL-2 was not dependent on regulation of c-FLIP. Alternatively, dysregulation of c-FLIP by IL-2, as observed in LGL leukemia samples, may contribute to apoptosis sensitivity because of the notable lack of increased levels of c-FLIP, which would protect against apoptosis. The mechanism of dysregulation of c-FLIP in LGL leukemia is not understood but is under investigation. The Fas pathway is activated at precise stages of the immune response, which is particularly critical for the depletion of autoreactive T cells during thymic development and elimination of activated T cells following an immune response.13 Alterations in this cell death program may lead to enhanced survival of potentially harmful autoreactive T lymphocytes.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Gary A. Chase and the Penn State Cancer Institute Biostatistical Core for assistance with the data analyses. We thank Lynn F. Ruiz, research studies coordinator and LGL registrar. We thank Kendall Thomas and Tylee Walck for blood preparation. We also thank the Flow Cytometry Core Facility at Penn State Cancer Institute for acquisition and analysis of flow cytometric data.
This work was supported by VA Merit Grant and National Institute of Health grants R01-CA94872, R01-CA112112, and R01-AI056213.
Authorship
Contribution: J.Y., P.K.E.-B., S.E.W., and T.P.L. designed research, analyzed data, and wrote the manuscript. J.Y., J.S.P., J.X.Z., and F.B. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jun Yang, MD, Penn State College of Medicine, Penn State Cancer Institute, H072, Set C6846, 500 University Drive, Hershey, PA 17033; e-mail: jyang@psu.edu.
References
Author notes
J.Y. and P.K.E.-B. are equal first authors.