Abstract
In order to investigate the biologic processes underlying and resulting from the megakaryocytic hyperplasia that characterizes idiopathic myelofibrosis (IMF), peripheral blood CD34+ cells isolated from patients with IMF, polycythemia vera (PV), and G-CSF–mobilized healthy volunteers were cultured in the presence of stem cell factor and thrombopoietin. IMF CD34+ cells generated 24-fold greater numbers of megakaryocytes (MKs) than normal CD34+ cells. IMF MKs were also shown to have a delayed pattern of apoptosis and to overexpress the antiapoptotic protein bcl-xL. MK hyperplasia in IMF is, therefore, likely a consequence of both the increased ability of IMF progenitor cells to generate MKs and a decreased rate of MK apoptosis. Media conditioned (CM) by CD61+ cells generated in vitro from CD34+ cells were then assayed for the levels of growth factors and proteases. Higher levels of transforming growth factor-β (TGF-β) and active matrix metalloproteinase-9 (MMP9) were observed in media conditioned with IMF CD61+ cells than normal or PV CD61+ cells. Both normal and IMF CD61+ cells produced similar levels of VEGF. MK-derived TGF-B and MMP-9, therefore, likely contribute to the development of many pathological epiphenomena associated with IMF.
Introduction
Idiopathic myelofibrosis (IMF) is a chronic myeloproliferative disorder (MPD) characterized by leukoerythroblastosis, teardrop red blood cells, varying degrees of bone marrow (BM) fibrosis, splenomegaly, and extramedullary hematopoiesis.1-3 At present, there is no curative treatment available for this disease except hematopoietic stem cell transplantation.4,5 IMF is characterized by a profound hyperplasia of morphologically abnormal megakaryocytes (MKs). The biologic basis for the MK hyperplasia in IMF remains unclear. Increased numbers of MK progenitor cells as well as progenitor cell cytokine independence have been thought to contribute to the MK hyperplasia in IMF. The formation of platelets from MKs and MK senescence in vitro has been shown to be associated with apoptosis.6 Zauli et al previously generated MKs in serum-free (SF) suspension cultures of normal CD34+ cells in the presence TPO.7 The peak generation of MKs occurred after 12 to 15 days of culture. A progressive increase in MKs undergoing apoptosis was observed after 15 days with more than 50% of the cells undergoing apoptosis after 18 days of culture.8,9 Changes in the levels of bcl-xL, an antiapoptotic protein,9 in MKs have been correlated with MK ultrastructure and platelet levels. High levels of expression of bcl-xL have been shown to impact the ability of MKs to fragment into platelets.10 Apoptosis has been previously reported to be dysregulated in the MPDs, including polycythemia vera (PV) and essential thrombocythemia (ET).11,12 Bcl-xL has been shown to be responsible for giving rise to erythropoietin-independent erythroid colony formation in PV.13 We hypothesized that a greater ability of IMF CD34+ cells to generate MKs as well as delayed MK apoptosis might contribute to the MK hyperplasia in IMF.
Recently, a mutation in the JH2 domain of the JAK2 tyrosine kinase (JAK2 617V>F) has been identified in the Philadelphia chromosome (Ph)-negative MPDs and has been shown to be associated with hypersensitivity of hematopoietic progenitor cells to cytokines.14 The JAK2 617V>F mutation is present in about 50% of patients with IMF, but its role in the pathogenesis of this disease remains uncertain.15 In addition, about 10% of IMF patients negative for JAK2 617V>F have been found to have an activating mutation in the c-MPL (MPL 515W>L), which also confers cytokine-independent proliferation, and results in constitutive phosphorylation of JAK2, STAT 3, STAT 5, AKT, and ERK.16 We therefore determined whether the JAK2 617V>F mutation in IMF CD34+ cells affected the behavior of IMF MKs.
In IMF, MKs have been suggested to be responsible for the BM fibrosis due to the local release of fibrogenic growth factors.17,18 TGF-β is an important growth factor, which promotes the extracellular matrix deposition, angiogenesis,19 and fibrosis.20,21 Other factors including platelet-derived growth factor (PDGF), basic fibroblast growth factor-2 (bFGF), and vascular endothelial growth factor (VEGF) have also been implicated in the generation of the BM fibrosis or the increased BM microvessel density characteristic of this disorder.22-24 In addition, we have previously reported that IMF patients have significantly higher plasma levels of proteases including neutrophil elastase (NE), and matrix metalloproteinase-9 (MMP-9) creating a proteolytic marrow microenvironment likely responsible for the constitutive mobilization of CD34+ cells into the peripheral blood in these patients.25 We therefore examined if these cytokines or proteases were produced preferentially by IMF MKs.
Patients, materials, and methods
Patient specimens and CD34+ cells isolation
Peripheral blood (PB) was collected from 10 healthy volunteers mobilized with granulocyte colony-stimulating factor (G-CSF) (MPB), 12 patients who fulfilled the Italian diagnostic criteria for the diagnosis of IMF,26 and 4 patients with PV diagnosed according to the Polycythemia Vera Study Group criteria.3 The PB from the PV patients was collected as part of a therapeutic phlebotomy. All patients had signed an informed consent approved by the institutional review board of the University of Illinois at Chicago in accordance with the Declaration of Helsinki.
Mononuclear cells (MNCs) were isolated by density gradient centrifugation using Ficoll-Paque (GE Healthcare Bio-Science AB, Uppsala, Sweden). CD34+ cells were then selected using a CD34+ Isolation Kit (Miltenyi Biotec, Auburn, CA). The purity of CD34+ cells was verified using CD34-PE flow cytometrically. A CD34+ cell population with purity of at least 85% was used for all studies.
