Hypomorphic mutations of the RAG genes in humans are associated with a spectrum of clinical and immunologic presentations that range from T− B− severe combined immune deficiency (SCID) to Omenn syndrome. In most cases, residual V(D)J recombination activity allows for development of few T-cell clones, which expand in the periphery and infiltrate target organs, resulting in tissue damage. Invariant natural killer T (iNKT) cells play an important immunoregulatory role and have been associated with protection against autoimmunity. We now report on 5 unrelated cases of combined immune deficiency due to hypomorphic RAG mutations, and demonstrate the absence of iNKT cells in all 5 patients. These findings suggest that lack of this important immunoregulatory cell population may contribute to the pathophysiology of Omenn syndrome.
Introduction
Omenn syndrome (OS) is a combined immunodeficiency characterized by early-onset erythroderma, lymphadenopathy, hepatosplenomegaly, and severe infections. Patients with OS have a variable number of autologous, oligoclonal, and activated T cells that infiltrate and damage target tissues.1,2
OS may be due to heterogeneous gene defects that impair, but do not abolish, thymic T-cell development. In particular, hypomorphic mutations of the RAG1 or RAG2 genes, involved in V(D)J recombination, are a common cause for OS.3,–5 The same defects may lead to a spectrum of clinical and immunologic phenotypes that also include typical T− B− severe combined immune deficiency (SCID) or leaky SCID with residual presence of activated T cells, in the absence of typical features of OS.4,6
The clinical and pathological features of OS are suggestive of T-cell–mediated autoimmunity. In keeping with this hypothesis, we have found that expression of autoimmune regulator (AIRE) and of AIRE-dependent tissue-specific transcripts is reduced in the thymus from patients with OS, possibly contributing to impaired central tolerance.7 In addition, defects of regulatory T cells have been also hypothesized in patients with OS.8 Both abnormalities have been confirmed in a newly generated murine model of OS.9 However, the possible contributory role of other immunomodulatory components in determining the phenotype of OS has not been carefully evaluated.
Natural killer T (NKT) cells represent a population of cells with significant immunomodulatory properties.10 In humans, most NKT cells recognize glycolipids presented in the context of CD1d molecules and express NK-cell markers along with an invariant T-cell receptor (TCR) with a Vα24-Jα18 rearrangement. NKT cells expressing the invariant TCR (iNKT) can be identified using α-galactosylceramide (α-GalCer)–loaded CD1d tetramers.11 iNKT cells are generated in the thymus from CD4+CD8+ thymocytes that rearrange the invariant TCR and are directed to the NKT lineage following cognate interactions with CD1d-expressing cortical thymocytes.12,13 Therefore, NKT cell development is absolutely dependent on V(D)J recombination, consistent with the observation that iNKT cells are severely reduced in a Rag2 knock-in mouse model of OS.9
In this study, we provide the first evidence that iNKT cells are absent in peripheral blood of patients with hypomorphic RAG mutations, a situation where substantial numbers of autoreactive T cells develop. We speculate that the absence of iNKT cells may contribute to the pathophysiology of OS.
Methods
Patients
Five unrelated patients with hypomorphic RAG defects were studied; 4 of them (patients 1, 2, 3, and 5) had typical OS, whereas patient 4 had the phenotype of T+B− leaky SCID (Table 1). Informed consent was obtained in accordance with the Declaration of Helsinki and according to protocols approved by Children's Hospital, Boston; the Department of Pediatrics, University, Brescia, Italy; and the Department of Pediatrics, Tor Vergata University, Rome, Italy. Maternal T-cell engraftment was ruled out by molecular analysis using microsatellite markers.
Mutation analysis
Genomic DNA was extracted by standard procedures from blood samples. The single exons of RAG genes were amplified by polymerase chain reaction (PCR) using 13 pairs of overlapping primers, and the presence of mismatches was revealed by denaturing high-performance liquid chromatography (DHPLC). When mismatches were found, direct sequencing was performed using the Big Dye Terminator kit (Applied Biosystems, Foster City, CA) on an ABI PRISM 3130 automatic sequencer (Applera, Norwalk, CT).
