Following antigen acquisition and maturation, dendritic cells (DCs) disengage from the extracellular matrix, cross basement membranes, and travel to draining lymph nodes to activate T cells. CCR7 expression is necessary but not sufficient for the directional migration of DCs. Prostaglandin E2 (PGE2), present in inflammatory sites, induces DC migration, presumably by enacting a migration-permissive gene expression program. Since regulation of DC migration is highly important for their use in vaccination and therapy, we examined the PGE2-induced changes in the expression of metalloproteinases (MMPs). Our results indicate that PGE2 significantly up-regulates MMP-9 expression, induces both secreted and membrane-bound MMP-9, and that in turn, DC-derived MMP-9 is essential for DC chemotaxis in response to the CCR7 ligand CCL19, Matrigel migration, and in vivo migration in both wild-type and MMP-9–deficient hosts. We conclude that DCs matured within inflammatory sites require both CCR7 and PGE2-induced MMP-9 for their directional migration to draining lymph nodes.
Introduction
Dendritic cells (DCs), generated from bone marrow and distributed as immature cells in tissues, have the unique ability to recognize antigen, process, and present it to cognate naive T cells following migration to draining lymph nodes. Migration to the secondary lymphoid organs and subsequent antigen presentation requires DC maturation, a process associated with up-regulation of MHC and costimulatory molecules, and with a switch in chemokine receptor expression.
CCR7 is induced upon DC maturation,1,–3 and its role in DC migration has been revealed through the use of CCR7-deficient mice or of CCR7-deficient DCs in adoptive transfer experiments.4,–6 However, expression of CCR7, although necessary, is not sufficient for DC migration.7,8 Factors present in inflammatory sites, such as lipid mediators, particularly prostaglandin E2 (PGE2),9 and most recently, the high mobility group box-1 (HMGB1) protein, have been shown to induce migration of maturing DCs.8,10,–12 The role of PGE2 in DC migration is strongly supported by the fact that mice deficient in the PGE2 receptor EP4 or wild-type mice treated with an EP4 antagonist exhibit reduced DC migration to lymph nodes.13
The PGE2-induced DC migration is not mediated through CCR7 up-regulation, since DCs matured in the presence or absence of PGE2 express similar CCR7 levels.11,12,14 Although it has been proposed that PGE2 increases CCR7 sensitivity to CCL19/CCL21 signaling, the mechanisms by which PGE2 alters CCR7 function are not clear. Although PGE2 was proposed to increase CCR7 signaling through PI3K/PKB activation, PI3K inhibitors did not affect PGE2-induced DC migration.12,15 Recently, Legler et al11 reported that PGE2 does not promote DC migration if present only during the last 12 hours of DC maturation, suggesting the requirement of PGE2-induced de novo gene expression. At the present time, the nature of the PGE2-dependent genes involved in DC migration has not been elucidated.
Here we report on the potent induction of matrix metalloproteinase 9 (MMP-9) expression by PGE2 in both immature DCs and DCs matured with proinflammatory cytokines (TNF-α and IFN-α) and on the essential role of PGE2-induced MMP-9 in DC migration in vitro and in vivo.
Methods
Mice
Six- to eight-week-old B10.A mice, MMP-9–deficient mice, and corresponding wild-type controls (FBV/NJ) were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained and bred (MMP-9–deficient mice) in the Temple University School of Medicine animal facility under pathogen-free conditions. Mice were handled and housed in accordance with the guidelines of the Temple University Animal Care and Use Committee.
Reagents
Lipopolysaccharide (LPS) (Escherichia coli O55:B5), prostaglandin E2 (PGE2), and ibuprofen were purchased from Sigma (St Louis, MO). Granulocyte-macrophage colony-stimulating factor (GM-CSF) was purchased from Peprotech (Rocky Hill, NJ). Butaprost, sulprostone, and misoprostol were purchased from Cayman (Ann Arbor, MI). H89, dibutiryl-cAMP, MMP-2 inhibitor I, and MMP-9 inhibitor I were purchased from Calbiochem (La Jolla, CA). The following antibodies were used for FACS analysis: fluorescein isothiocyanate (FITC)–conjugated anti-CD40, FITC-conjugated anti-CD80, FITC-conjugated anti-CD86, FITC-conjugated anti–I-Ek, FITC-conjugated anti-CD11b, and FITC-conjugated anti-CD44 were purchased from PharMingen (San Diego, CA). Phycoerythrin (PE)—anti–mouse CCR7 was purchased from eBioscience (San Diego, CA). Purified goat IgG anti–mouse MMP-9 and PE-conjugated donkey anti–goat IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Generation and purification of DCs from bone marrow
DCs were generated in vitro from bone marrow. Briefly, 2 × 106 bone marrow cells flushed out from femur and tibiae were cultured in 100-mm petri dishes containing 10 mL RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Atlanta Biologicals, Norcross, GA), 2 mM l-glutamine, and 20 ng/mL recombinant GM-CSF. After 3 days, another 10 mL of complete medium containing GM-CSF was added to each dish. On day 8 the nonadherent cells were harvested and purified by immunomagnetic sorting with anti–CD11c-coated magnetic beads using the autoMACS system according to the manufacturer's instructions (Miltenyi Biotech, Bergish-Gladbach, Germany). The purity of the sorted cells was determined by FACS analysis (> 96% for CD11c+ cells).
FACS analysis
Cells were subjected to FACS analysis in a 3-color FACSCalibur (BD Biosciences, Mountain View, CA), following staining with anti–CD40 FITC, anti–CD80 FITC, anti–CD86 FITC, anti–I-Ek FITC, anti–CD11b FITC, anti–CD11c PE, or anti–CD44 FITC, and the appropriate isotype controls. Data were collected for 10 000 cells and analyzed using Cellquest software from BD Biosciences. For membrane-associated MMP-9 expression, the cells were stained with goat anti–mouse MMP-9 for 40 minutes followed by PE-conjugated donkey anti–goat IgG for 40 minutes.
Chemotaxis assay
Purified DCs were treated for 48 hours with interferon-α (IFN-α) (1000 IU/mL) and tumor necrosis factor-α (TNF-α) (20 ng/mL), with or without PGE2 and assayed for migration in response to the chemokine CCL19 (100 ng/mL or 300 ng/mL). The lower chambers of Transwell plates (8.0-μm pore size; Corning, Acton, MA) were filled with 500 μL serum-free medium with or without CCL19. DCs (1 × 105 cells in 0.1 mL) resuspended in serum-free medium were deposited in the upper chambers of the Transwell plates and allowed to migrate for 3 hours at 37°C in 5% CO2. The numbers of migrating DCs harvested from the lower chambers were counted by FACS (60-second counts).
Mouse extracellular matrix and adhesion molecules PCR array
The expression of mouse extracellular matrix (ECM) and adhesion molecules was detected by the SYBR green-based real-time reverse transcriptase–polymerase chain reaction (RT-PCR) technique. RNA was prepared from purified CD11c+ DCs treated with PGE2 (10−6 M) for 24 hours. cDNA was obtained from 1 μg of total RNA in the presence of 200 units of MMLV-RT, 1 μg of random primers, 40 units of RNAsin, 3 μg of bovine serum albumin (BSA), 0.5 mM dNTPs, and 1xMMLV reaction buffer (Promega, Madison, WI) in a total volume of 30 μL at 42°C for 1 hour. cDNA was then diluted to a total of 100 μL with ddH2O. The 98 μL cDNA were mixed with 1225 μL SYBR green-containing PCR master mixture (2 ×) and 1127 μL ddH2O. Twenty-five microliters of this mixture was added to each well of a 96-well plate precoated with different primers (SuperArray Bioscience, Frederick, MD). The PCR array was performed using Stratagene Mx3005P (Stratagene, Cedar Creek, TX), and the cycling conditions were 95°C for 15 seconds, 60°C for 1 minute, for 40 cycles, followed by a melting point determination or dissociation curves. The expression level of each gene is indicated by the number of cycles needed for the cDNA amplification to reach a threshold. The amount of DNA is calculated from the number of cycles by using standard curves, and the results are normalized to the housekeeping gene β-actin.