Clinical characteristics
IMF patients were between 44 and 66 years of age (mean, 53 years). Fifty percent of these patients were JAK2 617V>F positive, and all patients were negative for the MPL 515W>L mutation. All IMF patients had moderate to massive splenomegaly, at least 1+ bone marrow fibrosis, peripheral blast count less than 3%, and a Lille score of 0 to 2. Half the patients had anemia and 20% had chromosomal abnormalities. Each of the PV patients was JAK2 617V>F positive, 2 were heterozygous, and 2 homozygous for the mutation. The average age of the PV patients was 50 (ranging between 39 and 60 years) and were diagnosed 1 to 4 years before the present evaluation. Three of the patients had palpable splenomegaly, while one of the patients had massive splenomegaly.
JAK2 617V>F and MPL 515W>L mutational analysis
The presence of JAK2 617V>F and MPL 515W>L mutations was determined in the PB MNCs of patients with IMF as previously described.27,28 Briefly, genomic DNA (gDNA) was extracted from MNCs using Easy-DNA Kit (Invitrogen, Carlsbad, CA) and was amplified using primers 5′-GATCTCCATATTCCAGGCTTACACA-3′ and 5′-TATTGTTTGGGCATTGTAACCTTCT-3′, which cover JAK2 617V>F mutation sites, as well as using primers 5′-TgggCCgAAgTCTgACCCTTT-3′ and 5′-ACAgAgCgAACCAAgAATgCCTgT-3′, which cover the MPL 515W>L mutational site. Polymerase chain reaction (PCR) was performed with DNA polymerase iTaq (BiO-Rad, Hercules, CA). The PCR products were gel purified and sequenced directly using the ABI 3730xl DNA analyzer (Applied Biosystems, Foster City, CA) at the DNA Sequencing Facility of the University of Illinois at Chicago. Among the 12 IMF patients used in this study, 6 patients were JAK2 617V>F positive and none was MPL 515W>L positive.
Suspension cultures
CD34+ cells were cultured in serum free media (StemSpan SFEM; Stem Cell Technologies, Vancouver, BC) at a concentration of 50 000 cells/mL in the presence or absence of cytokines.7 Every 4 days, half of the media was exchanged with fresh media containing cytokines. Individual cultures of CD34+ cells were performed with varying doses of TPO (0, 5, 50, 100, 200 ng/mL) and a fixed amount of SCF (100 ng/mL). On day 15, cells from the cultures performed with 100 ng/mL TPO and SCF were washed and CD61+ cells isolated using CD61+ Isolation Kit (Miltenyi Biotec) according to the manufacturer's recommendations. A purity of more than 90% was verified flow cytometrically using anti-CD61-PE antibodies (Miltenyi Biotec). Purified populations of MKs (> 90%) were cultured for an additional 7 days in the presence of maximal amounts of SCF and TPO. Conditioned media (CM) were prepared from the liquid cultures at days 8, 11, and 15 of primary cultures and on day 3 and 5 of secondary cultures by collecting half the supernatant obtained after the centrifugation of the cultured cells. The CM was stored at −80°C until evaluation.
Morphologic analysis
Primary cells after 8, 11, and 15 days of cultures and CD61+ selected cells after 3, 5, and 7 days of secondary cultures were spun using a Cytospin 3 (Thermo Shandon, Astmoor, England) onto glass slides. Cells were stained with May-Grunwald-Giemsa and MK morphology was evaluated using a light microscope (Olympus BX40F-3; Olympus, Melville, NY).
Flow cytometric analysis
CD41/propidium iodide (PI) (Sigma-Aldrich, St Louis, MO) staining.
On days 8, 11, and 15, 1 × 105 cultured cells were removed from the suspension cultures, washed, blocked with 40% human serum, and incubated for 15 minutes on ice. The cells were then labeled with either CD41a-FITC (BD Pharmingen, San Diego, CA) for the test samples or IgG1-FITC (BD Pharmingen) for the controls, for 30 minutes. Cells were fixed with 100% ethanol while vortexing, washed, and treated with ribonucleasese A solution (Sigma-Aldrich) for 30 minutes at 37°C. Cells were resuspended with 0.5μL/mL PI and analyzed for the percentage of CD41+ cells and the percentage CD41+ cells undergoing apoptosis using a FACSCalibur (BD Pharmingen). The ploidy of CD41+ cells was also evaluated on day 15 by analyzing the DNA content of cells stained with PI flow cytometrically as previously described.29
Bcl-xL intracellular staining.
CD61+ cells selected from the primary cultures on day 15 were washed, blocked with human serum, stained with CD41-PE or IgG-PE, and incubated on ice for 1 hour. Similar analyses were also performed on days 3, 5, and 7 of the secondary cultures. Cells were then washed and incubated for 20 minutes in Cytofix/Cytoperm solution (BD Pharmingen) and washed with Perm/Wash solution. bcl-xL-FITC antibody (100 μL; Santa Cruz Biotechnology, Santa Cruz, CA) was then added to the cell pellet and IgG1-FITC (BD Pharmingen) to the control samples, and the samples were incubated for 30 minutes at 4°C in the dark. Cells were washed, resuspended in magnetic-activated cell sorting (MACS) buffer, and analyzed flow cytometrically.
ELISA assays
Frozen samples of CM were thawed overnight at 4°C and analyzed using commercially available enzyme-linked immunosorbent assays (ELISA) kits for levels of various growth factors and proteases including TGF-β1, bFGF-2, PDGF, VEGF, MMP-9, and MMP-2 (R&D Systems, Minneapolis, MN) and NE (Cell Sciences, Canton, MA) according to the manufacturer's recommendations. Briefly, after appropriate dilution and standard curve preparation, plates coated with various antibodies were incubated for 2 hours at room temperature in the presence of CM from different samples. After incubation, the wells were washed with a wash buffer using an ELx50 washing machine (Bio-Tek, Winooski, VT) and a corresponding conjugate added to each sample followed by a reincubation. Samples were again washed and the substrate solution was added to each well. After a brief incubation, a stop solution provided with each kit was used to terminate the reaction and the plates were read using KC4 v.3.4 software and a Synergy HT microplate reader (Bio-Tek) set at 450- and 570-nm wavelengths. The concentrations of active MMP-9 were measured using a fluorometric assay in which MMP-9 was first immunoabsorbed to plastic wells coated with an anti-MMP-9 monoclonal antibody. The concentration of active MMP-9 was measured with a fluorogenic substrate cleaved by proteolitically active MMP-9 and read at 320-nm wavelengths.