Immunophenotyping of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Histopaque 1077 (Sigma-Aldrich, St Louis, MO). Immunophenotyping was performed by flow cytometry (BD FACSCanto) using the following antihuman antibodies: FITC-conjugated anti-CD4 (BD Pharmingen, San Diego, CA), fluorescein isothiocyanate (FITC)–conjugated anti-Vα24 (Immunotech, Miami, FL), FITC-conjugated mouse IgG1 isotype control (eBioscience, San Diego, CA), phycoerythrin (PE)–conjugated anti-Vβ11 (Immunotech), PE-conjugated anti-invariant NK T cells (clone 6B11; BD Pharmingen), PE-conjugated mouse IgG2a isotype control (BD Pharmingen), PE-conjugated mouse IgG1 (BD Pharmingen), PE-conjugated PBS57-loaded human CD1d tetramer or PE-conjugated unloaded human CD1d tetramer (National Institutes of Health [NIH] Tetramer Facility, Bethesda, MD), PE-Cy5 conjugated anti-CD45 (eBioscience), and Alexa750-conjugated anti-CD3 (eBioscience). Data were analyzed using the FlowJo 8.3.3 software (TreeStar, Ashland, OR). Leukocytes were gated on CD45+ cells. Lymphocytes were gated based on forward and side scatter. CD3+ lymphocytes were then analyzed for expression of invariant T-cell receptor (iTCR) of iNKT cells as determined by staining with PBS57-loaded CD1d tetramer (in comparison with unloaded CD1d tetramers). The percentage of iNKT cells in patients 3 and 5 was analyzed also by anti-Vα24 and anti-Vβ11 antibodies as well as by anti-invariant NKT cells (clone 6B11) antibody.
V(D)J recombination analysis
V(D)J recombination activity of the human RAG1 mutants G139R, G392E, and L732fs was assessed by a plasmid-based colony-forming assay by cotransfecting into 293T cells the expression vectors of wild-type hRAG1 and wild-type hRAG2 (or of the mutants) with substrates pGG49 and pGG51 to allow to measure signal joints and coding joint formation, respectively.14
Results and discussion
The 5 patients represent the spectrum of clinical and laboratory manifestations associated with hypomorphic RAG mutations. In all cases, the presence of at least one hypomorphic RAG allele allowed for residual T-cell development, with accumulation of activated and anergic autologous T cells. The M435V mutation in the RAG1 gene of patient 1 falls within the nonamer-binding domain, and has been previously reported in OS.6 The G392E mutation of patient 3 involves the first residue of the GGRPR motif of the nonamer-binding domain (NBD) and had not been previously reported. While double mutations of murine GG 389-390 (corresponding to human residues 392-393) have a dramatic impact on nonamer binding,15 single amino acid substitutions in the NBD allow for residual V(D)J recombination activity and have been identified in patients with OS.3,16 The R561H mutation of patient 4 has been previously reported.3,6 It affects the Rag-2 interacting domain of Rag-1 and reduces V(D)J recombination activity by 3- to 4-fold.3 The RAG1 L526R mutation of patient 5 is novel. Finally, the RAG2 G139R mutation of patient 2 falls in the first β strand of the third kelch motif within the β-propeller structure of the catalytic core of Rag-2, where several other disease-causing mutations have been identified.17,18 The novel mutations detected in patients 2 and 3 were found to severely reduce, but not completely abrogate, the V(D)J recombination activity (Table 1).
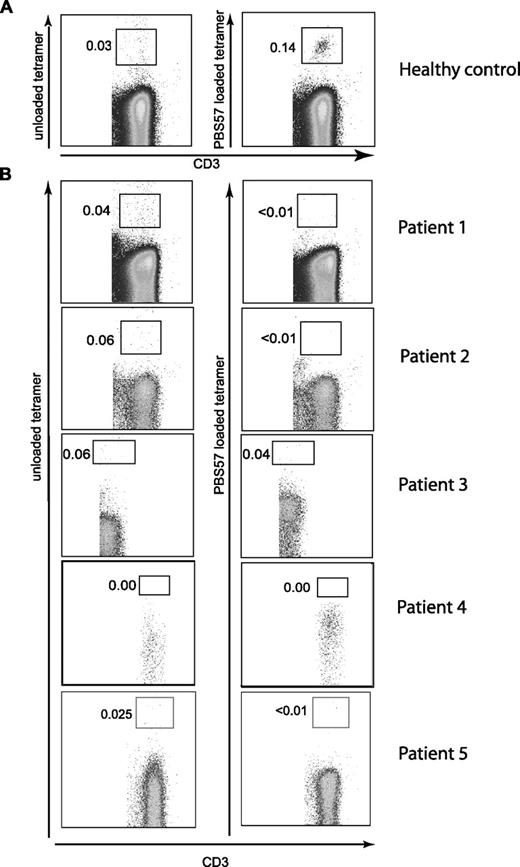
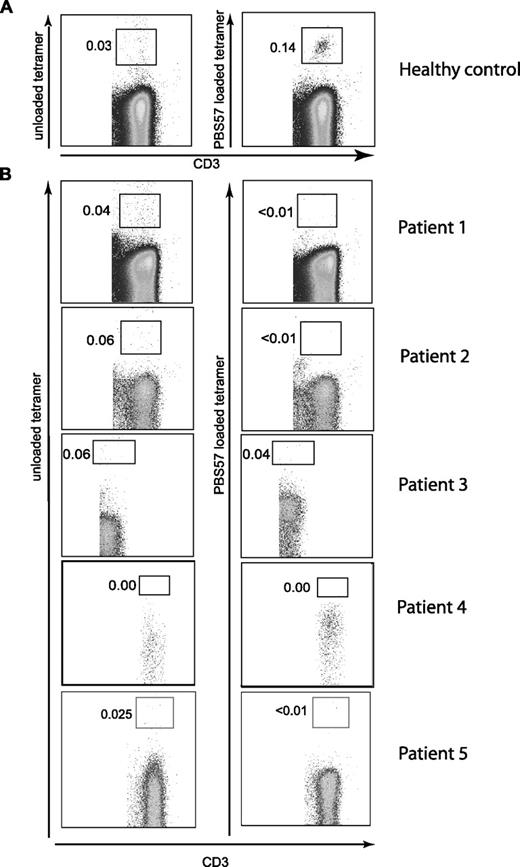