Real-time RT-PCR
The expression of CCR7, CD11b, MMP-9 and MMP-2 was detected by the SYBR green-based real-time RT-PCR technique. RNA was prepared from purified CD11c+ DCs, treated with or without PGE2 for various time periods. cDNA was prepared as described. The 20 μL (total volume) of the PCR mixture consists of 4 μL diluted cDNA, 10 μL SYBR green-containing PCR master mixture (2 ×), and 150 μM of each primer. The MMP-9 and MMP-2 primers for real-time RT-PCR were designed by using the Primer Express software from Applied Biosystems (Foster City, CA) and are as follows: MMP-9: sense 5′-AAAACCTCCAACCTCACGGA-3′ and antisense 5′-GCGGTACAAGTATGCCTCTGC-3′; MMP-2: sense 5′-CGCTCAGATCCGTG GTGA-3′ and antisense 5′-CGCCAAATAAACCGGTCCTT-3′; CCR7 sense: 5′-TTCCAGCTGCCCTACAATGG-3′ and antisense 5′-GAAGTTGGCCACCGTCT GAG-3′.
Real-time RT-PCR was performed using the Stratagene Mx3005P, and the cycling conditions used were 95°C for 15 seconds, 60°C for 1 minute, for 40 cycles, followed by a melting point determination or dissociation curves. The expression level of each gene is indicated by the cycle numbers needed for the cDNA to be amplified to reach a threshold. The amount of DNA is calculated from the cycle numbers by using standard curves, and the results are normalized to the housekeeping gene β-actin from the same sample.
Matrix metalloproteinase protein assay
Purified CD11c+ DCs (1 × 106 cells/mL) were seeded in 12-well plates and treated as described in “Results.” The amounts of pro-MMP-9 or total MMP-2 released in the medium 48 hours later were measured by using the mouse Pro-MMP-9 or mouse Total-MMP-2 Quantikine Kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The absorbance was determined using a Polarstar Optima Plate Reader at a wavelength of 450 nm.
Gelatinase activity assay
Supernatants were collected from DCs treated as described in “Results” and assayed for MMP-9 activity by using an MMP gelatinase activity assay (Chemicon, Temecula, CA). Briefly, supernatants are incubated with biotinylated gelatinase substrate, and 4 hours later the remaining biotinylated fragments are detected by using a biotin-binding 96-well plate and streptavidin-enzyme complex, followed by colorimetric substrate degradation. The analytical sensitivity of the MMP gelatinase assay is 5 ng MMP/mL.
Matrigel migration assay
Matrigel migration was quantified in Transwell inserts (6.5 mm) fitted with polycarbonate filters (8-μm pore size). The upper sides of the wells were coated with Matrigel (BD Biosciences) diluted in phosphate buffered saline (PBS) (70 μg/filter). CD11c+ DCs cultured with IFN-α (1000 IU/mL) and TNF-α (20 ng/mL), or with IFN-α, TNF-α, and PGE2 (10−6 M) with or without MMP-9 inhibitor I (10−6-10−7 M) or MMP-2 inhibitor I (10−5 M) for 48 hours were tested for migration to CCL19 (100 ng/mL). Briefly, the lower chambers of the plate were filled with 500 μL serum-free medium with or without (100 ng/mL) CCL19. DCs (1 × 105 cells in 0.1 mL) were deposited in the upper Transwell chambers and allowed to migrate for 5 hours at 37°C in 5% CO2. Migrated DCs harvested from the lower chambers were counted by FACS (60-second counts).
In vivo migration assay
Bone marrow (BM-DCs) generated from different strains (B10.A, wt FBV/NJ, or MMP-9−/−) were treated with TNF-α and IFN-α in the presence or absence of PGE2. Forty-eight hours later the DCs were labeled with PKH 26 red fluorescent dye (Sigma) according to the manufacturer's instructions, and 106 labeled DCs were inoculated subcutaneously in the footpads of mice preinjected 24 hours earlier with 40 ng TNF-α (subcutaneously in the footpads). At different time points (24, 48, and 72 hours), the numbers of labeled DCs collected from the draining popliteal lymph nodes were determined by FACS.
Statistical analysis
Results are given as means plus or minus standard error (± SE). Comparisons between 2 groups were done using the Student t test, whereas comparisons between multiple groups were done by ANOVA. Statistical significance was determined as P values less than .05.
Results
PGE2 promotes BM-DC migration in response to CCL19
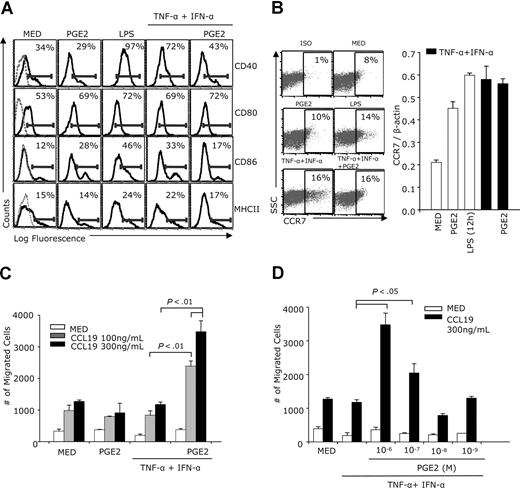
Previous studies showed that human monocyte-derived DCs, matured through exposure to the proinflammatory cytokines TNF-α + IFN-α, stimulation with CD40 ligand, or intact E coli migrate in response to the CCR7 ligand CCL19, as long as PGE2 is present during maturation.16 To confirm that PGE2 has a similar effect on murine bone marrow–derived DCs, purified CD11c+ DCs were treated with IFN-α and TNF-α in the presence or absence of PGE2. Similar to LPS (used as a positive control), the TNF-α + IFN-α treatment induced increases in surface CD80, CD86, and MHCII, and particularly in CD40 expression. Addition of PGE2 to TNF-α + IFN-α–treated DCs decreased CD40, CD86, and MHCII expression and had no effect on CD80 (Figure 1A). Treatment of immature DCs with PGE2 induced CCR7 expression (Figure 1B). CCR7 expression also was up-regulated following DC maturation with either LPS or TNF-α + IFN-α, and PGE2 did not alter CCR7 levels in TNF-α + IFN-α–treated DCs (Figure 1B). Immature DCs treated with PGE2, and DCs matured with TNF-α + IFN-α in the absence of PGE2, although expressing CCR7, did not migrate in response to CCL19 (Figure 1C). In contrast, although PGE2 did not up-regulate CCR7 expression in TNF-α + IFN-α–matured DCs, it increased CCL19-induced chemotaxis in a dose-dependent manner (Figure 1C,D).