Statistical analysis
The results were expressed as the mean plus or minus the standard deviation (SD) or standard error of the mean (SEM) of data obtained from varying numbers of individual experiments. Differences between the experimental groups were assessed using unpaired 2-sided Student t test. Statistical significance was defined as P value of less than .05.
Results
IMF CD34+ cells generate greater numbers of megakaryocytes in the presence and absence of TPO
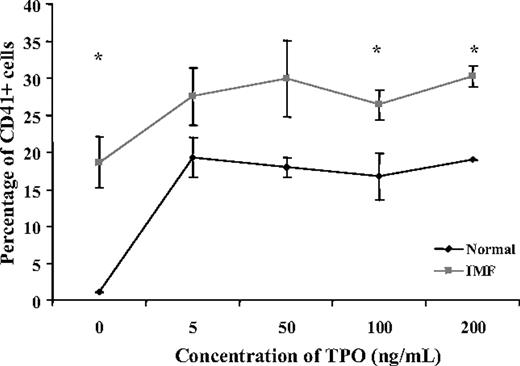
In order to determine the dependency of IMF CD34+ cell differentiation to MKs on TPO, normal and IMF CD34+ cells were incubated in the absence of TPO as well as the presence of varying concentrations of TPO for different periods of time, and the percentage of CD41+ cells was determined flow cytometrically. As can be seen in Figure 1A, after 11 days of culture, in the absence of TPO, the percentage of CD41+ cells was significantly higher in IMF (18.2% ± 3.4%) compared with normal samples (1.1% ± 0.1%). MK formation by normal CD34+ cells was largely dependent on the addition of TPO, while IMF CD34+ cells generated a significant fraction (62%) of the maximum percentage of CD41+ cells in the absence of TPO. The addition of 5 ng/mL TPO resulted in a small additional increase in the percentage of IMF CD41+ cells (7.9% ± 2.1%), while a marked increase occurred following the addition of a similar concentration of TPO to normal samples (18.2% ± 2.8%).
Effect of different concentrations of TPO on the generation of CD41+cells in vitro. This figure represents the percentage of CD41+ cells generated in suspension culture from normal and IMF CD34+ cells in the presence of 100 ng/mL SCF and various concentrations of TPO (0-200 ng/mL) (n = 3) after 11 days of culture. In the absence of TPO, the percentage of CD41+ cells was significantly greater in cultures of IMF CD34+ cells compared with normal (P < .05). The addition of 5 ng/mL TPO resulted in a marked increase in the percentage of normal CD41+ cells (> 95% of the maximal output), while causing a far smaller increment in the percentage of CD41+ cells in the IMF samples (38% of the maximal output). A relative plateau in the percentage of CD41+ cells was observed at higher doses of TPO. The * indicates significant differences between the percent of normal CD41+ cells in normal and IMF cultures (P < .05). Values are shown as the mean ± SD.
Effect of different concentrations of TPO on the generation of CD41+cells in vitro. This figure represents the percentage of CD41+ cells generated in suspension culture from normal and IMF CD34+ cells in the presence of 100 ng/mL SCF and various concentrations of TPO (0-200 ng/mL) (n = 3) after 11 days of culture. In the absence of TPO, the percentage of CD41+ cells was significantly greater in cultures of IMF CD34+ cells compared with normal (P < .05). The addition of 5 ng/mL TPO resulted in a marked increase in the percentage of normal CD41+ cells (> 95% of the maximal output), while causing a far smaller increment in the percentage of CD41+ cells in the IMF samples (38% of the maximal output). A relative plateau in the percentage of CD41+ cells was observed at higher doses of TPO. The * indicates significant differences between the percent of normal CD41+ cells in normal and IMF cultures (P < .05). Values are shown as the mean ± SD.
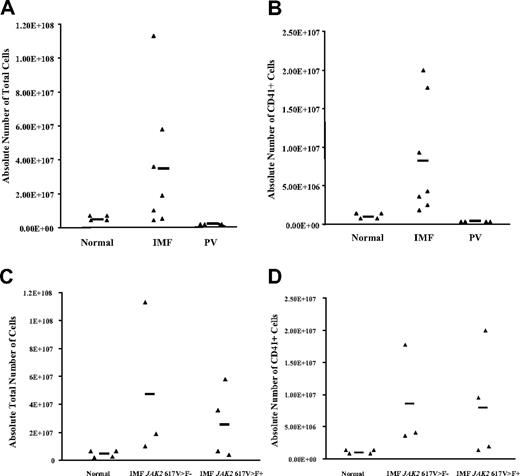
In the presence of maximal cytokine concentrations (100 ng/mL SCF and 100 ng/mL TPO), the total number of cells generated after 15 days of culture by IMF CD34+ cells (3.5 × 107 ± 1.3 × 107cells, n = 7) was 22.5-fold greater than healthy controls (1.5 × 106 ± 6.8 × 105 cells, n = 4; P < .05) and more than 300-fold greater than PV CD34+ cells (N = 4) (Figure 2A), while the absolute number of IMF CD41+ cells (8.2 × 106 ± 2.9 × 106, n = 7) was more than 24-fold greater compared with healthy samples (3.4 × 105 ± 0.7 × 105, n = 4; P < .05) and more than 800-fold greater than the PV samples (Figure 2B). The total number of cells as well as the total number of CD41+ cells generated were similar when cultures were initiated with CD34+ cells isolated from IMF patients with or without the JAK2 617V>F mutation (Figure 2C,D), suggesting that this mutation did not exclusively account for the in vitro behavior of IMF CD34+ cells.