The 5 patients examined had variable numbers of autologous peripheral blood CD4+ and CD8+ T cells (Table 1), indicating that at least limited V(D)J recombination occurred in these patients. Since CD4+ T cells in OS have been shown to produce Th2 cytokines,19 and CD4+ iNKT cells also produce Th2 cytokines,20 we asked whether any of the peripheral blood T cells in OS patients were iNKT cells. Using α-GalCer–loaded CD1d tetramers, we were unable to detect iNKT cells (Figure 1), while iNKT cells were present in healthy controls (Figure 1; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Lack of iNKT cells in OS patients was confirmed by staining with the 6B11 antibody (which binds to the CDR3 region of human invariant TCR) and by analysis of Vα24+/Vβ11+ T cells (Figure S2).
Flow cytometric analysis of peripheral blood iNKT cells from patients with hypomorphic RAG mutations. Mononuclear cells (PBMCs) from a healthy control (representative of 6 donors) (A) and 5 patients with hypomorphic mutations of the RAG genes (B) were analyzed for the presence of iNKT cells. Leukocytes were enumerated by gating on CD45+CD3+ cells. iNKT cells were identified using PBS57-loaded CD1d tetramers (right) using cells stained with unloaded CD1d tetramers as control (left).
Flow cytometric analysis of peripheral blood iNKT cells from patients with hypomorphic RAG mutations. Mononuclear cells (PBMCs) from a healthy control (representative of 6 donors) (A) and 5 patients with hypomorphic mutations of the RAG genes (B) were analyzed for the presence of iNKT cells. Leukocytes were enumerated by gating on CD45+CD3+ cells. iNKT cells were identified using PBS57-loaded CD1d tetramers (right) using cells stained with unloaded CD1d tetramers as control (left).
iNKT cells are important in protecting against autoimmune manifestations21 and graft-versus-host disease (GvHD).22 Moreover, they can suppress pathogenic Th1 cells.23 Numeric or functional defects of iNKT cells have been reported in numerous autoimmune conditions in humans and in mice.10,23 Therefore, the absence of iNKT cells in OS may contribute to the development of the skin disease and colitis frequently observed in these patients.
In conclusion, our data demonstrate that hypomorphic mutations of the RAG genes in humans are not permissive for development of iNKT cells. The contribution of this abnormality to the pathophysiology of OS could be addressed by adoptive transfer of purified iNKT cells into mice expressing hypomorphic mutations of the Rag genes.9,24
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the NIH Tetramer Facility for providing CD1d tetramers for this study.
This work was partially supported by MIUR-PRIN 2006 and European Union (project EURO-POLICY-PID; L.D.N.), by RO1AI26322 from the NIH (D.T.U.), by NIH (P01 AI61093; P.C.), by the American Cancer Society (RSG-04-191-01-LIB; P.C.), and by Nobel Cariplo (L.D.N. and A.V.).
National Institutes of Health
Authorship
Contribution: P.M. and M.P. performed analysis of iNKT cells and contributed to the writing and critical revision of the paper; E.M. and A.F. were involved in clinical care of patients 2, 3, 4, and 5; D.E.S. performed the in vitro V(D)J recombination analysis under the supervision of P.C. S.G. performed mutation analysis at the RAG loci in patients 2, 3, and 4; A.V. and C.S. identified the mutations in patient 5; D.T.U. and L.D.N. conceived the study and wrote the paper.
P.M. and M.P. contributed equally to this work.
D.T.U. and L.D.N. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Luigi D. Notarangelo, Division of Immunology, Children's Hospital, Karp Bldg 9th Floor, Rm 9120, 1 Blackfan Circle, Boston, MA 02115; e-mail: luigi.notarangelo@childrens.harvard.edu.