Effects of PGE2 on DC phenotype and CCL19-induced chemotaxis. CD11c+ DCs were treated with IFN-α (1000 IU/mL) plus TNF-α (20 ng/mL) with or without PGE2 (10−6 M) (A-C) or with different PGE2 concentrations (D) for 48 hours. Controls consisted of DCs cultured in medium, treated with PGE2 alone, or with LPS (1 μg/mL). (A) FACS analysis for MHCII, CD40, CD80, and CD86 expression. Dotted lines in the left panels represent isotype controls. (B) CCR7 expression was analyzed by FACS (24 hours for LPS and 48 hours for all other treatments) and real-time RT-PCR (12 hours for LPS and 24 hours for all other treatments). (C) DCs (105 cells in 0.1 mL) were placed in the upper chambers of a 8.0-μm pore size Transwell plate. The bottom chambers were filled with serum-free medium with or without CCL19 (100 ng/mL and 300 ng/mL). After 3 hours of incubation at 37°C, the migrating cells were collected from the lower chambers and counted by FACS (60-second counts). (D) DCs were treated with TNF-α +IFN-α in the absence and presence of various PGE2 concentrations. Chemotaxis in response to CCL19 (300 ng/mL) was determined as described in panel C. Data are representative of 3 independent experiments.
Effects of PGE2 on DC phenotype and CCL19-induced chemotaxis. CD11c+ DCs were treated with IFN-α (1000 IU/mL) plus TNF-α (20 ng/mL) with or without PGE2 (10−6 M) (A-C) or with different PGE2 concentrations (D) for 48 hours. Controls consisted of DCs cultured in medium, treated with PGE2 alone, or with LPS (1 μg/mL). (A) FACS analysis for MHCII, CD40, CD80, and CD86 expression. Dotted lines in the left panels represent isotype controls. (B) CCR7 expression was analyzed by FACS (24 hours for LPS and 48 hours for all other treatments) and real-time RT-PCR (12 hours for LPS and 24 hours for all other treatments). (C) DCs (105 cells in 0.1 mL) were placed in the upper chambers of a 8.0-μm pore size Transwell plate. The bottom chambers were filled with serum-free medium with or without CCL19 (100 ng/mL and 300 ng/mL). After 3 hours of incubation at 37°C, the migrating cells were collected from the lower chambers and counted by FACS (60-second counts). (D) DCs were treated with TNF-α +IFN-α in the absence and presence of various PGE2 concentrations. Chemotaxis in response to CCL19 (300 ng/mL) was determined as described in panel C. Data are representative of 3 independent experiments.
PGE2 up-regulates MMP-9 expression at mRNA and protein levels
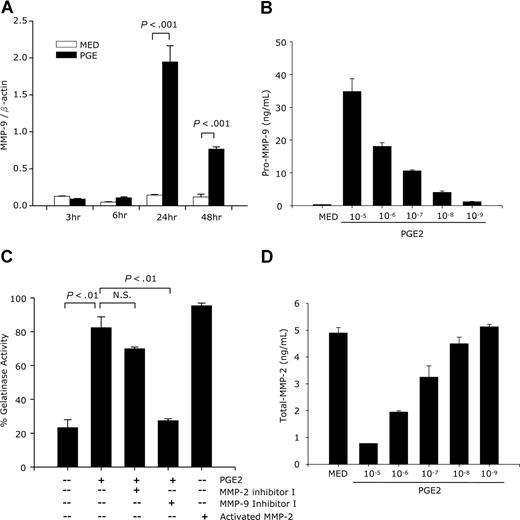
DC migration to inflammatory sites and to neighboring lymph nodes is a complex process that involves interactions with the ECM and crossing of basement membranes. MMPs play an essential role in these processes. To determine the effects of PGE2 on the expression of MMPs, we first performed an RT-PCR array. PGE2-treated DCs expressed higher levels of MMP-3, -7, -11, -13, and TIMP-1, MMP-10, and particularly MMP-9; in contrast, MMP-2 levels were decreased by PGE2. The PGE2 treatment did not change the expression of MMP-1, -8, -12, -14, or of TIMP-2 and -3 (Table 1). Both MMP-2 and -9 are involved in the degradation and remodeling of basement membranes and have been reported to play essential roles in DC migration.17 Since PGE2 induced the highest increase in MMP-9 (26-fold), we focused our investigation on PGE2-induced MMP-9 expression. The effect of PGE2 was confirmed by real-time RT-PCR. MMP-9 mRNA was significantly up-regulated 24 hours after PGE2 treatment and remained high at 48 hours (Figure 2A). At protein level, PGE2 induced pro–MMP-9 secretion in a dose-dependent manner (Figure 2B). We tested the activity of the secreted MMP-9 by using a gelatinase activity assay. Supernatants collected from DCs treated with PGE2 had high gelatinase activity, which was abolished by the MMP-9 selective inhibitor I and only slightly reduced by a selective MMP-2 inhibitor (Figure 2C).
The effect of PGE2 on the expression of MMPs and TIMPs
| Extracellular matrix proteins . | Other name . | Fold change* . |
|---|---|---|
| MMP-1 | Collagenase-1 | 1.96 |
| MMP-2 | Gelatinase A | -2.29 |
| MMP-3 | Stromelysin-1 | 2.51 |
| MMP-7 | Uterine metalloproteinase | 2.67 |
| MMP-8 | Collagenase-2 | 1.36 |
| MMP-9 | Gelatinase B | 26.51 |
| MMP-10 | Stromelysin-2 | 4.89 |
| MMP-11 | Stromelysin-3 | 3.12 |
| MMP-12 | Metalloelastase | -1.01 |
| MMP-13 | Collagenase-3 | 2.37 |
| MMP-14 | Membrane-type 1 MMP (MT1-MMP) | 1.11 |
| MMP-15 | Membrane-type 2 MMP (MT2-MMP) | 2.88 |
| TIMP-1 | Tissue inhibitor of metalloproteinase 1 | 2.58 |
| TIMP-2 | Tissue inhibitor of metalloproteinase 2 | 1.27 |
| TIMP-3 | Tissue inhibitor of metalloproteinase 3 | 1.80 |
| Extracellular matrix proteins . | Other name . | Fold change* . |
|---|---|---|
| MMP-1 | Collagenase-1 | 1.96 |
| MMP-2 | Gelatinase A | -2.29 |
| MMP-3 | Stromelysin-1 | 2.51 |
| MMP-7 | Uterine metalloproteinase | 2.67 |
| MMP-8 | Collagenase-2 | 1.36 |
| MMP-9 | Gelatinase B | 26.51 |
| MMP-10 | Stromelysin-2 | 4.89 |
| MMP-11 | Stromelysin-3 | 3.12 |
| MMP-12 | Metalloelastase | -1.01 |
| MMP-13 | Collagenase-3 | 2.37 |
| MMP-14 | Membrane-type 1 MMP (MT1-MMP) | 1.11 |
| MMP-15 | Membrane-type 2 MMP (MT2-MMP) | 2.88 |
| TIMP-1 | Tissue inhibitor of metalloproteinase 1 | 2.58 |
| TIMP-2 | Tissue inhibitor of metalloproteinase 2 | 1.27 |
| TIMP-3 | Tissue inhibitor of metalloproteinase 3 | 1.80 |
Purified CD11c+ bone marrow–derived DCs were stimulated with PGE2 (10−6 M) for 24 hours. RNA was extracted and converted to cDNA. cDNA was diluted according to the manufacturer's instruction and subjected to (RT)2-profiler PCR array. One representative experiment of 3 is shown.
Gene expression levels of PGE2-treated DCs divided by gene expression levelsof untreated DCs.
Effects of PGE2 on MMP-9 and MMP-2 expression and activity. (A) CD11c+ DCs were treated with PGE2 (10−6 M), and RNA was extracted at different time points and subjected to MMP-9 real-time RT-PCR. One representative experiment of 2 is shown. (B,D) CD11c+ DCs were treated with different concentrations of PGE2 for 48 hours, followed by ELISA for secreted pro–MMP-9 and total-MMP-2. Data are representative of 3 independent experiments. (C) DCs were treated with PGE2 (10−6 M) for 48 hours in the presence or absence of MMP-2 inhibitor I (10−5 M) or MMP-9 inhibitor I (10−6 M). Supernatants were collected and subjected to a gelatinase activity assay. p-aminophenylmercuric acetate-activated human MMP-2 was used as a positive control. Data are representative of 3 independent experiments.