Number of cells generated from normal and IMF CD34+cell cultures. (A) The absolute total number of cells generated by normal, IMF, and PV CD34+ cells after 15 days of culture in the presence of maximal cytokine combination (100 ng/mL TPO and 100 ng/mL SCF) favoring megakaryocyte development. Each ▴ represents the result of a single experiment with specimens from different patients. The bar represents the mean number of values for each group. The total number of cells generated from CD34+ cells was significantly greater in IMF samples (n = 7) compared with normal samples (n = 4) and PV samples (n = 4) (P < .05). (B) The absolute number of CD41+ cells generated after 15 days in the same cultures. The numbers of CD41+ cells generated by IMF CD34+ cells was statistically greater than the numbers generated by normal CD34+ cells (n = 4) or PV CD34+ cells (P < .05). No significant difference (C) in the absolute numbers of cells or (D) the absolute number of CD41+ cells generated by the CD34+ cells obtained from patients with JAK2 617V>F–positive and –negative IMF was found (P > .05).
Number of cells generated from normal and IMF CD34+cell cultures. (A) The absolute total number of cells generated by normal, IMF, and PV CD34+ cells after 15 days of culture in the presence of maximal cytokine combination (100 ng/mL TPO and 100 ng/mL SCF) favoring megakaryocyte development. Each ▴ represents the result of a single experiment with specimens from different patients. The bar represents the mean number of values for each group. The total number of cells generated from CD34+ cells was significantly greater in IMF samples (n = 7) compared with normal samples (n = 4) and PV samples (n = 4) (P < .05). (B) The absolute number of CD41+ cells generated after 15 days in the same cultures. The numbers of CD41+ cells generated by IMF CD34+ cells was statistically greater than the numbers generated by normal CD34+ cells (n = 4) or PV CD34+ cells (P < .05). No significant difference (C) in the absolute numbers of cells or (D) the absolute number of CD41+ cells generated by the CD34+ cells obtained from patients with JAK2 617V>F–positive and –negative IMF was found (P > .05).
IMF megakaryocytes are characterized by a decreased rate of apoptosis
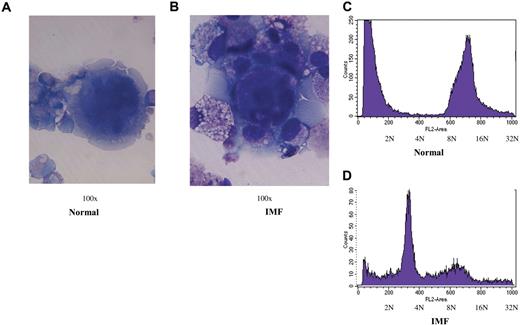
The morphology of CD41+ cells was evaluated on days 8, 11, and 15 of culture. Normal MKs were larger, multilobulated and by day 15 began to show evidence of apoptosis, including vacuolization of the cytoplasm, nuclear condensation, disruption of the integrity of the cell membrane, and cytoplasmic blebbing (Figure 3A). By contrast, IMF MKs were smaller, had an open nucleus, and had an intact cell membrane (Figure 3B). Flow cytometric analysis of the CD41+ cell DNA content after 15 days of culture was evaluated as the percentage of cells with less than 2N and more than 2N. Of the normal CD41+ cells, 51.4% (± 12.7%) had a ploidy more than 2N (n = 9), while only 15.5% (± 1.8%) of the CD41+ cells from IMF patients were more than 2N (n = 11; P < .05, Figure 3C,D).
MK morphology and ploidy after 15 days of culture. (A,B) Representative photomicrographs demonstrating the morphology (100 ×/0.75 NA oil objective, Olympus BH-2 microscope, Nikon digital sight DS-L1 camera) of normal and IMF MKs generated from CD34+ cell cultures after 15 days of incubation. (C,D) demonstrate DNA content of CD41+ cells generated from a representative sample of normal and IMF CD34+ cells as determined flow cytometrically.
MK morphology and ploidy after 15 days of culture. (A,B) Representative photomicrographs demonstrating the morphology (100 ×/0.75 NA oil objective, Olympus BH-2 microscope, Nikon digital sight DS-L1 camera) of normal and IMF MKs generated from CD34+ cell cultures after 15 days of incubation. (C,D) demonstrate DNA content of CD41+ cells generated from a representative sample of normal and IMF CD34+ cells as determined flow cytometrically.