Effects of PGE2 on MMP-9 and MMP-2 expression and activity. (A) CD11c+ DCs were treated with PGE2 (10−6 M), and RNA was extracted at different time points and subjected to MMP-9 real-time RT-PCR. One representative experiment of 2 is shown. (B,D) CD11c+ DCs were treated with different concentrations of PGE2 for 48 hours, followed by ELISA for secreted pro–MMP-9 and total-MMP-2. Data are representative of 3 independent experiments. (C) DCs were treated with PGE2 (10−6 M) for 48 hours in the presence or absence of MMP-2 inhibitor I (10−5 M) or MMP-9 inhibitor I (10−6 M). Supernatants were collected and subjected to a gelatinase activity assay. p-aminophenylmercuric acetate-activated human MMP-2 was used as a positive control. Data are representative of 3 independent experiments.
This indicates that PGE2 induces high levels of MMP-9 activity, with MMP-2 playing only a minor role. In agreement with this conclusion, the RT-PCR array indicated a PGE2-induced decrease in MMP-2 levels. This was confirmed at protein level, with MMP-2 production being inhibited by PGE2 in a dose-dependent manner (Figure 2D).
PGE2 induces both membrane-associated and soluble MMP-9 protein expression
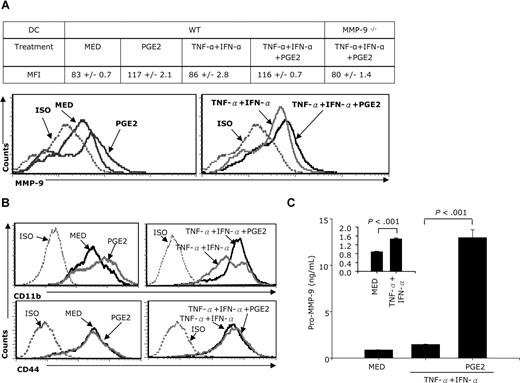
MMP-9 is present both as CD44 or CD11b-associated membrane-bound and as secreted protein. To evaluate whether PGE2 induces membrane-associated MMP-9, CD11c+ DCs were stimulated with TNF-α + IFN-α in the absence or presence of PGE2, and membrane-bound MMP-9 expression was analyzed by FACS 24 hours later. Compared with DCs cultured in medium, PGE2 increased MMP-9 surface expression in both immature and TNF-α + IFN-α–matured DCs (Figure 3A). No MMP-9 surface expression was observed in DCs generated from MMP-9–deficient mice even following TNF-α + IFN-α + PGE2 treatment (Figure 3A). Together with CD11b, CD44 provides the docking site for membrane-associated MMP-9. To investigate whether an increase in the PGE2-induced membrane-associated MMP-9 correlates with an up-regulation of CD44 or CD11b, DCs were treated with PGE2 for 24 hours and analyzed for CD44 and CD11b expression by FACS. PGE2 up-regulated CD11b expression in both immature and TNF-α + IFN-α–matured DCs (Figure 3B, top panels). In contrast, CD44 expression does not appear to be affected by PGE2 (Figure 3B bottom panels).
PGE2 induces both membrane-associated and soluble MMP-9 expression. (A) CD11c+ DCs were treated with TNF-α plus IFN-α in the presence or absence of PGE2 for 24 hours in serum-free conditions. Cells were stained with goat anti–mouse MMP-9 antibody for 40 minutes, followed by PE-donkey anti–goat IgG for 40 minutes. Expression of membrane-associated MMP-9 was measured by FACS. (B) CD11c+ DCs were treated with PGE2 (10−6 M) or TNF-α IFN-α plus or minus PGE2 for 24 hours followed by FACS for CD11b and CD44 expression. (C) CD11c+ DCs were stimulated with TNF-α plus IFN-α (in the presence or absence of PGE2 (10−6 M) for 48 hours. Supernatants were collected, and the amounts of secreted pro–MMP-9 were determined by using the pro–MMP-9 Quantikine assay. Data are representative of 3 independent experiments for panels A and C, and of 2 representative experiments for panel B.
PGE2 induces both membrane-associated and soluble MMP-9 expression. (A) CD11c+ DCs were treated with TNF-α plus IFN-α in the presence or absence of PGE2 for 24 hours in serum-free conditions. Cells were stained with goat anti–mouse MMP-9 antibody for 40 minutes, followed by PE-donkey anti–goat IgG for 40 minutes. Expression of membrane-associated MMP-9 was measured by FACS. (B) CD11c+ DCs were treated with PGE2 (10−6 M) or TNF-α IFN-α plus or minus PGE2 for 24 hours followed by FACS for CD11b and CD44 expression. (C) CD11c+ DCs were stimulated with TNF-α plus IFN-α (in the presence or absence of PGE2 (10−6 M) for 48 hours. Supernatants were collected, and the amounts of secreted pro–MMP-9 were determined by using the pro–MMP-9 Quantikine assay. Data are representative of 3 independent experiments for panels A and C, and of 2 representative experiments for panel B.
To determine whether PGE2 induces MMP-9 secretion, we treated DCs with PGE2 for 48 hours and measured secreted pro–MMP-9 by ELISA. The TNF-α + IFN-α treatment doubled MMP-9 secretion (Figure 3C, inset). PGE2, however, had a tremendous effect, raising the levels of secreted MMP-9 12- to 13-fold (Figure 3C).
MMP-9 up-regulation by PGE2 is mediated through EP2/EP4 receptors
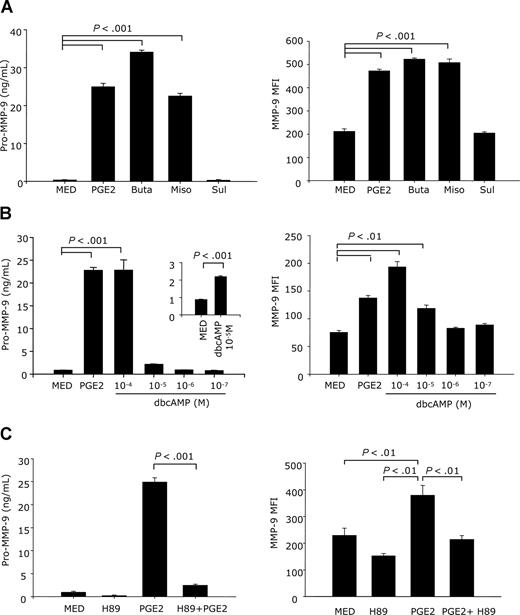
To determine which PGE2 receptors are involved in the up-regulation of MMP-9 by PGE2, DCs were stimulated with the following EP receptor agonists: butaprost (a selective agonist for EP2), sulprostone (EP1/EP3 agonist), or misoprostol (EP2/EP4/EP3 agonist). Similar to PGE2, butaprost and misoprostol greatly enhanced both membrane and soluble MMP-9 levels. In contrast, sulprostone did not affect either surface of secreted MMP-9 levels (Figure 4A). This suggests that the effect of PGE2 on MMP-9 expression and secretion is mediated through the EP2/EP4 receptors.
MMP-9 up-regulation by PGE2 is mediated through the EP2/EP4 receptors and the cAMP→PKA signaling pathway. (A) CD11c+ DCs were treated with butaprost (10−5 M) (an EP2 agonist), misoprostol (10−5 M) (an EP2/EP4/EP3 agonist), or sulprostone (10−5 M) (an EP1/EP3 agonist) for 24 hours in serum-free medium for membrane-bound MMP-9 and for 48 hours in complete medium for secreted MMP-9. Cells were stained with goat anti–mouse MMP-9 antibody for 40 minutes, followed by PE-donkey anti–goat IgG for 40 minutes. Membrane-associated MMP-9 was measured by FACS. The amounts of secreted pro–MMP-9 were measured in supernatants by using the pro–MMP-9 Quantikine assay. (B) CD11c+ DCs were treated with different concentrations of dbcAMP (10−4-10−7 M) for 24 hours (membrane MMP-9) or 48 hours for secreted MMP-9. Membrane-bound and secreted MMP-9 were determined as in panel A. (C) CD11c+ DCs were pretreated with H89 (10−5 M) for 30 minutes, followed by PGE2 (10−6 M) for 24 hours for membrane MMP-9 and 48 hours for secreted MMP-9. Membrane-bound and secreted MMP-9 were determined as in panel A. Data are representative of 2 independent experiments for membrane-bound MMP-9 and 3 independent experiments for secreted MMP-9.
MMP-9 up-regulation by PGE2 is mediated through the EP2/EP4 receptors and the cAMP→PKA signaling pathway. (A) CD11c+ DCs were treated with butaprost (10−5 M) (an EP2 agonist), misoprostol (10−5 M) (an EP2/EP4/EP3 agonist), or sulprostone (10−5 M) (an EP1/EP3 agonist) for 24 hours in serum-free medium for membrane-bound MMP-9 and for 48 hours in complete medium for secreted MMP-9. Cells were stained with goat anti–mouse MMP-9 antibody for 40 minutes, followed by PE-donkey anti–goat IgG for 40 minutes. Membrane-associated MMP-9 was measured by FACS. The amounts of secreted pro–MMP-9 were measured in supernatants by using the pro–MMP-9 Quantikine assay. (B) CD11c+ DCs were treated with different concentrations of dbcAMP (10−4-10−7 M) for 24 hours (membrane MMP-9) or 48 hours for secreted MMP-9. Membrane-bound and secreted MMP-9 were determined as in panel A. (C) CD11c+ DCs were pretreated with H89 (10−5 M) for 30 minutes, followed by PGE2 (10−6 M) for 24 hours for membrane MMP-9 and 48 hours for secreted MMP-9. Membrane-bound and secreted MMP-9 were determined as in panel A. Data are representative of 2 independent experiments for membrane-bound MMP-9 and 3 independent experiments for secreted MMP-9.
PGE2 up-regulation of MMP-9 expression is mediated through the cAMP→PKA pathway
EP2/EP4 signaling stimulates adenylate cyclase, resulting in increases in intracellular cAMP. To further confirm the role of cAMP in the up-regulation of MMP-9 expression, we used the stable cAMP analog dbcAMP. DbcAMP induced MMP-9 expression and secretion (Figure 4B). cAMP activates both PKA-dependent and -independent signal pathways. To assess the role of PKA [protein kinase A] in PGE2-induced MMP-9 expression, CD11c+ DCs were pretreated with the PKA inhibitor H89 for 30 minutes, followed by treatment with PGE2. H89 completely abolished the PGE2-induced increase in membrane-associated and soluble MMP-9 protein (Figure 4C), without affecting cell viability. These results indicate that PGE2 induces MMP-9 production and release through the cAMP→PKA signaling pathway.
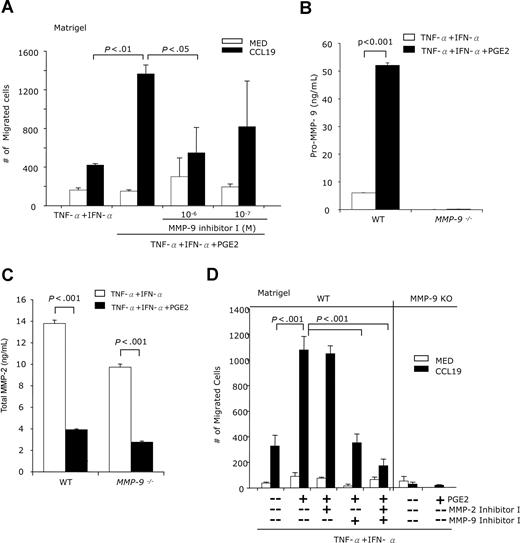
MMP-9 involvement in the PGE2-induced BM-DC migration through Matrigel
To examine the involvement of MMP-9 in DC migration, we used a Matrigel barrier as an in vitro model for basement membranes. We determined the capacity of DCs, treated with TNF-α + IFN-α with or without PGE2, to cross Matrigel-coated membranes in response to CCL19. A substantially higher number of PGE2-treated DCs migrated across the Matrigel barrier (Figure 5A). PGE2-induced DC migration was inhibited by the selective MMP-9 inhibitor MMP-9 inhibitor I (Figure 5A). The results confirm the role of MMP-9 in the PGE2-induced chemotaxis of DCs through Matrigel.
PGE2 promotes BM-DC chemotactic migration through Matrigel in an MMP-9–dependent manner. (A) CD11c+ DCs were treated with IFN-α + TNF-α with or without PGE2 (10−6 M), in the presence or absence of MMP-9 inhibitor I for 48 hours. DCs (105 cells in 0.1 mL) were placed in the upper Transwell chambers precoated with Matrigel (70 μg). The bottom chambers were filled with serum-free medium with or without CCL19 (100 ng/mL). Following incubation at 37°C for 4 hours, the migrating cells were collected from the lower chambers and counted by FACS (60-second counts). (B,C) DCs generated from wild-type (WT) FBV/NJ or MMP-9–deficient mice were treated with IFN-α + TNF-α with or without PGE2 (10−6 M) for 48 hours. The amounts of pro–MMP-9 (B) and total MMP-2 (C) were quantified by ELISA. (D) DCs generated from wild-type (WT) FBV/NJ or MMP-9–deficient mice were treated with IFN-a + TNF-α with or without PGE2 (10−6 M), in the presence or absence of the MMP-9 inhibitor I (10−6 M) or MMP-2 inhibitor I (10−5 M) for 48 hours. The cells were placed in the Transwell upper chambers precoated with Matrigel. The bottom chambers were filled with serum-free medium with or without CCL19 (100 ng/mL). After 5 hours of incubation at 37°C, the migrating cells were collected from the lower chambers and counted by FACS (60-second counts). Data are representative of 3 independent experiments.
PGE2 promotes BM-DC chemotactic migration through Matrigel in an MMP-9–dependent manner. (A) CD11c+ DCs were treated with IFN-α + TNF-α with or without PGE2 (10−6 M), in the presence or absence of MMP-9 inhibitor I for 48 hours. DCs (105 cells in 0.1 mL) were placed in the upper Transwell chambers precoated with Matrigel (70 μg). The bottom chambers were filled with serum-free medium with or without CCL19 (100 ng/mL). Following incubation at 37°C for 4 hours, the migrating cells were collected from the lower chambers and counted by FACS (60-second counts). (B,C) DCs generated from wild-type (WT) FBV/NJ or MMP-9–deficient mice were treated with IFN-α + TNF-α with or without PGE2 (10−6 M) for 48 hours. The amounts of pro–MMP-9 (B) and total MMP-2 (C) were quantified by ELISA. (D) DCs generated from wild-type (WT) FBV/NJ or MMP-9–deficient mice were treated with IFN-a + TNF-α with or without PGE2 (10−6 M), in the presence or absence of the MMP-9 inhibitor I (10−6 M) or MMP-2 inhibitor I (10−5 M) for 48 hours. The cells were placed in the Transwell upper chambers precoated with Matrigel. The bottom chambers were filled with serum-free medium with or without CCL19 (100 ng/mL). After 5 hours of incubation at 37°C, the migrating cells were collected from the lower chambers and counted by FACS (60-second counts). Data are representative of 3 independent experiments.