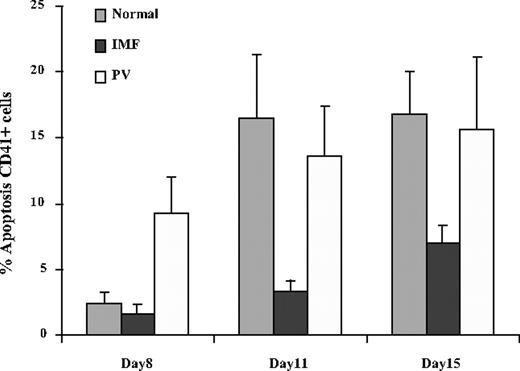
The percentage of normal, PV, and IMF MKs undergoing apoptosis was evaluated by flow cytometry using an anti-CD41 antibody and PI staining after 8, 11, and 15 days of culture. The apoptotic fraction of CD41+ cells was measured by monitoring the fraction of CD41+ cells in the sub-G0/G1 fraction. The percentage of normal and IMF CD41+ cells undergoing apoptosis gradually increased during the period of culture (Figure 4). However, this increment in the percentage of cells undergoing apoptosis was dramatically lower in IMF MKs (3.4% ± 0.7%) compared with normal MKs (14.6% ± 5.2%) after 11 days of culture (n = 8), and 6.7% (± 1.2%) compared with 16.8% (± 3.1%) after 15 days of culture (N = 12; P < .05 for both time points). On day 15, CD61+ cells from IMF, normal, and PV CD34+ cell cultures were immunoselected using an anti-CD61 antibody, cultured in similar conditions and analyzed on days 3, 5, and 7 of secondary cultures using an antibody to bcl-xL. After 18 days of culture, the mean percentage of normal CD41+ cells expressing bcl-xL was approximately 40%, while more than 80% of IMF and PV CD34+ cells expressed bcl-xL (Figure 5A). The decay of bcl-xL expression in CD41+ cells is illustrated in Figure 5B. One can see that the percentage of IMF and PV CD41+ cells expressing bcl-xL persisted for far greater periods of time than that observed for normal CD41+ cells. In cultures of normal CD41+ cells, the expression of bcl-xL dramatically decreased after 11 days of culture, while bcl-xL expression by IMF and PV CD41+ cells persisted until day 18 before decreasing. The expression of bcl-xL in IMF CD41+ cells was similar in cultures initiated with CD41+ cells obtained from IMF patients with or without JAK2 617V>F mutation (83.2% ± 3% vs 87.5% ± 3.5% in JAK2 617V>F negative and positive, respectively) (Figure 5C).
Apoptosis of normal and IMF CD41+cells. The percentages of normal, IMF, and PV CD41+ cells undergoing apoptosis after 8, 11, and 15 days of culture are shown here. A progressive increase in the number of CD41+ cells undergoing apoptosis was observed in normal, IMF, and PV CD34+ cell cultures. The number of IMF CD41+ cells undergoing apoptosis was significantly smaller than that of normal CD41+ cells after 11 and 15 days of culture (P < .05; n = 8-12). Values are expressed as the mean ± SD.
Apoptosis of normal and IMF CD41+cells. The percentages of normal, IMF, and PV CD41+ cells undergoing apoptosis after 8, 11, and 15 days of culture are shown here. A progressive increase in the number of CD41+ cells undergoing apoptosis was observed in normal, IMF, and PV CD34+ cell cultures. The number of IMF CD41+ cells undergoing apoptosis was significantly smaller than that of normal CD41+ cells after 11 and 15 days of culture (P < .05; n = 8-12). Values are expressed as the mean ± SD.
Expression of bcl-xL by normal and IMF megakaryocytes. (A) depicts a representative flow cytometric analysis of bcl-xL expression by normal and IMF CD41+ cells after 3 days of secondary culture (day 18). Percentages represent the percentage of CD41+ cells expressing Bcl-xL. (B) shows the decay of bcl-xL expression in the percentage of CD41+ cells present in normal, IMF, and PV cultures. After 11 days of culture, there was a marked decline in the expression of bcl-xL in the normal CD41+ cells, while the expression of bcl-xL remained persistently elevated until day 18 in the IMF and PV CD41+ cells and then decreased after that. A significant difference in the expression of bcl-xL was observed between the normal and IMF-cultured cells (P < .01) (n = 5 and 7, respectively). Values are shown as the mean ± SD. (C) represents the expression of bcl-xL by CD41+ cells derived from CD34+ cells isolated from patients with IMF. The overexpression of bcl-xL was independent of JAK2 617V>F mutational status (P > .05). Values are shown as the mean ± SD.
Expression of bcl-xL by normal and IMF megakaryocytes. (A) depicts a representative flow cytometric analysis of bcl-xL expression by normal and IMF CD41+ cells after 3 days of secondary culture (day 18). Percentages represent the percentage of CD41+ cells expressing Bcl-xL. (B) shows the decay of bcl-xL expression in the percentage of CD41+ cells present in normal, IMF, and PV cultures. After 11 days of culture, there was a marked decline in the expression of bcl-xL in the normal CD41+ cells, while the expression of bcl-xL remained persistently elevated until day 18 in the IMF and PV CD41+ cells and then decreased after that. A significant difference in the expression of bcl-xL was observed between the normal and IMF-cultured cells (P < .01) (n = 5 and 7, respectively). Values are shown as the mean ± SD. (C) represents the expression of bcl-xL by CD41+ cells derived from CD34+ cells isolated from patients with IMF. The overexpression of bcl-xL was independent of JAK2 617V>F mutational status (P > .05). Values are shown as the mean ± SD.
Taken together, these data indicate a significant decrease in the rate of IMF MKs undergoing apoptosis, which was associated with an increased expression of the antiapoptotic protein bcl-xL. These findings, in addition to the preferential programming of IMF CD34+ cells to generate MKs, might explain the accumulation of MKs in the BM from IMF patients.
TGF-β, active MMP-9, and VEGF levels are increased in the conditioned media prepared from IMF cell cultures
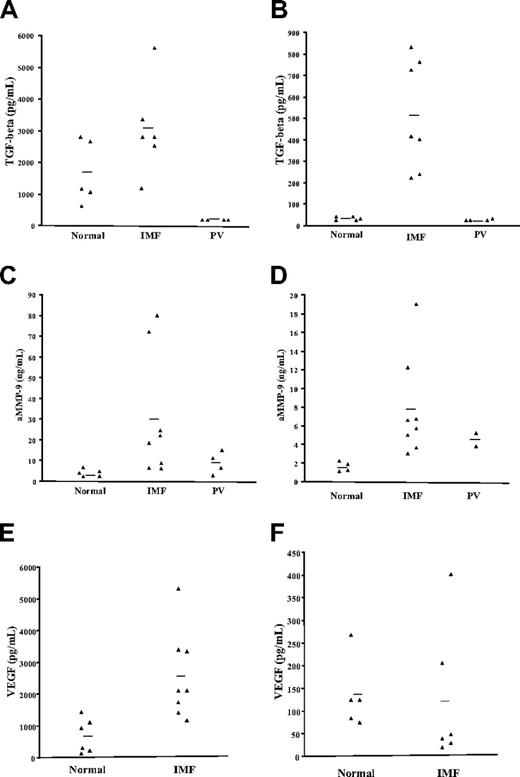
CM from normal and IMF MK cultures were collected and evaluated for the presence of growth factors and proteases thought to be involved in the pathogenesis of IMF. The levels of TGF-β, VEGF, bFGF, PDGF, active MMP-9, MMP-2, and NE were quantitated in the CM obtained after 8, 11, and 15 days of primary cultures and in the CM prepared using CD61+ selected cells after 3, 5, and 7 additional days of culture (secondary cultures) using the appropriate ELISA assays. Greater amounts of TGF-β were present in the IMF CM after 8, 11, and 15 days of primary culture. After 15 days, IMF CM contained greater levels of TGF-β levels then that those found in normal samples (mean, 3080.6 ± 585.7 pg/mL vs 1688.3 ± 462.1 pg/mL, P > .05, Figure 6A). After CD61+ selection and 3 days of secondary culture (day 18), the amount of TGF-β was significantly greater in the CM prepared with cells from IMF samples compared with normal control cells (mean, 513 ± 96.2 vs 43 ± 4 pg/mL, P < .01, Figure 6B). No TGF-β was found in the CM from the PV samples, which might be related to the limited number of MKs generated from PV CD34+ cells (Figure 6A,B). The presence of TGF-β in normal CD61+ cell CM confirms the previously reported ability of normal megakaryocytes to produce TGF-β.19 Moreover, our experiments indicate that significantly greater amounts of TGF-β are generated by equal numbers of IMF MKs compared with normal MKs and that the TGF-β production appears to be characteristic of IMF compared with other Ph-negative MPDs such as PV.
TGF-β, active MMP-9, and VEGF levels in the CM from normal and IMF cell cultures. The levels of TGF-β, active MMP-9, and VEGF were quantified in the CM after 15 days of primary cultures and 3 days of secondary cultures by ELISA. Each point represents the concentration of a growth factor or protease in the CM. The bars represent the mean values for each group. (A,B) represent the levels of TGF-β in the CM from normal, IMF, and PV samples at the 2 time points mentioned above. Significantly greater levels of TGF-β were found in the IMF CM from secondary cultures (B) (P < .01). (C,D) represent the levels of active MMP-9 in the CM from normal, IMF, and PV cultures in primary and secondary cultures. Significantly greater levels of active MMP-9 were found in the IMF CM both after 15 days of primary cultures and 3 days of secondary cultures compared with normal samples (P < .05). (E-F) VEGF levels were also measured in the CM from these cultures. In contrast to TGF-β or active MMP-9, the levels of VEGF were not found to be elevated in the IMF CM after 3 days of secondary cultures (F), however, greater levels of VEGF were found in the IMF CM from primary cultures (E) (P < .05), probably related to the greater numbers of MKs found in these cultures.
TGF-β, active MMP-9, and VEGF levels in the CM from normal and IMF cell cultures. The levels of TGF-β, active MMP-9, and VEGF were quantified in the CM after 15 days of primary cultures and 3 days of secondary cultures by ELISA. Each point represents the concentration of a growth factor or protease in the CM. The bars represent the mean values for each group. (A,B) represent the levels of TGF-β in the CM from normal, IMF, and PV samples at the 2 time points mentioned above. Significantly greater levels of TGF-β were found in the IMF CM from secondary cultures (B) (P < .01). (C,D) represent the levels of active MMP-9 in the CM from normal, IMF, and PV cultures in primary and secondary cultures. Significantly greater levels of active MMP-9 were found in the IMF CM both after 15 days of primary cultures and 3 days of secondary cultures compared with normal samples (P < .05). (E-F) VEGF levels were also measured in the CM from these cultures. In contrast to TGF-β or active MMP-9, the levels of VEGF were not found to be elevated in the IMF CM after 3 days of secondary cultures (F), however, greater levels of VEGF were found in the IMF CM from primary cultures (E) (P < .05), probably related to the greater numbers of MKs found in these cultures.
The levels of active MMP-9 were also quantitated in the CM prepared with normal and IMF cells. In primary cultures after 15 days, the IMF CM contained 29.8 (± 10) ng/mL compared with the normal CM, which contained 2.7 (± 0.5 ng/mL, P < .05). Secondary cultures of IMF CD61+ cells after 3 days contained 7.6 (± 1.9) ng/mL, while normal CD61+ CM prepared with similar number of MKs contained 3.76 (± 0.21) ng/mL MMP-9 (P < .05). By contrast, PV cultures contained intermediate levels of MMP-9 (Figure 6C,D). These data demonstrate that the intrinsic behavior of IMF MKs is characterized by excessive production of TGF-β and active MMP-9.
The concentration of VEGF was also found to be significantly increased in the CM from IMF samples compared with normal controls. Four times greater concentrations of VEGF were found in the IMF CM after 15 days of CD34+ cell culture (2552.2 ± 672.5 pg/mL) compared with normal samples (672.5 ± 220.4 pg/mL, P < .01, Figure 6B). Equal numbers of IMF and normal CD61+ cells selected after 15 days produced similar amounts of VEGF after 3 additional days of secondary cultures (120.7 ± 62.2 pg/mL vs 137 ± 34.1 pg/mL, Figure 6C). Our experiments indicate that IMF cultures generate greater amounts of VEGF, but, unlike TGF-β or MMP-9, IMF MKs do not appear to possess a greater ability to produce VEGF than normal MKs. The higher levels of VEGF found in CM prepared with IM CD34+ cells after 15 days of culture can, likely, be attributed to the greater absolute number of MKs present in such cultures. We did not identify a significant difference in the levels of TGF-β, active MMP-9, and VEGF between the CM prepared from primary and secondary cultures of patients with JAK2 617V>F-positive or -negative IMF (data not shown).
In addition, the ability of IMF MKs to produce other growth factors and proteases was examined. PDGF, bFGF, MMP-2, and NE were not detected in CM generated from both the primary and the secondary cultures of either normal or IMF cells performed under the conditions described in “Patients, materials, and methods.”
Correlation of MK abnormalities with clinical phenotype
No significant correlations were found between the number of total cells or CD41+ cells generated in vitro from IMF CD34+ cells, the presence or absence of JAK2 617V>F mutation, the levels of bcl-xL overexpression, and the clinical features of the IMF patients (the degree of BM fibrosis, the spleen size, the percentage of blast count in the peripheral blood, Lille score). Both JAK2 617V>F–positive and –negative IMF patients had similar degrees of splenomegaly, degrees of BM fibrosis, and Lille scores. The ability to generate total cells and CD41+ cells from PV CD34+ cells tended to be greater in 2 samples isolated from patients homozygous for JAK2 617V>F.
Discussion
IMF is a Ph-chromosome–negative MPD characterized by MK hyperplasia, BM fibrosis, increased BM microvessel density,30 and constitutive mobilization of CD34+ cells in the circulation.31 BM hyperplasia of morphologically abnormal MKs is a hallmark of IMF. We hypothesize that MK proliferation is at the center of the pathogenesis of this disease, and that MKs play a crucial role in the disease progression through the secretion of different growth factors and proteases involved in the generation of BM fibrosis, marrow microvessel density, and stem cell mobilization. It has long been suspected that different growth factors might be involved in the pathogenesis of IMF, in particular, TGF-β, VEGF, PDGF, and bFGF. A proteolytic environment has been reported to disrupt the adhesive interactions between hematopoietic stem cells/hematopoietic progenitor cells (HSCs/HPCs) and the stem cell niche leading to mobilization of CD34+ cells. Higher levels of proteases including MMP-9 and NE have been found in the plasma of patients with IMF,25 suggesting that these ligand-receptor interactions that result in the retention of HSCs/HPCs within the BM.32
Previously, several investigators have reported the results of studies using suspension cell cultures, which favored the differentiation of normal CD34+ cells into MK in vitro using media supplemented with SCF and TPO.7,8 Progressive MK apoptosis was observed after 15 days of culture.7,8 MK apoptosis was correlated with the decreased expression of an antiapoptotic protein bcl-xL but not with changes in bcl-2 expression.33 Bcl-xL is an important regulatory protein associated with normal MK development. Levels of this protein have been shown to decrease progressively according to the stages of normal MK differentiation.8 In addition, Kaluzhny et al have shown that overexpression of MK bcl-xL resulted in increased MK numbers and decreased apoptosis and the ability of these cells to generate proplatelets.10
We investigated the origins of MK hyperplasia in IMF as well as the production of growth factors and proteases by MKs using a modification of the “2-step” MK culture system described by Majka et al.34 In this system, MK formation by normal CD34+ cells was dependent on the addition of exogenous TPO. Previously, MK colony formation in semisolid media was, however, reported to occur in the absence of TPO.35 We were able to confirm this characteristic of IMF MKs using suspension cell cultures. We have found that PB IMF CD34+ cells generated more than 16-fold greater numbers of CD41+ cells in the absence of TPO compared with normal CD34+ cells. Adding TPO to IMF CD34+ cell cultures caused only a modest further increase in the percentage of CD41+ cells compared with CD34+ cells derived from PB of healthy volunteer donors. These results strongly suggest that the differentiation of a subpopulation of IMF CD34+ cells to CD41+ cells occurs in the absence of TPO, while a smaller second subpopulation of CD34+ cells requires TPO to differentiate into CD41+ cells. In addition, equal numbers of IMF CD34+ cells generated far greater MKs than normal CD34+ cells in vitro. This capability of CD34+ cells to generate far greater numbers of MKs surely contributes to the MK hyperplasia in IMF.
In addition, the possibility that MK accumulation might partially account for the MK hyperplasia in IMF was explored. MK apoptosis in vitro was found to be delayed in IMF. This delay was associated with the overexpression of bcl-xL. CD41+ cells derived from normal and IMF CD34+ cells uniformly expressed bcl-xL for the first 11 days of culture. After that, the normal CD41+ cells experienced a rapid decline in the expression of this antiapoptotic protein. Both IMF and PV CD41+ cells, however, had persistent expression of bcl-xL until day 18, after which the bcl-xL expression diminished. Overexpression of bcl-xL, in addition to the increased ability of IMF CD34+ cells to generate MKs might explain the megakaryocytic hyperplasia in IMF.
IMF CD34+ cells have a greater ability to generate CD41+ cells that overexpress bcl-xL and undergo apoptosis in vitro in a delayed manner. The properties of PV CD34+ cells are, however, quite different. Under identical culture conditions, PV CD34+ cells generated far fewer numbers of total cells and MKs than IMF or normal CD34+ cells. This finding is surprising since MK hyperplasia is a pathological hallmark of bone marrow biopsies of patients with PV. PV CD41+ cells, however, clearly resemble IMF CD41+ cells in having a delayed pattern of undergoing in vitro apoptosis and are characterized by increased bcl-xL. Teofili et al have recently shown that PV cells are characterized by increased phosphorylation of STAT-3 and STAT-5,36 which is correlated with bcl-xL overexpression,37 suggesting the importance of a JAK-STAT-bcl-xL activation pathway in this disease. By contrast, STAT-3 and STAT-5 activation was not documented in IMF marrow cells.36 In this report, the bcl-xL overexpression was observed in IMF MKs regardless of the presence of JAK2 617V>F mutation. These findings, in light of the data presented by Teofili et al, suggest that a different mechanism might be responsible for the greater bcl-xL expression in IMF than in PV.
MKs have been hypothesized to contribute to the pathogenesis of IMF by the production of profibrotic and proangiogenetic growth factors. Multiple growth factors have been implicated in this process but conclusive evidence exists for a central role of TGF-β. TGF-β is a multifunctional cytokine that may stimulate the secretion of extracellular matrix, which has been linked to human diseases as promoter of fibrosis. TGF-β can also act as a regulator of cellular proliferation, migration, survival and differentiation. Genetic studies in humans have also revealed the role of TGF-β as well as its signaling components in angiogenesis.38 TGF-β has a dual role in cancer progression. Although TGF-β is a potent tumor suppressor, it has been reported to enhance tumor growth and metastasis in advanced stages of cancer. TGF-β signaling in tumor cells has been shown to promote angiogenesis by up-regulating MMP-9.39 TGF-β has been shown to cause BM fibrosis and osteosclerosis in mouse models.40 Our work showed that IMF MKs are a major source of TGF-β, and that abnormal MKs in IMF, due to their increased ability to produce TGF-β, likely play an important role in the generation of the BM fibrosis in these patients.
Matrix metalloproteinases have been implicated in promoting tumor angiogenesis and metastases41 and have been shown to play an important role in angiogenesis, collagen degradation, and mobilization of HSC/HPC. MMPs are secreted as latent proenzyme forms, which require activation in order for the proteolysis to occur.41 We have previously shown that active MMP-9 is increased in the plasma of patients with IMF.25 We now demonstrate that IMF CMs from both primary and secondary MK cultures contain significantly greater amounts of MMP-9. The increased production of active MMP-9 by IMF MKs might also contribute to the release of progenitor cells into the circulation of patients with IMF by degrading SDF-1 or cleaving its receptor CXCR-4.33
VEGF is a potent proangiogenic growth factor responsible for endothelial cell proliferation and differentiation, shown by Mohle et al to be expressed by normal MKs and myeloid progenitors.42 Serum VEGF levels have been reported to be 10 times higher in the PB of IMF patients compared with healthy subjects.24 Although IMF and normal MKs appear to be equivalent in their ability to produce VEGF, our results indicate an increased production of VEGF in the IMF CD34+ cell cultures compared with normal controls after 15 days of culture, a likely consequence of increased number of MKs in IMF samples. The increased levels of VEGF characteristic of IMF MK cultures could contribute to the increased microvascular density found in IMF BM.43
Several laboratories have previously described a role for PDGF and bFGF in the pathogenesis of BM fibrosis in IMF.21-23 Our data indicate that PDGF and bFGF levels were not detectable in the media conditioned by IMF CD61+ cells. Furthermore, MMP-2 and NE have been shown also to play a role in the cytokine-mediated mobilization of CD34+. Levels of these proteases were not detected in the media conditioned by IMF CD61+ cells, indicating that MKs are not the primary cellular source of these proteases and growth factors in IMF.
A number of activating mutations of intracellular and receptor tyrosine kinases have recently been described in the Ph-chromosome-negative MPDs.14,16 The JAK2 617V>F mutation has been found in about 50% patients with IMF,14 while about 10% of JAK2 617V>F-negative samples have been shown to be characterized by the presence of MPL 515W>L mutation.16 These genetic abnormalities have also shown to cause cytokine sensitivity in MPD patients. In the present study, we did not observe a difference in the proliferative capacity of CD34+ cells, numbers of MKs generated, or overexpression of bcl-xL between specimens obtained from IMF JAK2 617V>F-negative or -positive specimens. Garcon et al have hypothesized that JAK2-dependent activation of the STAT5-bcl-xL pathway might account for the endogenous erythroid colony formation observed in MPDs.13 Our findings suggest that JAK2 617V>F is not solely responsible for the overexpression of bcl-xL in the IMF MKs and that additional yet-unknown genetic events might be responsible for the overexpression of bcl-xL.
In conclusion, our studies demonstrate that MKs play a central role in the pathogenesis of IMF. The greater number of MKs observed in the cultures of IMF CD34+ cells can be attributed to the increased ability of IMF CD34+ cells to generate MKs as well as decreased the rate of MK apoptosis. This intrinsic abnormality of IMF MKs is also accompanied by a greater ability to generate TGF-β and active MMP-9 than normal MKs. Therapeutic strategies targeting these unique properties of IMF MKs may provide the basis for future treatment of this disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by grants from National Cancer Institute (1P01CA108671 to R.H.), the Department of Defense (MP048010 to R.H. and MP048007 to M.X.), and the Myeloproliferative Disorders Foundation (R.H. and M.X.).
National Institutes of Health
Authorship
Contribution: S.O.C. performed the experiments and wrote the paper; D.M. performed some of the experiments; N.M. helped perform flow cytometric analysis; T.I., Y.Z., and W.H. performed the genotyping of JAK2 617V>F and MPL 515W>L and the ELISA experiments; E.B. performed cell separation procedures; M.X. and R.H. designed the experiments, interpreted the data, and wrote the paper.
A complete list of the members of the Myeloproliferative Diseases Research Consortium is provided in Document S1 as a data supplement to the online version of this article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald Hoffman, University of Illinois College of Medicine at Chicago, COMRB, 909 S Wolcott, Chicago, IL 60612; e-mail: ronhoff@uic.edu.