DCs generated from MMP-9–deficient mice exhibit impaired Matrigel migration
To confirm the fact that PGE2 affects DC migration primarily through the induction of MMP-9, we compared the migration of wild-type and MMP-9–deficient DCs through Matrigel. DCs generated from wild-type and MMP-9–deficient mice were matured with TNF-α + IFN-α in the presence or absence of PGE2. We monitored first the expression of MMP-9 and MMP-2. In contrast to wild-type controls, where PGE2 induces MMP-9 expression and secretion, DCs from MMP-9–deficient mice do not produce MMP-9 (Figure 5B). In contrast, both wild-type and MMP-9–deficient DCs produce MMP-2 upon maturation with TNF-α + IFN-α, and PGE2 reduces MMP-2 production in both types of DCs (Figure 5C). Both MMP-2 and MMP-9 contribute to epidermal and dermal DC migration from skin,18 and therefore could play a role in DC migration in response to CCL19. Our data indicate that PGE2 has opposite effects on the 2 MMPs, up-regulating MMP-9 while down-regulating MMP-2. To evaluate the role of MMP-2 and MMP-9 in DC migration, we treated DCs with TNF-α + IFN-α + PGE2 in the absence and presence of MMP-2 and MMP-9 inhibitors. MMP-9–deficient DCs migrated through Matrigel in very low numbers, which were similar for PGE2-treated or -untreated samples, and similar in response to CCL19 or medium (Figure 5D right panel). This suggested that MMP-2 plays a minor role, if any, in the in vitro Matrigel migration of MMP-9–deficient DCs. As expected, wild-type DCs treated with PGE2 migrate in high numbers (Figure 5D left panel). In contrast to the MMP-2 inhibitor that does not significantly affect migration, the MMP-9 inhibitor reduces migration to the level observed for DCs not treated with PGE2, supporting our hypothesis that PGE2-induced MMP-9 is the major player in DC migration. The fact that the inhibition of both MMP-2 and MMP-9 reduced migration even further suggests a minor role for MMP-2 as well. To show that MMP-9−/− DCs do not have an intrinsic migration defect, we subjected wild-type and MMP-9−/− DCs to CCL19-induced chemotaxis instead of Matrigel migration. Without PGE2 treatment, wild-type and MMP-9−/− DCs migrated in similar numbers (2535 ± 593 vs 3525 ± 241); a similar result was obtained for the chemotactic migration of wild-type and MMP-9−/− DCs after PGE2 treatment (5888 ± 753 vs 6255 ± 938).
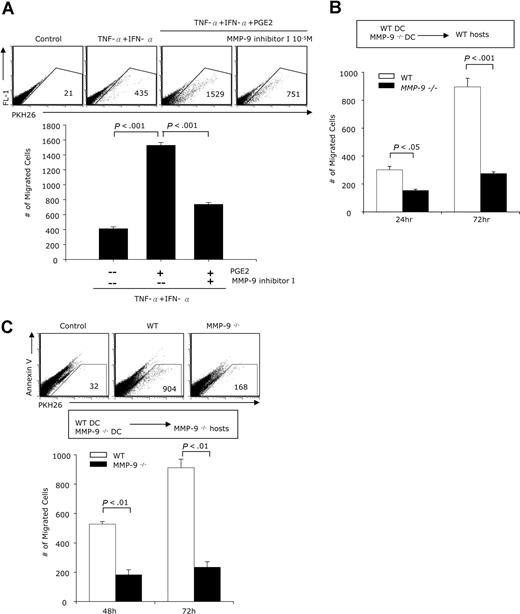
PGE2-induced MMP-9 promotes DC migration in vivo
The migration of mature DCs to neighboring lymph nodes represents the initiating step in the activation of the adaptive T-cell response. To investigate the role of the PGE2-induced MMP-9 expression in the in vivo migration of DCs, we treated DCs with TNF-α + IFN-α in the presence or absence of PGE2 for 48 hours, fluorescently labeled the mature DCs, and injected them subcutaneously into footpads of B10.A mice preinjected 24 hours earlier with TNF-α to activate vascular/lymphatic endothelial cells. Twenty-four hours after DC inoculation, the labeled DCs harvested from the draining lymph nodes were counted by FACS. Lymph nodes from control mice (no injected DCs) were used to establish the gates to eliminate autofluorescence. DCs treated with TNF-α + IFN-α and PGE2 migrated in much higher numbers than cells cultured without PGE2 (Figure 6A). When DCs treated with TNF-α + IFN-α and PGE2 were cultured in the presence of the MMP-9 inhibitor, the numbers migrating to the draining lymph nodes were reduced by 50% (Figure 6A). These results confirm that, similar to the in vitro experiments, exposure to PGE2 stimulates subsequent migration in vivo and that the effect of PGE2 is mediated, at least partially, through MMP-9.
In vivo migration of PGE2-treated DCs is MMP-9 dependent. (A) DCs generated from B10.A mice were treated with IFN-α + TNF-α with or without PGE2 (10−6 M), in the presence or absence of the MMP-9 inhibitor I (10−5 M) for 48 hours. DCs were collected and labeled with the PKH 26 red fluorescence dye. Two groups of B10.A mice (n = 5) were preinjected with 40 ng TNF-α subcutaneously in the footpads of each of the hind legs. Twenty-four hours later the mice were inoculated with DCs (1 × 106 cells in 60 μL, subcutaneously in the footpads) as follows: mice in group I received untreated DCs in the right leg, and TNF-α + IFN-α–treated DCs in the left leg; mice in group II received TNF-α + IFN-α + PGE2–treated DCs in the right leg and TNF-α + IFN-α + PGE2 + MMP-9 inhibitor I in the left leg. The draining popliteal lymph nodes were harvested 48 hours later, and the numbers of labeled cells were determined by FACS. Results are expressed as numbers of fluorescent cells per 100 000 lymph node cells. (B) DCs generated from WT (FBV/NJ) or MMP-9–deficient mice were treated with IFN-α + TNF-α + PGE2 for 48 hours. Following labeling with the PKH 26 red fluorescence dye, 1 × 106 DCs were injected subcutaneously in the footpads of WT mice (n = 10). The mice were preinjected with TNF-α as described. Each mouse received WT DC in the right leg and MMP-9–deficient DCs in the left leg. Five mice were killed 24 hours later, and the rest were killed 72 hours after the DC inoculation. The number of labeled cells in the draining popliteal lymph nodes was determined by FACS. (C) DCs generated from WT (FBV/NJ) or MMP-9–deficient mice were treated with IFN-α + TNF-α with or without PGE2 and injected into 2 groups (n = 5) of MMP-9–deficient mice. Each mouse in group I received WT DC treated with IFN-α + TNF-α in the right leg and WT DC treated with IFN-α + TNF-α + PGE2 in the left leg. Mice in group 2 received MMP-9–deficient DCs treated with IFN-α + TNF-α in the right leg and MMP-9–deficient DCs treated with IFN-α + TNF-α + PGE2 in the left leg. All mice were killed 48 hours later; draining lymph node cells were collected and stained with Annexin V and the numbers of labeled cells in the Annexin V–negative population were determined by FACS. Results are expressed as numbers of PHK26+ cells per 100 000 cells.
In vivo migration of PGE2-treated DCs is MMP-9 dependent. (A) DCs generated from B10.A mice were treated with IFN-α + TNF-α with or without PGE2 (10−6 M), in the presence or absence of the MMP-9 inhibitor I (10−5 M) for 48 hours. DCs were collected and labeled with the PKH 26 red fluorescence dye. Two groups of B10.A mice (n = 5) were preinjected with 40 ng TNF-α subcutaneously in the footpads of each of the hind legs. Twenty-four hours later the mice were inoculated with DCs (1 × 106 cells in 60 μL, subcutaneously in the footpads) as follows: mice in group I received untreated DCs in the right leg, and TNF-α + IFN-α–treated DCs in the left leg; mice in group II received TNF-α + IFN-α + PGE2–treated DCs in the right leg and TNF-α + IFN-α + PGE2 + MMP-9 inhibitor I in the left leg. The draining popliteal lymph nodes were harvested 48 hours later, and the numbers of labeled cells were determined by FACS. Results are expressed as numbers of fluorescent cells per 100 000 lymph node cells. (B) DCs generated from WT (FBV/NJ) or MMP-9–deficient mice were treated with IFN-α + TNF-α + PGE2 for 48 hours. Following labeling with the PKH 26 red fluorescence dye, 1 × 106 DCs were injected subcutaneously in the footpads of WT mice (n = 10). The mice were preinjected with TNF-α as described. Each mouse received WT DC in the right leg and MMP-9–deficient DCs in the left leg. Five mice were killed 24 hours later, and the rest were killed 72 hours after the DC inoculation. The number of labeled cells in the draining popliteal lymph nodes was determined by FACS. (C) DCs generated from WT (FBV/NJ) or MMP-9–deficient mice were treated with IFN-α + TNF-α with or without PGE2 and injected into 2 groups (n = 5) of MMP-9–deficient mice. Each mouse in group I received WT DC treated with IFN-α + TNF-α in the right leg and WT DC treated with IFN-α + TNF-α + PGE2 in the left leg. Mice in group 2 received MMP-9–deficient DCs treated with IFN-α + TNF-α in the right leg and MMP-9–deficient DCs treated with IFN-α + TNF-α + PGE2 in the left leg. All mice were killed 48 hours later; draining lymph node cells were collected and stained with Annexin V and the numbers of labeled cells in the Annexin V–negative population were determined by FACS. Results are expressed as numbers of PHK26+ cells per 100 000 cells.
To further evaluate the role of MMP-9 in DC migration in vivo, DCs generated from wild-type and MMP-9–deficient mice were matured with TNF-α + IFN-α in the presence of PGE2, fluorescently labeled with PKH26, and injected subcutaneously into the footpads of wild-type mice. The numbers of fluorescent DCs in the draining lymph nodes were determined at 24 hours and 72 hours. Wild-type DCs migrated in significantly higher numbers than MMP-9–deficient DCs (Figure 6B), indicating that MMP-9 production by the migrating DCs is essential for migration in vivo. To confirm the role of DC-derived MMP9, we inoculated fluorescently labeled DCs derived from wild-type and MMP-9–deficient DCs in MMP-9–deficient mice. Cells collected from the draining lymph nodes 48 and 72 hours later were labeled with Annexin V to identify apoptotic cells and analyzed for PKH26+AnnexinV− cells. Wild-type, but not MMP-9–deficient, DCs migrated in significant numbers to the draining lymph node (Figure 6C). We conclude that the determining factor in the in vivo migration to lymph nodes is the expression of MMP-9 by the migratory DCs, regardless of the capacity of neighboring tissues to express MMP-9.
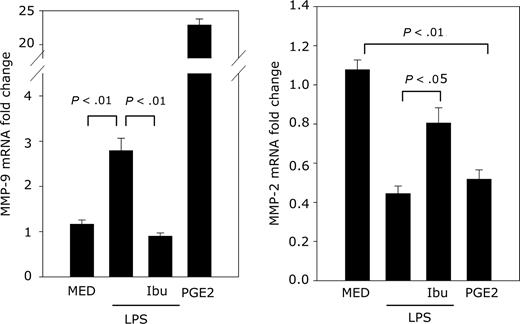
Endogenous PGE2 up-regulates MMP-9 expression
To determine whether endogenous PGE2 plays a role in MMP-9 production, DCs were treated with LPS in the presence or absence of ibuprofen, a Cox 1/2 inhibitor, followed by real-time RT-PCR for MMP-9 and MMP-2 expression. LPS induces endogenous PGE2 release from DCs. Therefore, we expected LPS to induce MMP-9 and reduce constitutive MMP-2 expression. This was indeed the case (Figure 7). When LPS-induced PGE2 release was inhibited by the Cox1/2 inhibitor ibuprofen, MMP-9 was reduced to control levels, and MMP-2 expression increased to almost control level (Figure 7). These results indicate that, similar to exogenous PGE2, endogenous PGE2 released from activated DCs induces MMP-9 expression.
Endogenous PGE2 up-regulates MMP-9 expression. DCs were pretreated with ibuprofen (10−5 M) for 30 minutes followed by treatment with LPS (1 μg/mL). DCs were treated with ibuprofen every 12 hours (total time in culture, 60 hours). The negative controls consisted of DCs cultured in medium (with or without ibuprofen treatment). Addition of ibuprofen to cells cultured in medium did not affect the levels of MMP-9 or -2. The positive control consisted of DCs exposed to exogenous PGE2 (10−6 M) for 24 hours. MMP-9 and MMP-2 expression was determined by real-time RT-PCR.
Endogenous PGE2 up-regulates MMP-9 expression. DCs were pretreated with ibuprofen (10−5 M) for 30 minutes followed by treatment with LPS (1 μg/mL). DCs were treated with ibuprofen every 12 hours (total time in culture, 60 hours). The negative controls consisted of DCs cultured in medium (with or without ibuprofen treatment). Addition of ibuprofen to cells cultured in medium did not affect the levels of MMP-9 or -2. The positive control consisted of DCs exposed to exogenous PGE2 (10−6 M) for 24 hours. MMP-9 and MMP-2 expression was determined by real-time RT-PCR.
Discussion
Although the role of PGE2 in the chemotactic response of mature DCs to the CCR7 ligands CCL19/21 is well established, the mechanisms involved in PGE2-induced DC migration are not understood. Scandella et al12 proposed that PGE2 is essential for the CCL19/21-induced calcium flux required for DC mobilization. However, a recent report indicates that PGE2 addition during the final stage does not promote a DC chemotactic response,11 suggesting that PGE2 induces the migratory phenotype by modifying the gene expression profile in maturing DCs. In agreement with this hypothesis, our results indicate that PGE2 plays an essential role in the up-regulation of MMP-9 gene expression in bone marrow–derived DCs and that, in turn, DC-derived MMP-9 is essential for Matrigel DC migration in response to CCL19 and for the in vivo migration in both wild-type and MMP-9–deficient hosts.
MMPs are major participants in cell migration through the degradation of extracellular matrix and of basement membranes.17,19 MMP-2 and -9 are especially important in migration, since they cleave collagen IV, a major component of basement membranes. MMP-9 is secreted primarily by activated cells of the macrophage/monocyte lineage as an inactive proenzyme, which upon cleavage of the prodomain can function either as secreted or membrane-bound proteinase. The role of MMP-9 in DC migration both in response to proinflammatory chemokines such as CCL5 and lymph node–derived chemokines such as CCL19 has been demonstrated in vitro, in skin explant models, and in vivo following epicutaneous hapten stimulation and treatment with anti–MMP-9 Abs, as well as in MMP-9–deficient mice.18,20,,,,–25 Recently, DC transmigration through brain capillary endothelial cell monolayers also has been shown to depend on MMPs.26 In addition, DCs from multiple sclerosis (MS) patients have been shown to secrete high levels of MMP-2, -3, and -9 and to exhibit increased spontaneous migration over ECM-coated filters.27 These are important findings, since central nervous system (CNS) perivascular DCs migrating from the periphery play an essential role in the restimulation of encephalitogenic T cells in experimental autoimmune encephalomyelitis.28
Treatment of DCs with proinflammatory cytokines/chemokines, exposure to ECM-coated filters, and in vivo exposure to epicutaneous hapten stimulation results in MMP-9 expression and increased transmigration.22,27,29,–31 Interestingly, DCs exposed to ECM-coated filters also secrete active MMP-3, a major MMP-9 activator.27 This suggests that, in certain conditions, activated DCs can secrete and activate pro–MMP-9 without the involvement of other cell types.
PGE2 can play opposite roles in MMP-9 expression, depending on the cell type. PGE2 inhibits MMP-9 expression in the breast cancer cell line MCF-7, in peritoneal macrophages isolated from women with endometriosis, and in interleukin-1 (IL-1)–stimulated rabbit articular chondrocytes.32,–34 In contrast, exogenous PGE2 promotes MMP-9 expression in macrophages stimulated with phorbol myristate acetate (PMA) or plated on ECM and in LPS-stimulated trophoblasts.35,,–38 In addition, endogenous PGE2 was shown to play an important role in MMP-9 expression in macrophages exposed to ECM or stimulated with LPS.35,39
In comparison to monocytes/macrophages, very little is known about the role of PGE2 in the regulation of MMPs in DCs. We report here for the first time that exogenous PGE2 induces MMP-9 expression in both immature bone marrow–derived DCs and in DCs matured in the presence of the proinflammatory cytokines TNF-α and IFN-α. MMP-9 expression is associated with increased Matrigel migration in response to CCL19. Previously, Baratelli et al reported that, in immature human monocyte-derived DCs, PGE2 induced a slight increase (2 ×) in the MMP-9 inhibitor TIMP-1 but not in MMP-9.14 The authors also reported a slight increase in MMP-9 production in TNF-α–matured DCs treated with PGE2.14 In contrast, although we saw a slight increase in TIMP-1 expression in immature BM-DCs, we observed a high increase in MMP-9 mRNA and protein expression in both immature and TNF-α + IFN-α–matured DCs.
The TIMP family of endogenous MMP inhibitors consists of 4 members, which regulate MMP activity and play a major role in balancing the degradation of matrix components in physiological and pathological conditions.17,40 Pro–MMP-9 can form stable complexes with, and be inactivated by, TIMP-1 and -3.41,42 We observed slight increases in TIMP-1 and -3 (Table 1). However, the increase in MMP-9 expression was much higher, suggesting that PGE2 tilts the balance in favor of active MMP-9. This was supported by the gelatinase activity assay, which in contrast to the classical zymography technique, measures only active secreted gelatinases.
Both MMP-2 and MMP-9 degrade collagen IV and act as major players in cell migration. Although the gelatinase assay cannot distinguish between MMP-2 and MMP-9 activity, specific ELISA assays and inhibitors can be used to distinguish between them. To our surprise, PGE2 had opposite effects on the 2 gelatinases; that is, it strongly induced MMP-9 expression, whereas down-regulating MMP-2 expression. The PCR array data were confirmed at protein level in DCs from both wild-type and MMP-9–deficient mice. Also, the Matrigel migration of wild-type DCs was shown to depend primarily on MMP-9, with only a minor contribution from MMP-2.
Secreted pro–MMP-9 can re-associate either in an active or inactive form on the cell surface. Both human and murine DCs express surface MMP-9 bound to CD44 and CD11b.18,21,22 Interestingly, we found that PGE2 induces an increase in both surface MMP-9 and CD11b expression. It is tempting to speculate that CD11b up-regulation is related to increased capture of secreted MMP-9 that would allow activated DCs to use MMP-9 activity in a targeted manner for directional migration.
PGE2 can signal through the EP1-4 receptors, leading to the activation of different signaling pathways. We have shown previously that BM-DCs express primarily EP2 and EP4 receptors,43,44 which mediate increases in intracellular cAMP and downstream activation of PKA and/or PI3K.45 The effect of PGE2 on MMP-9 induction is mediated through the EP2 and possibly EP4 receptor, since butaprost (EP2 agonist) and misoprostol (EP2/EP4/EP3 agonist), but not sulprostone (EP1/EP3 agonist), induced MMP-9. The effect of PGE2 is mimicked by dibutiryl cAMP and reversed by H89, a PKA inhibitor, indicating that PGE2 induces MMP-9 expression in DCs through the EP2/EP4→cAMP→PKA signaling pathway.
Kabashima et al reported that only EP4-deficient mice exhibit impaired migration of Langerhans cells (LCs) to draining lymph nodes.13 If both EP2 and EP4 receptors mediate the effect of PGE2 on DC MMP-9 expression, why do only EP4-deficient mice show reduced migration? One possible explanation is the difference between LC and bone marrow–derived DCs. Also, although both receptors induce cAMP and activate PKA, only EP4 mediates the cAMP-dependent activation of PI3K.46 It is possible that, in addition to MMP-9, other factors induced by PGE2 through the EP4→cAMP→PI3K pathway are required for DC migration. Recently, PGE2-induced high-speed migration of human monocyte–derived DCs was shown to depend on rapid β1 integrin inactivation and subsequent podosome dissolution.47 Although there is no information on β1 regulation by PI3K in DCs, decreased β1 integrin expression and reduction in cell adhesion was reported in renal epithelial cells transfected with PI3K-C2beta.48
Similar to the in vitro Matrigel assay, DCs matured with TNF-α + IFN-α in the presence of PGE2 migrated in vivo in significantly higher numbers to the draining lymph node compared with DCs matured in the absence of PGE2. The PGE2-induced migration was dependent on MMP-9, since the selective MMP-9 inhibitor I reduced migration almost to control level. In vivo, MMP-9 could be provided by the migrating DCs or by neighboring cells. We addressed this by using DCs generated from MMP-9–deficient mice and wild-type controls. When maturing DCs exposed to PGE2 were inoculated in wild-type hosts, the wild-type DCs migrated, whereas the MMP-9–deficient DCs did not. The same pattern was observed upon inoculation in MMP-9–deficient hosts. This suggests that the determining factor in PGE2-induced DC migration is the MMP-9 produced by the migrating DCs.
The effect of PGE2 on MMP-9 expression and DC transmigration has physiological relevance. Several reports using Cox2 inhibitors or Cox2-deficient cells indicate a role for endogenous PGE2 in MMP-9 induction in monocytes/macrophages.35,37,49 Here we showed that ibuprofen, a Cox1/2 inhibitor, reversed the effect of LPS on MMP-9/MMP-2 expression, suggesting that LPS acted through endogenous PGE2. In addition, EP4-deficient and MMP-9–deficient mice exhibit impaired transmigration of DCs.13,18,25 These observations suggest a role for endogenous PGE2 in DC transmigration. PGE2 released within inflammatory sites promotes the directed migration of resident and/or incoming DCs matured in inflammatory or pathogenic conditions. This could occur through an early PGE2-induced rapid inactivation of β1 integrins, resulting in the dissolution of podosomes and less adherence to the extracellular matrix, followed by a change in DC gene expression profile, which includes a potent up-regulation of MMP-9. Both secreted and surface-bound MMP-9 would then allow CCR7-expressing DCs to migrate through the ECM, cross the basement membrane, and enter the lymphatic vessels leading to the lymph nodes.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases grant RO1AI052306 (D.G.) and by Rutgers University Dissertation Fellowships (J.-H.Y., T.K.).
National Institutes of Health
Authorship
Contribution: J.-H.Y. designed and performed experiments and analyzed data; T.K. performed experiments; D.G. designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Doina Ganea, Temple University School of Medicine, Department of Microbiology and Immunology, 3400 N Broad St, Philadelphia, PA 19140; e-mail: doina.ganea@temple.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal