Abstract
Epo's erythropoietic capacity is ascribed largely to its antiapoptotic actions. In part via gene profiling of bone marrow erythroblasts, Epo is now shown to selectively down-modulate the adhesion/migration factors chemokine receptor-4 (Cxcr4) and integrin alpha-4 (Itga4) and to up-modulate growth differentiation factor-3 (Gdf3), oncostatin-M (OncoM), and podocalyxin like-1 (PODXL). For PODXL, Epo dose–dependent expression of this CD34-related sialomucin was discovered in Kit+CD71high proerythroblasts and was sustained at subsequent Kit−CD71high and Ter119+ stages. In vivo, Epo markedly induced PODXL expression in these progenitors and in marrow-resident reticulocytes. This was further associated with a rapid release of PODXL+ reticulocytes to blood. As studied in erythroblasts expressing minimal Epo receptor (EpoR) alleles, efficient PODXL induction proved dependence on an EpoR-PY343 Stat5 binding site. Moreover, in mice expressing an EpoR-HM F343 allele, compromised Epo-induced PODXL expression correlated with abnormal anucleated red cell representation in marrow. By modulating this select set of cell-surface adhesion molecules and chemokines, Epo is proposed to mobilize erythroblasts from a hypothesized stromal niche and possibly promote reticulocyte egress to blood.
Introduction
To maintain tissue oxygenation, red cell formation from progenitors in bone marrow, spleen, and fetal liver is strictly regulated.1 In early progenitors, erythroid lineage commitment is directed by a unique set of DNA binding and transcription factors (eg, GATA-1, EKLF-1, and FOG-1).2 Subsequent proerythroblast expansion is likewise sharply controlled, in part by the glycoprotein hormone erythropoietin (Epo).3 Epo is expressed in adult kidney via hypoxia-inducible transcription factor pathways.4 Epo's subsequent interactions with its single transmembrane receptor (EpoR) are then thought to selectively support erythroblast survival.5-7 Redundant EpoR-activated survival pathways, in fact, have been described that depend on PI3 kinase– and AKT-dependent regulation of Foxo3a8 and (m)TOR,9 as well as EpoR/Jak2/Stat5-dependent induction of Pim1 kinase10 and the Bcl2 orthologue Bcl-xl.7 These response pathways probably contribute in important ways to Epo's clinical utility as an antianemia agent and apparent cytoprotective actions on injured cardiac, endothelial, neuronal, and renal cells.11
Less attention has been paid to other possible key Epo action modes. Recently, our laboratory described a core EpoR/Jak2 signaling axis that supports steady-state erythropoiesis but interestingly fails to support accelerated red cell production during anemia.12 Efficient stress erythropoiesis is rescued, however, upon the selective restoration of EpoR/PY343/Stat5 signaling.12 These findings prompted a search for new Epo- (and Stat5-) response genes that might promote stress erythropoiesis. This initially involved the first-time profiling of such genes in purified bone marrow–derived primary erythroblasts. Outcomes reveal Epo regulation of several chemokines and adhesion/migration factors including growth differentiation factor-3 (Gdf3),13 oncostatin-M (OncoM),14 chemokine receptor-4 (Cxcr4),15 integrin alpha-4 (Itga4),16,17 and the CD34-related sialomucin podocalyxin like-1 (PODXL).18
Among this novel set of Epo-modulated factors, Onco-M acts via its JAK- and Stat-coupled heterodimeric receptor19 and can affect cell growth, differentiation, and/or migration in tissue- and context-specific fashions.14,20 Gdf3 acts as a TGF-beta receptor family antagonist and is best characterized by its effects on embryonic dorsal axis formation.13,21 Cxcr4 and Itga4, respectively, are the 7 transmembrane receptors for the Cxc chemokine SDF-122 and an integrin alpha-4 subunit that (as associated with beta-1 integrin) mediates binding to vascular cell adhesion molecule 1 (VCAM-1), fibronectin, and paxillin.16,23,24
PODXL is a sulphated sialomucin that is expressed at high levels by renal podocytes and supports glomerular diaphragm slit formation via antiadhesive and/or charge repulsion effects.18,25 However, PODXL also is a marker for developing hemangioblasts and hematopoietic stem cells26 and can apparently exert antiadhesive effects in cell migratory contexts.18 Within the erythroid lineage, the marked Epo induction of PODXL found in these experiments (and the induction of the above chemokines and adhesion/migration factors) in erythroblasts is therefore proposed to promote transit from a hypothesized early stage niche as well as reticulocyte egress from marrow, especially during anemia.
In bone marrow, niches for stem cells have been characterized at sinusoidal endothelia, which affect self-renewal versus differentiation.27 Similarly, B-cell progenitor fates have been shown to depend on niche-associated interactions with sinusoidal reticular and CXCL12high stromal cells.28 Erythroid islands also clearly exist, are broadly distributed in marrow, and are composed of approximately 10 erythroid cells plus a central resident macrophage.29 Island formation depends in part on ICAM4 and alpha-V integrin interactions and appears to affect primarily late-stage erythroblast maturation.29,30 By comparison, the presently proposed erythroid niche includes early stage erythroblasts, predicted stromal components, and several previously undescribed Epo target genes. Overall, findings suggest that Epo functions as more than a simple survival factor and may dynamically modify the erythroblast cell surface and its microenvironment.
Materials and methods
Mice
Mice expressing EpoR-HM and EpoR-H alleles (and congenic controls), as described,12,31 were used in Institutional Animal Care and Use Committee (IACUC)–approved procedures at age 8 to 12 weeks. Hematocrits and reticulocytes were assayed by microcentrifugation and flow cytometry (ReticCount-Reagent; BD Biosciences, San Jose, CA).12 Epoietin-alpha was administered intraperitoneally at 1 and 24 hours at the doses indicated.
Primary erythroblast preparations
Marrow was flushed from femora and tibiae in Iscove modified Dulbecco medium (IMDM; Invitrogen, Carlsbad, CA) containing 2% fetal bovine serum (FBS), passed through a 40-μm strainer, washed, and resuspended in 1 mL of phosphate-buffered saline (PBS; Invitrogen). After a 2-minute exposure to 9 mL of buffered 0.8% ammonium chloride (Stem Cell Technologies, Vancouver, BC, Canada), 10 × PBS (1.1 mL) was added and cells were collected through 50% FBS in PBS and washed in IMDM. Ex vivo culture was at 8 × 105 cells/mL in StemPro-34 (Invitrogen) supplemented with 2.5 U/mL Epo, 100 ng/mL mSCF, 1 μM dexamethasone, 1 μM beta-estradiol, 75 μg/mL h-transferrin (Sigma, St Louis, MO), 0.5% BSA (Stem Cell Technologies), 0.1 mM 2-mercaptoethanol, and 1.5 mM l-glutamine (ie, “SP34-EX” medium).12 At day 3 of expansion, CD71+Ter119− erythroblasts were isolated by 2 rounds of Lin+ cell depletion (Stem Cell Technologies, as biotinylated antibodies to CD5 [Ly-1], CD45R/B220, CDllb [Mac1], Ter119, and Ly6G [Gr1]). Kit+CD71high erythroblasts were then purified further by CD117 magnetic-activated cell separation (MACS) selection (Miltenyi Biotech, Auburn, CA).
Gene profiling, data analysis, and RT-PCR
Purified Kit+CD71+ cells were cultured for 6 hours in IMDM containing 0.5% BSA, transferrin (10 μg/mL; Sigma), and insulin (15 ng/mL; Invitrogen). Cells then were exposed to Epo (± 5 U/mL) for 90 minutes, and RNA was isolated using Trizol reagent (Invitrogen) and robotic extraction (Autogen Prep245).12 Biotin-cRNA syntheses used 3 μg RNA, and hybridizations were to Affymetrix 430–2.0 arrays (Santa Clara, CA). Signals were processed via GeneChip 3000 scanning and GCOS software (Affymetrix). In data mining, GeneTraffic (Iobion, La Jolla, CA), exploratory visual analysis (EVA), ChipInspector, BiblioSphere Pathway-Edition software (Genomatix, Munich, Germany), and SAM (Significance Analysis of Microarrays) were used.32,33 Reverse transcription RT used TURBO DNase (Ambion, Austin, TX) and Superscript III (Invitrogen). Polymerase chain reaction (PCR) primer pairs (SuperArray Bioscience, Frederick, MD) were as follows: OncoM, NM001013365; Gdf3, NM008108; Podxl, NM013723; Itga4, NM010576; Cxcr4, NM009911; Cis, NM009895; beta-actin, NM007393. Quantitative PCR used iQ SYBR Green and an i-Cycler (BIO-RAD, Hercules, CA).
Flow cytometry
In flow cytometry (BD FACScalibur), 106 cells were incubated at 4°C with 1 μg rat IgG in 0.2 mL PBS, 0.5% BSA (15 minutes), and for 45 minutes with 1 μg of primary antibodies as follows: APC-Ter119 or APC–anti-Kit; phycoerythrin (PE)–anti-CD71 (BD Biosciences); and biotin anti-PODXL (R&D Systems, Minneapolis, MN) (or biotin goat IgG as a negative control). Bound PODXL antibodies were detected using either AlexaFluor-488 or AlexaFluor-647 streptavidin (Molecular Probes, Eugene, OR). FITC–anti-Cxcr4 and PE-CD49d were from BD Biosciences and Southern Biotech (Birmingham, AL). Nucleated erythroblasts were assayed by costaining with PE-Ter119 (BD Biosciences,) and DRAQ5 (10 μM; Alexis Biochemicals, San Diego, CA). Reticulocytes were costained with anti-PODXL and Retic-COUNT. In all experiments, equivalent numbers of gated events were analyzed.
Microscopy
Cytospin analyses (105 cells) involved slide-centrifugation (15 minutes, 10 × g; Hettich Universal-16A cyto-centrifuge, Tuttlingen, Germany) and Dip-Stain reagent staining (Volu-Sol Inc., Salt Lake City, UT). In confocal microscopy (Leica, Heidelberg, Germany; LTCS-SP), staged erythroblasts were isolated, immunostained, washed, fixed in 4% paraformaldehyde, and costained with Hoechst 34580 (Molecular Probes).
Biotin-sialyl-Epo cell lysates and Western blotting
Bioactive biotin-sialyl-Epo was prepared as detailed by Wojchowski and Caslake.32 This reagent was used to assay cell-surface EpoR levels on purified Kit+CD71highTer119− and Kit−CD71highTer119+ erythroblasts as follows. Cells were incubated (at 2°C for 3 hours) with biotin-sialyl-Epo at 10 U/mL in the presence or absence of unlabeled Epo at 300 U/mL. Cells were then washed and lysed as detailed by Menon et al,33 with the exception that Igepal was decreased to 0.3%. Biotin-sialyl-Epo/EpoR complexes were retrieved from cleared lysates using streptavidin CL4B agarose (Pierce, Rockford, IL). EpoR levels were then assayed by Western blotting (Santa Cruz anti-EpoR antibody; Santa Cruz, CA).33
Results
Primary erythroblast system and array-based discovery of Epo-modulated chemokines and cell-surface adhesion/migration factors
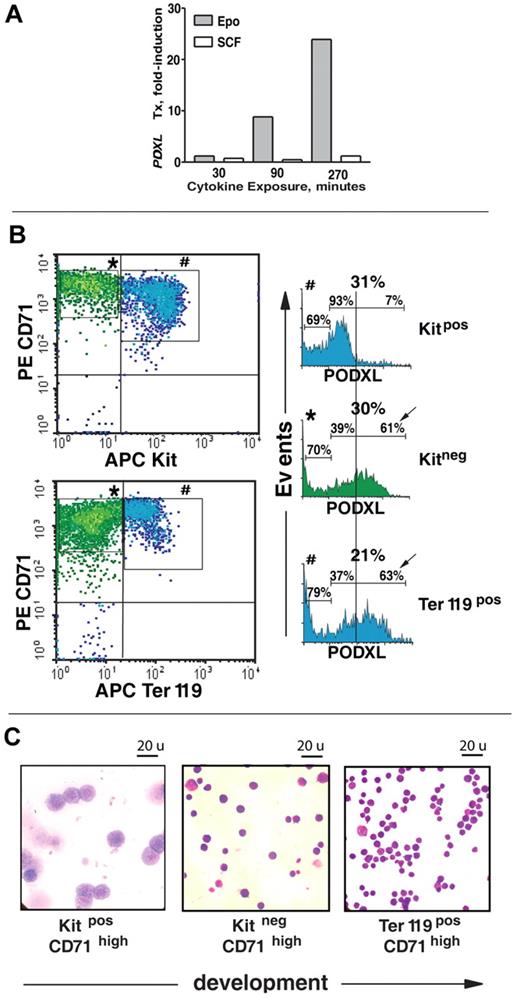
Experiments first investigated possible Epo regulation of novel response genes in murine bone marrow–derived erythroblasts. This primary target population was generated via short-term expansion of erythroid progenitor cells in an optimized serum-free SP34-EX system.12 At day 3.5, Kit+CD71highTer119− erythroblasts were isolated (from n = 4 mice at ≥ 99% purity) via lin+ depletion and Kit+ cell selection (Figure 1A). These staged (and maximally Epo-responsive) erythroblasts were then cultured for 6 hours in the absence of hematopoietic cytokines and stimulated with Epo for 90 minutes. From these and parallel unstimulated cultures, biotin cRNAs and DNAs were prepared. Quantitative PCR also was used to confirm high-level induction of Cis1 (Figure 1B), a known Epo-response gene.34 In Figure 1C, relative differences for microarray outcomes are illustrated. Overall, approximately 200 Epo-response genes were identified with high statistical significance.
Gene array–based discovery of Epo-modulated cytokines and cell-surface adhesion factors in murine bone marrow–derived erythroblasts. (A) Illustrated are steps used to expand and isolate Kit+CD71highTer119− erythroblasts together with a representative flow cytometric profile. (B) For erythroblasts prepared from independent bone marrow preparations (n = 4), hematopoietic cytokines were withdrawn for 6 hours and cells were then exposed to Epo (± 5 U/mL). At 90 minutes, RNA was prepared and used in transcriptome analyses. As a control, levels of Epo-induced Cis1 transcript levels (right panel) were analyzed by quantitative RT-PCR. (C) For Epo-regulated genes, genome-wide outcomes are illustrated by relative difference analyses. Cel files were analyzed using ChipInspector. Significantly changed probes were then defined by SAM analysis (left panel) at a false discovery rate of 0.0% (and corresponding significantly changed transcripts needed to be covered by at least 3 such probes). The SAM analysis method is described by Tusher et al69 and Storey and Tibshirani.70 The false discovery rate is a measure for discrimination of significant features. (D) Affymetrix 430–2.0 array-based analyses of Epo-regulated cytokine and cell-surface adhesion factors. Values are mean fold-modulation by Epo (± a standard error [SE], n = 4). (E) Quantitative RT-PCR analyses of Epo regulation of OncoM, Gdf3, Cxcr4, Itga4, and PODXL. Values are means (± SE) and are normalized to beta-actin. (F) For PODXL, Cxcr4, and Itga4, cell-surface levels were also assayed (by flow cytometry) among Kit+CD71high erythroblasts following their isolation and subsequent 24-hour culture in Epo at 0.1, 0.4, and 1.6 U/mL in the presence or absence of SCF (50 ng/mL).
Gene array–based discovery of Epo-modulated cytokines and cell-surface adhesion factors in murine bone marrow–derived erythroblasts. (A) Illustrated are steps used to expand and isolate Kit+CD71highTer119− erythroblasts together with a representative flow cytometric profile. (B) For erythroblasts prepared from independent bone marrow preparations (n = 4), hematopoietic cytokines were withdrawn for 6 hours and cells were then exposed to Epo (± 5 U/mL). At 90 minutes, RNA was prepared and used in transcriptome analyses. As a control, levels of Epo-induced Cis1 transcript levels (right panel) were analyzed by quantitative RT-PCR. (C) For Epo-regulated genes, genome-wide outcomes are illustrated by relative difference analyses. Cel files were analyzed using ChipInspector. Significantly changed probes were then defined by SAM analysis (left panel) at a false discovery rate of 0.0% (and corresponding significantly changed transcripts needed to be covered by at least 3 such probes). The SAM analysis method is described by Tusher et al69 and Storey and Tibshirani.70 The false discovery rate is a measure for discrimination of significant features. (D) Affymetrix 430–2.0 array-based analyses of Epo-regulated cytokine and cell-surface adhesion factors. Values are mean fold-modulation by Epo (± a standard error [SE], n = 4). (E) Quantitative RT-PCR analyses of Epo regulation of OncoM, Gdf3, Cxcr4, Itga4, and PODXL. Values are means (± SE) and are normalized to beta-actin. (F) For PODXL, Cxcr4, and Itga4, cell-surface levels were also assayed (by flow cytometry) among Kit+CD71high erythroblasts following their isolation and subsequent 24-hour culture in Epo at 0.1, 0.4, and 1.6 U/mL in the presence or absence of SCF (50 ng/mL).
For Affymetrix 430–2.0 array-profiling outcomes, a focus was narrowed to transcripts that were modulated 2-fold or more by Epo and in addition corresponded to chemokine and adhesion/migration factors. This selectively included 2 secreted cytokines, Gdf313 and OncoM,14 together with Cxcr4,15 Itga4,16,17 and PODXL18 (Figure 1D). Profiling data specifically indicated 10.8- and 3.1-fold induction of OncoM and Gdf3; 1.3- and 2.2-fold down-modulation of Itga4 and Cxcr4; and 15.3-fold induction of PODXL. Follow-up quantitative RT-PCR analyses confirmed 16.3- and 2.2-fold induction of OncoM and Gdf3, respectively, 2.3- and 2.9-fold down-modulation of Itga4 and Cxcr4, respectively, and 8.9-fold induction of PODXL (Figure 1E). In Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article), several additional functional subsets of Epo-response genes are outlined to further illustrate the robust nature of transcriptome profiling and the utility of this approach.
Gdf3 is a TGF-beta antagonist that inhibits classical TGF-beta and BMP signaling13 and is expressed predominantly in bone marrow, spleen, thymus, and adipocytes.13 Its expression by erythroid progenitor cells has not previously been described. Onco-M is a pleiotropic cytokine,14 but disruption of its receptor selectively decreases erythro-megakaryocytic potentials.19 Onco-M and Gdf3 therefore are implicated as uniquely activated chemokine components of an Epo signaling axis. Cxcr4 and Itga4 were each rapidly down-modulated several-fold by Epo (Figure 1D-E). Cxcr4 is a 7-transmembrane receptor for stromal derived factor-1 (SDF-1) and can support niche homing by several stem and progenitor cell types.15,35 Within bone marrow, SDF-1 can remain further associated with stromal cell surfaces and recruit Cxcr4+ cells.15 Itga4 preferentially binds VCAM1 and fibronectin (and is also important for early hematopoietic progenitor cell migration and development).17 For both Cxcr4 and Itga4, Epo-dependent down-modulation in Kit+CD71high cells therefore suggests potential roles in promoting erythroblast transit from stromal cell compartments (as does the ensuing down-modulation of Kit per se in Kit−CD71high erythroblasts; see Figure 7D).
The CD34-related sialomucin PODXL, in contrast, was strongly up-modulated by Epo in Kit+CD71high erythroblasts (Figure 1D-E). PODXL is best known to be expressed by renal podocytes and to support filtration slit formation.18 However, PODXL also marks developing vascular endothelial cells and hematopoietic stem cells.18,26 For PODXL, Cxcr4, and Itga4, modulation at the erythroblast cell surface was further examined by flow-cytometric assays. In keeping with transcript analyses, Epo up-modulated PODXL and down-modulated Cxcr4 with dose dependency (Figure 1F). Cell surface levels of Itga4, however, were not significantly affected by Epo over a 24-hour time frame. For Itga4, this might reflect a long half-life (or possibly the opposing actions of factors that promote Itga4 expression).
Epo-specific induction of PODXL in developing erythroblasts, in part via an EpoR/PY343/Stat5 signaling axis
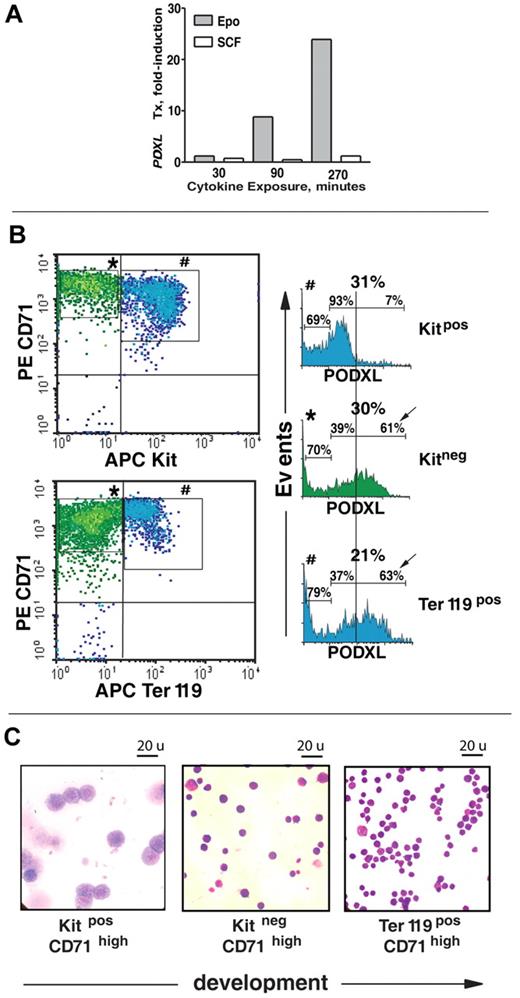
The extent to which PODXL's strong up-modulation might be affected specifically by Epo was next assessed. Kit+CD71high cells were isolated, cultured in the absence of hematopoietic growth factors, and stimulated with either Epo or SCF. Time-course analyses revealed an approximate 25-fold induction of PODXL by Epo (Figure 2A). No such increase was affected by SCF. Epo's ability to modulate PODXL expression within a staged series of erythroblasts also was examined. This included Kit+CD71high, Kit−CD71high, and Kit−CD71highTer119+ cells (see Figure 2B,C and Table 1 for flow cytometry and cytospin characterizations; larger-scale images of stained cytospins also are provided in Figure S2). In Kit−CD71high cells, PODXL expression increased markedly and was sustained in late-stage Ter119+CD71high erythroblasts (Figure 2B; Table 1).
Epo-specific PODXL induction in developmentally staged marrow erythroblasts. (A) In MACS-purified Kit+CD71high erythroblasts, time courses of EPO and SCF induction of PODXL expression (after cytokine withdrawal) were assessed by quantitative RT-PCR. (B) In SP34-EX expansion cultures, cell-surface PODXL expression among Kit+CD71high, Kit−CD71high, and Kit−CD71highTer119+ erythroblasts was analyzed by flow cytometry. Frequencies of PODXL+ cells are indicated for bisected PODXLlow and PODXLhigh subpopulations. (C) For the above stages of maturing erythroblasts, cytospin morphologies for MACS and/or FACS-purified populations also are shown. (Images were seen with a Zeiss (Oberkochen, Germany) model Axioskop 2 microscope with a 100 ×/1.3 oil lens, Immersol 518 (Zeiss) imaging solution, and Volu-sol (Volu-Sol Inc.) dip stain solution. Images were photographed with a Spotflex (Diagnostic Instruments Inc., Sterling Heights, MI) model 15.2 camera and processed with Spot advance (Diagnostic Instruments Inc.) version 4.6 and Photoshop (Adobe, San Jose, CA) version CS2 software.
Epo-specific PODXL induction in developmentally staged marrow erythroblasts. (A) In MACS-purified Kit+CD71high erythroblasts, time courses of EPO and SCF induction of PODXL expression (after cytokine withdrawal) were assessed by quantitative RT-PCR. (B) In SP34-EX expansion cultures, cell-surface PODXL expression among Kit+CD71high, Kit−CD71high, and Kit−CD71highTer119+ erythroblasts was analyzed by flow cytometry. Frequencies of PODXL+ cells are indicated for bisected PODXLlow and PODXLhigh subpopulations. (C) For the above stages of maturing erythroblasts, cytospin morphologies for MACS and/or FACS-purified populations also are shown. (Images were seen with a Zeiss (Oberkochen, Germany) model Axioskop 2 microscope with a 100 ×/1.3 oil lens, Immersol 518 (Zeiss) imaging solution, and Volu-sol (Volu-Sol Inc.) dip stain solution. Images were photographed with a Spotflex (Diagnostic Instruments Inc., Sterling Heights, MI) model 15.2 camera and processed with Spot advance (Diagnostic Instruments Inc.) version 4.6 and Photoshop (Adobe, San Jose, CA) version CS2 software.
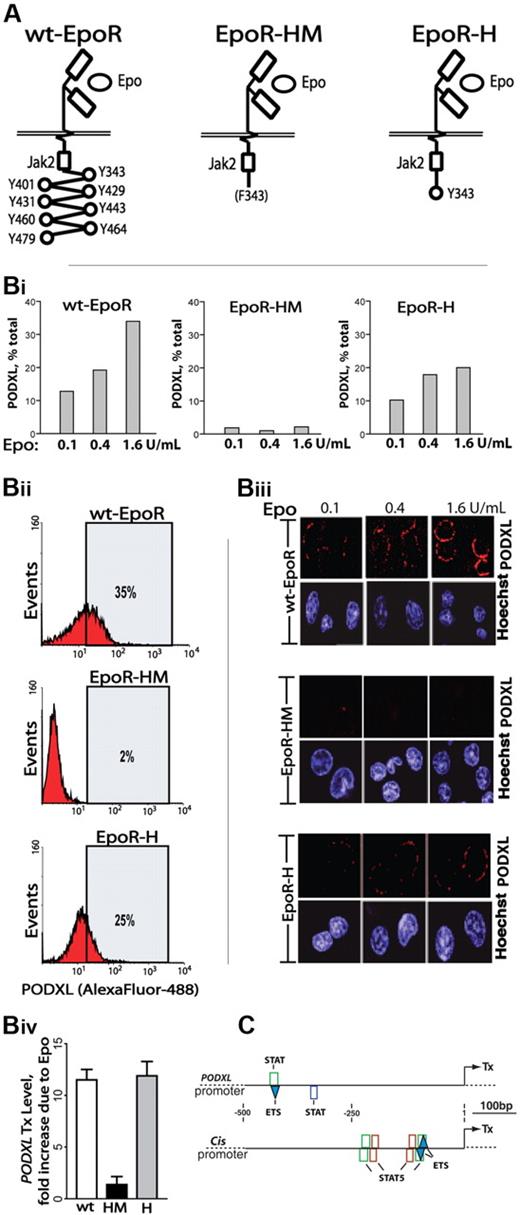
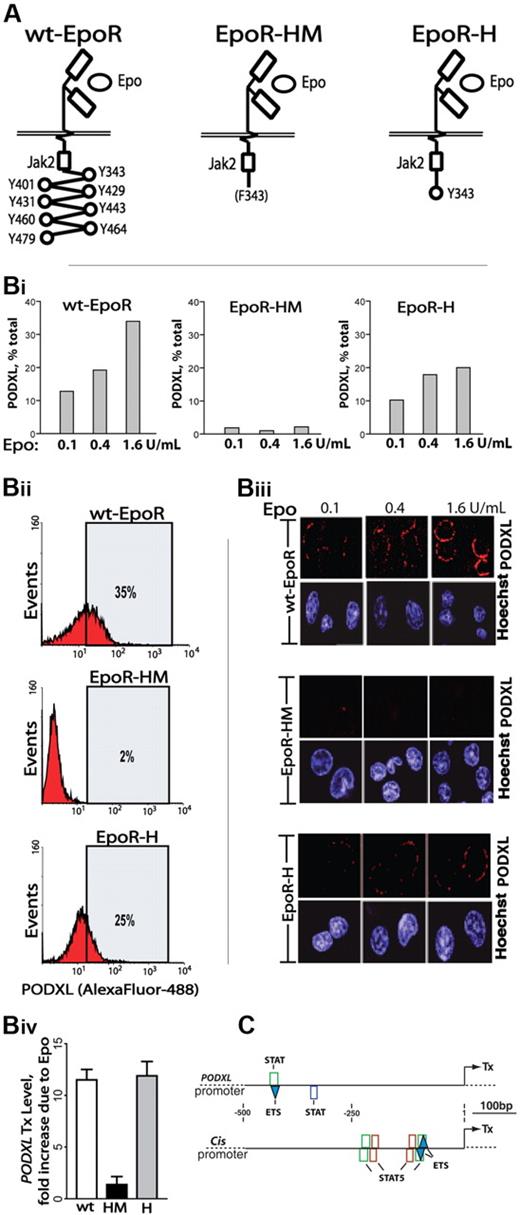
To more mechanistically consider Epo-regulated PODXL expression, induction via knocked-in EpoR-HM and EpoR-H alleles was studied. EpoR-HM retains a membrane proximal box-1 motif, activates JAK2, couples to MEK1/2 and ERK1/2,33 but otherwise lacks cytoplasmic PY (phosphotyrosine) signal transduction factor docking sites. EpoR-H is related but possesses a selectively restored PY343 Stat5 binding site (Figure 3A).12 Erythroid cells were expanded from wild-type EpoR (wt-EpoR), EpoR-HM, and EpoR-H marrow preparations. Kit+CD71high erythroblasts were then isolated and analyzed for PODXL expression at the transcript and cell-surface levels (Figure 3B). Each analysis revealed deficient expression in EpoR-HM erythroblasts (PY-null allele) together with a substantial rescue of PODXL expression upon PY343 site restoration in EpoR-H erythroblasts.
Epo-induced PODXL expression depends on EpoR/PY343/Stat5 signaling. (A) wt-EpoR and minimal knocked-in EpoR-HM and EpoR-H alleles are diagrammed. (B) EpoR-HM fails to support efficient Epo-induced PODXL expression; Kit+CD71high erythroblasts from wild-type, EpoR-HM, and EpoR-H marrow were expanded. At day 2.5, expansion cultures were shifted to SP34-EX medium lacking SCF and containing Epo at 0.1, 0.4, and 1.6 U/mL. At day 3.5, lin+-depleted cultures were analyzed for PODXL expression by flow cytometry (i-ii) and confocal microscopy (iii) (Images were seen with a Leica model TCS-SP confocal microscope with a 63 ×/1.32-0.6 oil lens, Vectashield (Vector, Burlingame, CA) imaging solution, and Streptavidin, Alexafluor 647, and Hoexchst 35480 (Molecular Probes, Carlsbad, CA) stains. Images were acquired using Leica TCS-NT version 1.6 and Photoshop (Adobe) version CS2 software.). Also graphed for wt-EpoR, EpoR-HM, and EpoR-H erythroblasts is the fold-induction of PODXL due to Epo (1.6 U/mL; iv). (C) In silico analyses of predicted STAT elements and STAT/ETS modules in murine PODXL and Cis1 promoters. The occurrences of consensus elements were predicted using Genomatix ChipInspector software.
Epo-induced PODXL expression depends on EpoR/PY343/Stat5 signaling. (A) wt-EpoR and minimal knocked-in EpoR-HM and EpoR-H alleles are diagrammed. (B) EpoR-HM fails to support efficient Epo-induced PODXL expression; Kit+CD71high erythroblasts from wild-type, EpoR-HM, and EpoR-H marrow were expanded. At day 2.5, expansion cultures were shifted to SP34-EX medium lacking SCF and containing Epo at 0.1, 0.4, and 1.6 U/mL. At day 3.5, lin+-depleted cultures were analyzed for PODXL expression by flow cytometry (i-ii) and confocal microscopy (iii) (Images were seen with a Leica model TCS-SP confocal microscope with a 63 ×/1.32-0.6 oil lens, Vectashield (Vector, Burlingame, CA) imaging solution, and Streptavidin, Alexafluor 647, and Hoexchst 35480 (Molecular Probes, Carlsbad, CA) stains. Images were acquired using Leica TCS-NT version 1.6 and Photoshop (Adobe) version CS2 software.). Also graphed for wt-EpoR, EpoR-HM, and EpoR-H erythroblasts is the fold-induction of PODXL due to Epo (1.6 U/mL; iv). (C) In silico analyses of predicted STAT elements and STAT/ETS modules in murine PODXL and Cis1 promoters. The occurrences of consensus elements were predicted using Genomatix ChipInspector software.
Epo dosing effects on PODXL expression were also examined. Primary erythroblasts from wt-EpoR, EpoR-HM, and EpoR-H were isolated and expanded. At day 2.5, cells were transferred to SP34-EX medium with Epo at 0.1, 0.4, or 1.6 U/mL (without SCF). After 24 hours, lin+-depleted cell populations were analyzed. In wt-EpoR cells, PODXL expression in low-level Epo (0.1 U/mL) was visible via confocal microscopy and in flow cytometry was detected on approximately 12% of erythroblasts (Figure 3Bi,iii). Higher-dose Epo (1.6 U/mL) boosted frequencies of positive erythroblasts (to ∼35%; Figure 3Bii) as well as PODXL cell-surface densities. In contrast, in EpoR-HM cells, little PODXL was detectable. For EpoR-H cells, confocal images and flow cytometry revealed a substantial (yet partial) restoration of PODXL expression. These experiments, together with PODXL transcript analyses (Figure 3Biv), indicate that EpoR PY343 (and Stat5) signals are important for Epo regulation of PODXL, but that expression is enhanced by EpoR C-terminal signals. Finally, in silico analyses indicated the occurrence of 2 consensus STAT elements within the PODXL proximal promoter (Figure 3C); 1 further occurred within a STAT/ETS module, which was also represented within the Cis1 gene promoter.
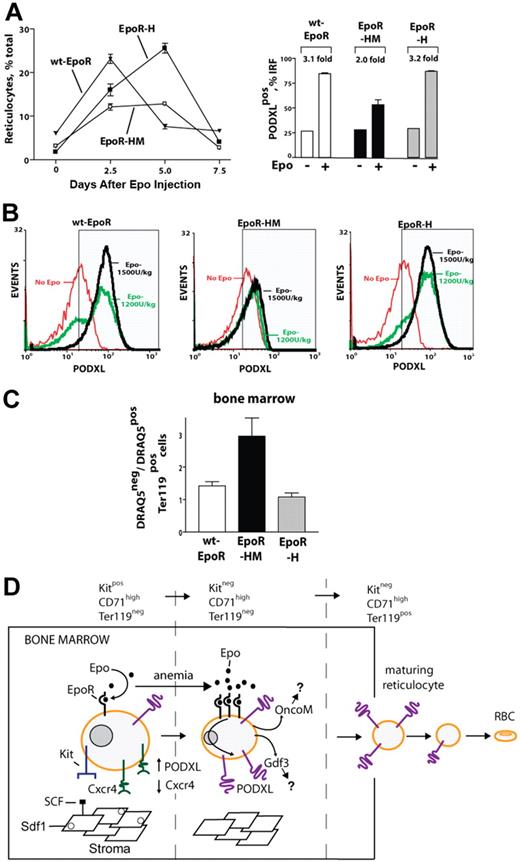
Epo dose–dependent PODXL expression in vivo is sustained among immature circulating and marrow-resident reticulocytes and rapidly induced at R5 and R4 stages
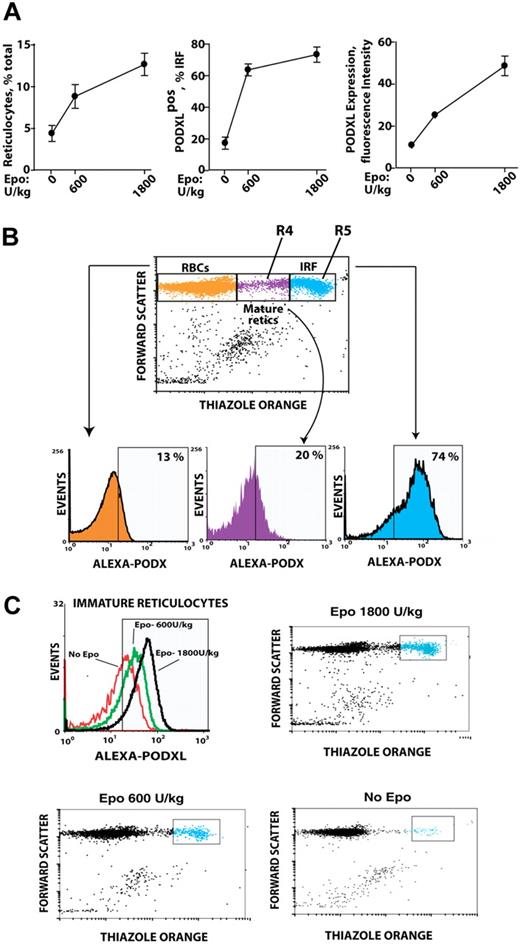
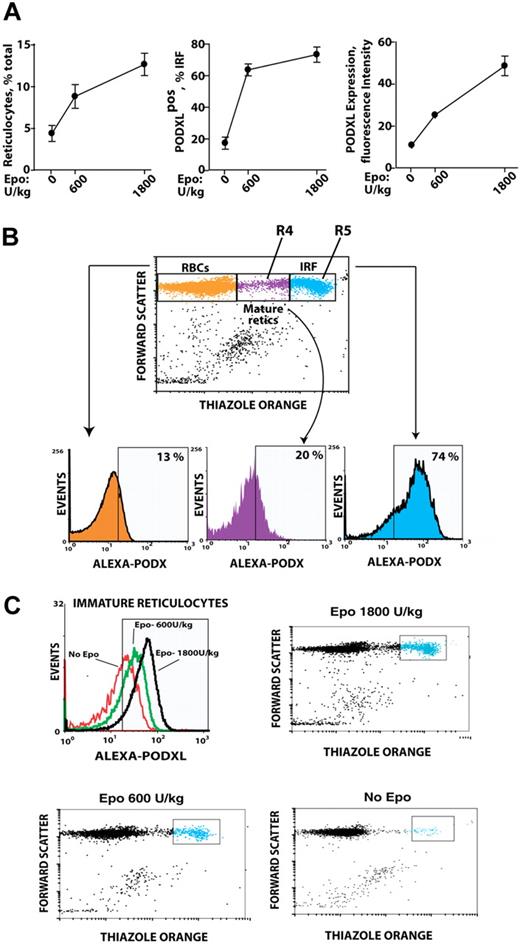
Based on Epo induction of PODXL by late-stage erythroblasts ex vivo, whether PODXL expression might persist among reticulocytes in vivo was next tested. Specifically, an Epo dose–response relationship for reticulocyte production was first defined, and peripheral red cells from Epo-injected mice were analyzed for PODXL expression (Figure 4A). In Epo-injected mice, a low percentage of red blood cells (RBCs; ∼13%) stained as PODXL+; 20% of mature reticulocytes (R4 stage) were PODXL+ and stained up to 5-fold brighter; and 74% of immature reticulocytes (R5 stage) were PODXL+ and stained at high intensity (immature reticulocyte fraction [IRF]; Figure 4B). Within the IRF, PODXL densities interestingly also increased in a sharply Epo dose–dependent fashion (Figure 4C).
In vivo Epo dose–dependent expression of PODXL by immature reticulocytes. (A) Epo dose–dependent increases in reticulocytes, PODXL+ immature reticulocytes, and PODXL expression levels in wild-type mice. At day 3 after Epo injection (0, 600, 1800 U/kg), increases in peripheral blood reticulocytes and in PODXL+ immature reticulocytes (immature reticulocyte fraction; IRF) were assayed by flow cytometry (left and center panels). Average PODXL expression levels within the IRF also were assayed based on fluorescence intensity (right panel). (B) PODXL staining intensities specifically within the IRF compartment and among stage mature R4 and immature R5 reticulocytes are illustrated. Here, wild-type mice were injected with Epo (1200 U/kg). For peripheral blood sampled at day 3, frequencies of PODXL+ cells within reticulocyte compartments were then determined. (C) Epo dose–dependent increases in PODXL+ expression levels (cell surface densities) by R5-stage reticulocytes also are illustrated.
In vivo Epo dose–dependent expression of PODXL by immature reticulocytes. (A) Epo dose–dependent increases in reticulocytes, PODXL+ immature reticulocytes, and PODXL expression levels in wild-type mice. At day 3 after Epo injection (0, 600, 1800 U/kg), increases in peripheral blood reticulocytes and in PODXL+ immature reticulocytes (immature reticulocyte fraction; IRF) were assayed by flow cytometry (left and center panels). Average PODXL expression levels within the IRF also were assayed based on fluorescence intensity (right panel). (B) PODXL staining intensities specifically within the IRF compartment and among stage mature R4 and immature R5 reticulocytes are illustrated. Here, wild-type mice were injected with Epo (1200 U/kg). For peripheral blood sampled at day 3, frequencies of PODXL+ cells within reticulocyte compartments were then determined. (C) Epo dose–dependent increases in PODXL+ expression levels (cell surface densities) by R5-stage reticulocytes also are illustrated.
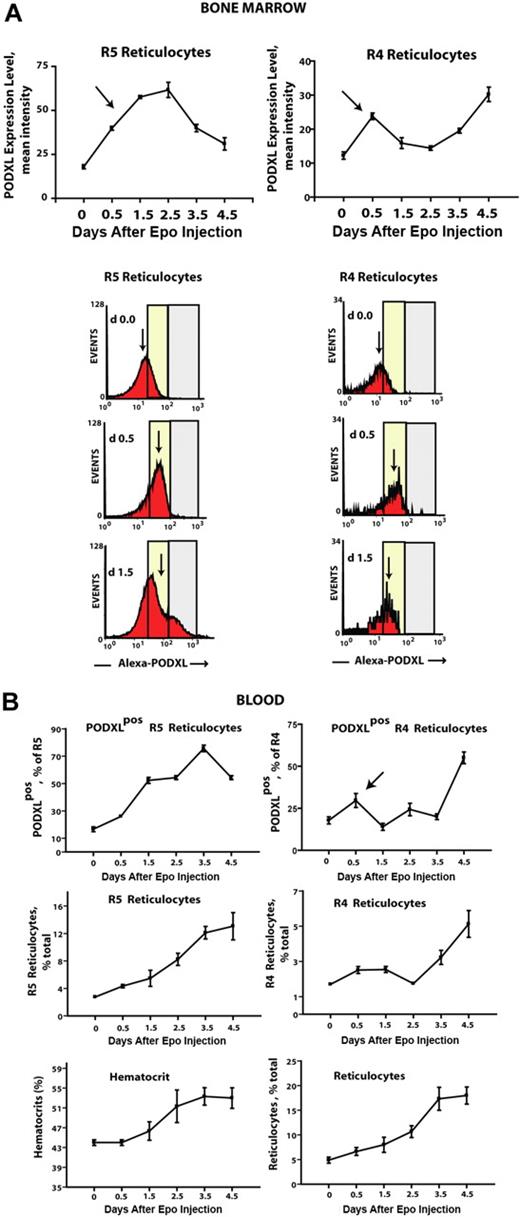
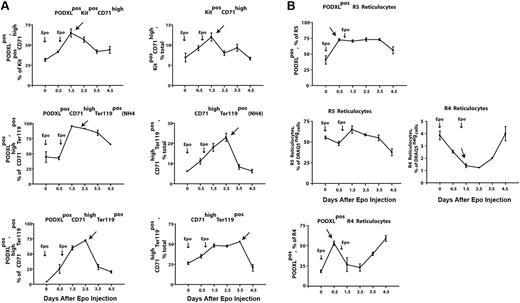
In vivo findings for PODXL+ expression among circulating reticulocytes prompted analyses of Epo-induced PODXL-positive erythroid progenitor cell production in marrow. Here, Epo was observed to stimulate sequential increases in the production of PODXL+Kit+CD71high proerythroblasts, NH4Cl-resistant early stage PODXL+CD71highTer119+ erythroblasts, and maturing PODXL+CD71highTer119+ erythroblasts (Figure 5A top left panels). For these PODXL+ cohorts, peak representation occurred sequentially at days 1.5, 1.5 to 2.5, and 2.5, respectively. These analyses therefore establish these (pro)erythroblast populations as in vivo targets for Epo-modulation of PODXL. Overall levels of total Kit+CD71high, NH4Cl-resistant CD71highTer119+, and total CD71highTer119+ erythroblasts also were analyzed (Figure 5B top right panels). For total populations of R4 reticulocytes (Figure 5B bottom right panel), overall increases in frequencies after day 2.5 were consistent with a wave of Epo-induced erythroblast production (and PODXL expression), as were parallel decreases in early stage R5 reticulocyte pools (Figure 5B bottom left panel). Based on a transient pulse of PODXL+ reticulocytes at day 0.5 (Figure 5B top panels), frequencies of Epo-induced PODXL+ subpopulations of R5 and R4 reticulocytes, however, suggested a perhaps less-straightforward relationship. This Epo response is not well understood but could reflect rapid effects on the release of a late-stage erythroblast pool and/or possible Epo modulation of PODXL among late-stage cells. This prospect that Epo might modulate PODXL expression at late stages prompted analyses of EpoR expression levels in Ter119+ erythroblasts. This was accomplished by using biotin-sialyl-Epo to retrieve cell-surface EpoRs from purified Kit+CD71highTer119− and Kit−CD71highTer119+ cells. Ter119+ cells, in fact, were proven to express EpoR at levels comparable with Kit+ proerythroblasts (Figure S3).
Epo rapidly induces the formation of PODXL+(pro)erythroblasts and reticulocytes within bone marrow. (A) Epo induction of the sequential formation of PODXL+ bone marrow Kit+CD71high and CD71highTer119+ erythroblasts. Wild-type mice were injected with Epo (1500 U/kg) at 1 and 24 hours. At days 0.5, 1.5, 2.5, 3.5, and 4.5, levels of marrow resident PODXL+Kit+CD71high (pro)erythroblasts, NH4CI-resistant PODXL+CD71highTer119+ erythroblasts, and PODXL+CD71highTer119+ erythroblasts were determined (left panels). For these populations, note the sequential waves of Epo-induced PODXL+ erythroblast formation (arrows). Total levels of these erythroblast cohorts also were determined (right panels). (B) Epo-induced formation of marrow-resident R5- and R4-PODXL+ reticulocytes (top panels). For R4 reticulocytes, also note the rapid Epo-induced decrease of this cohort within marrow (arrow, bottom right panel).
Epo rapidly induces the formation of PODXL+(pro)erythroblasts and reticulocytes within bone marrow. (A) Epo induction of the sequential formation of PODXL+ bone marrow Kit+CD71high and CD71highTer119+ erythroblasts. Wild-type mice were injected with Epo (1500 U/kg) at 1 and 24 hours. At days 0.5, 1.5, 2.5, 3.5, and 4.5, levels of marrow resident PODXL+Kit+CD71high (pro)erythroblasts, NH4CI-resistant PODXL+CD71highTer119+ erythroblasts, and PODXL+CD71highTer119+ erythroblasts were determined (left panels). For these populations, note the sequential waves of Epo-induced PODXL+ erythroblast formation (arrows). Total levels of these erythroblast cohorts also were determined (right panels). (B) Epo-induced formation of marrow-resident R5- and R4-PODXL+ reticulocytes (top panels). For R4 reticulocytes, also note the rapid Epo-induced decrease of this cohort within marrow (arrow, bottom right panel).
Using a combination of Draq5 staining and light scatter properties (in parallel with thiazole orange), it was also possible to assay the formation of bone marrow–resident early R5– and late R4–stage reticulocytes. In this compartment, 2 unexpected Epo-induced events were observed. First, in both R5 and R4 populations, frequencies of PODXL+ reticulocytes increased several-fold within 0.5 days of Epo exposure (Figure 5B top panels). (For details on flow cytometry and gating of reticulocytes, also see Figure S4.) Second, this was paralleled by an apparent decrease in overall numbers of marrow-resident R4 reticulocytes. This latter event is consistent with rapid Epo-dependent (and possibly PODXL-dependent) effects on R4 reticulocyte release to blood. Epo's apparent ability to modulate PODXL expression in marrow reticulocytes was analyzed further based on levels of Epo-induced expression (as assayed via relative fluorescence intensities of PODXL staining; Figure 6A). In R5 reticulocytes, and to a lesser yet significant extent in R4 reticulocytes, surface cell levels of PODXL expression were up-modulated several-fold by Epo within 0.5 to 1.5 days. In parallel analyses of blood, frequencies of circulating PODXL+ R5 and R4 reticulocytes increased overall by approximately 6-fold and approximately 4-fold, respectively (Figure 6B), and this included a rapid pulse in PODXL+ R4 reticulocyte levels at day 0.5 (top right panel, arrow).
Epo rapidly increases cell-surface densities of PODXL expression in R5 and R4 bone marrow reticulocytes. (A) In Epo-injected wild-type mice, cell-surface levels (ie, densities) of PODXL expression were assayed among bone marrow–resident R5 and R4 reticulocytes. Values (relative fluorescent intensities) are means (± SE) for n = 3 independent mice. Arrows indicate up-modulation of PODXL expression by Epo within 0.5-1.5 days. Bottom panels illustrate representative flow cytometry profiles at days 0, 0.5, and 1.5. (B) R5 and R4 reticulocytes in peripheral blood also were assayed in Epo-treated mice, including total and PODXL+ reticulocytes populations. For R4 reticulocytes, note the rapid pulse of PODXL positivity at day 0.5 (top right panel, arrow). Error bars represent SEM for n = 3 mice.
Epo rapidly increases cell-surface densities of PODXL expression in R5 and R4 bone marrow reticulocytes. (A) In Epo-injected wild-type mice, cell-surface levels (ie, densities) of PODXL expression were assayed among bone marrow–resident R5 and R4 reticulocytes. Values (relative fluorescent intensities) are means (± SE) for n = 3 independent mice. Arrows indicate up-modulation of PODXL expression by Epo within 0.5-1.5 days. Bottom panels illustrate representative flow cytometry profiles at days 0, 0.5, and 1.5. (B) R5 and R4 reticulocytes in peripheral blood also were assayed in Epo-treated mice, including total and PODXL+ reticulocytes populations. For R4 reticulocytes, note the rapid pulse of PODXL positivity at day 0.5 (top right panel, arrow). Error bars represent SEM for n = 3 mice.
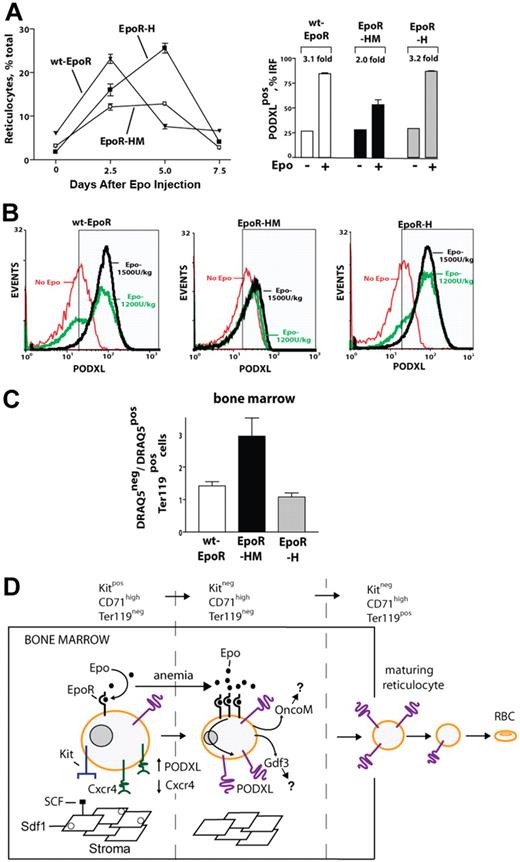
EpoR-HM mice exhibit deficient Epo-induced PODXL expression and circulating reticulocyte production, and accumulate mature erythroblasts within the bone marrow compartment
Finally, Epo-induced PODXL+ erythroid cell formation was examined in wt-EpoR, EpoR-HM, and EpoR-H mice. As described by Zang et al,31 EpoR-HM mice possess approximately wild-type erythroid burst-forming unit (BFUe) and erythroid colony-forming unit (CFUe) levels and generate a near-normal red cell mass at steady-state. As described by Menon et al,12 however, this EpoR allele selectively fails to support efficient stress erythropoiesis. Therefore, during Epo-induced reticulocyte formation, possible in vivo correlations with diminished PODXL expression levels in EpoR-HM mice were sought. Interestingly, EpoR-HM mice failed to generate normal levels of circulating reticulocytes, even at high Epo doses (1800 U/kg), and this paralleled a deficient representation of PODXL+ reticulocytes within an IRF compartment (Figure 7A). Furthermore, EpoR-H mice exhibited clear Epo dose–dependent PODXL expression in the IRF, whereas in EpoR-HM mice the percentage of PODXL-expressing immature reticulocytes remained largely unchanged in response to increasing Epo doses (1200 U/kg and 1500 U/kg; Figure 7B). Beyond this, when frequencies of nucleated marrow-resident red cells were analyzed (via Draq5 and Ter119 costaining), abnormally elevated levels were observed in EpoR-HM marrow (Figure 7C). This latter finding is consistent with an aberrant retention of late-stage red cells and correlates well with deficiencies in PODXL expression.
Deficient EpoR-HM reticulocyte production in response to Epo, and abnormal representation of anucleated red cells in EpoR-HM marrow. (A) For wt-EpoR, EpoR-HM, and EpoR-H mice, time courses of Epo induced in vivo reticulocyte production are graphed (left panel; means ± SE, n = 5 per group, 1200 U/kg). Frequencies of PODXL+ immature reticulocytes (IRF; day 3 after Epo) also are illustrated (right panel). (B) Wild-type (wt-EpoR), EpoR-HM, and EpoR-H mice were treated with Epo at 0, 1200, and 1800 U/kg. At day 3.5, levels of PODXL expression among immature reticulocytes were determined. (C) In bone marrow of wt-EpoR, EpoR-HM, and EpoR-H mice (at day 3 after Epo injection, 1500 U/kg), relative frequencies (ratios) of anucleated versus nucleated Ter119+ cells were determined based on DRAQ5 staining of Ter119+ cells. Error bars indicate the standard error from the mean value for n = 5 mice examined per group. (D) Model for Epo regulation of erythroid progenitor cell adhesion and migration within a proposed stromal niche. Epo's actions on Kit+CD71high proerythroblasts are depicted to involve an Epo dose–dependent repression of Cxcr4 expression and an induction of PODXL. This Epo response is sustained as progenitors advance to a Kit−CD71high erythroblast stage and exit a proposed stromal niche. Epo-dependent PODXL expression further persists among immature reticulocytes and is hypothesized to enhance their release to blood.
Deficient EpoR-HM reticulocyte production in response to Epo, and abnormal representation of anucleated red cells in EpoR-HM marrow. (A) For wt-EpoR, EpoR-HM, and EpoR-H mice, time courses of Epo induced in vivo reticulocyte production are graphed (left panel; means ± SE, n = 5 per group, 1200 U/kg). Frequencies of PODXL+ immature reticulocytes (IRF; day 3 after Epo) also are illustrated (right panel). (B) Wild-type (wt-EpoR), EpoR-HM, and EpoR-H mice were treated with Epo at 0, 1200, and 1800 U/kg. At day 3.5, levels of PODXL expression among immature reticulocytes were determined. (C) In bone marrow of wt-EpoR, EpoR-HM, and EpoR-H mice (at day 3 after Epo injection, 1500 U/kg), relative frequencies (ratios) of anucleated versus nucleated Ter119+ cells were determined based on DRAQ5 staining of Ter119+ cells. Error bars indicate the standard error from the mean value for n = 5 mice examined per group. (D) Model for Epo regulation of erythroid progenitor cell adhesion and migration within a proposed stromal niche. Epo's actions on Kit+CD71high proerythroblasts are depicted to involve an Epo dose–dependent repression of Cxcr4 expression and an induction of PODXL. This Epo response is sustained as progenitors advance to a Kit−CD71high erythroblast stage and exit a proposed stromal niche. Epo-dependent PODXL expression further persists among immature reticulocytes and is hypothesized to enhance their release to blood.
Discussion
The present investigations reveal that Epo's effects on bone marrow erythroblasts include a rapid and substantial modulation of a novel set of cell migration and/or adhesion factors. In part, the discovery of these Epo-modulated factors was accomplished through the use of a unique ex vivo system for bone marrow–derived erythroblast development. In profiling experiments, a Kit+CD71high population was specifically used based on analyses that demonstrated maximum Epo responsiveness for this (pro)erythroblast cohort. In this regard, and based on the colony-forming properties of this progenitor pool,36 these Kit+CD71high erythroblasts correspond most closely to CFUe's. In this system, the ability of progenitor cells to develop to Kit−CD71highTer119− and Kit−CD71highTer119+ erythroblast stages further enabled developmental analyses.
In Kit+CD71high erythroblasts, profiling selectively identified 5 novel Epo-response factors that may affect progenitor cell migration; 2 are Epo-modulated cytokines, Onco-M and Gdf3. For Onco-M, this pleiotropic cytokine is known to induce bFGF-independent endothelial cell migration,37,38 as well as leukocyte migration across endothelial cell monolayers.39 In Kit+CD71high erythroblasts, OncoM was notably among the most highly induced Epo-response genes. Disruption of the Onco-M receptor-beta (OSMR) leads to specific defects in erythropoiesis, including decreased BFUe's and CFUe's and mild anemia.19 Reconstitution studies also suggest possible stromal cell effects, in that transplantation of control hematopoietic cells into an OSMR−/− background yields decreased erythroid cell pools.19 Therefore, as secreted by Epo-stimulated erythroid progenitors, Onco-M might also modulate stromal components (or possibly later-stage erythroid blood islands). Due to its potential multiple effects on diverse target cells, however, the nature of Onco-M's candidate effects on erythroblast migration and/or development are presently speculative. For growth/differentiation factor 3 (Gdf3), this TGF-beta and BMP antagonist has not previously been associated with erythropoiesis or Epo's actions. TGF-beta, however, has been shown to modulate human erythroblast proliferation and promote differentiation.40 Therefore, Gdf3 may dampen this TGF-beta effect. In addition, BMP4 appears to be selectively important for stress splenic erythropoiesis41 and therefore Gdf3 may limit BMP4-dependent proerythroblast pools within this distinct niche. As illustrated by red cell–derived TGFbeta-1 inhibition of neutrophil chemotaxis,42 TGF-beta ligands can also affect cell migration, and therefore Epo-induced Gdf3 might more directly modulate developing erythroblasts.
In Kit+CD71high erythroblasts, 2 cell-surface adhesion and/or migration factors, Cxcr4 and Itga4, were rapidly down-modulated by Epo. Cxcr4 is a 7-transmembrane receptor for SDF-1 and is known to facilitate homing for hematopoietic, neural, smooth muscle, and mesenchymal progenitors.35,43-45 Furthermore, studies of bone marrow compartments have shown that SDF-1 can remain associated with stromal cell surfaces and act to recruit Cxcr4+ cells.46 For early stage erythroid cells, Cxcr4 may therefore support niche homing and adhesion. Epo-mediated down-modulation of Cxcr4 is then predicted to facilitate erythroblast release from a stromal niche. Notably, this appears to occur in parallel with an observed rapid loss of Kit expression, which is similarly predicted to promote release from SCF-positive stromal cells.47-49 During the preparation of this manuscript, Kim et al50 interestingly reported that GCSF-induced mobilization of myeloid Gr1+ progenitors may similarly involve an active repression of Cxcr4 expression.50 In Kit+CD71high erythroblasts, Epo also down-modulated the expression of integrin alpha 4 (Itga4/VLA4/cd49d). As expressed in multiple hematopoietic lineages, Itga4 heterodimerizes with beta-1 integrin and preferentially binds fibronectin, VCAM-1, and paxillin. Via these routes, Itga4 can regulate hematopoietic progenitor cell adhesion to stroma,51 and Itga4 deletion in hematopoietic tissues mobilizes hematopoietic progenitor cells.16 The presently indicated Epo down-modulation of Itga4 therefore might also contribute to erythroblast release from a stromal niche.
In contrast to Cxcr4 and Itga4, PODXL expression was sharply up-modulated by Epo. To our knowledge, connections between Epo and PODXL have not previously been reported (nor has cytokine regulation of PODXL). Analyses also indicated specificity, in that Epo, but not SCF, rapidly up-modulated PODXL up to 25-fold in Kit+CD71high marrow-derived erythroblasts. Investigations using erythroblasts with knocked-in EpoR-H or EpoR-HM alleles further indicated an important role for PY343-dependent signals for PODXL induction. Within the EpoR, PY343 previously has been shown to specifically mediate Stat5 binding and activation.52,53 PODXL induction by Epo therefore appears to require EpoR/PY343/Stat5 signals. Enhanced PODXL expression via the full-length EpoR, however, indicates that additional circuits to PODXL gene activation exist. Previously, PODXL gene expression has been shown in embryonic kidney cells to be stimulated by Wilms tumor-1 via a conserved −1615 element54 and to be repressed by p53.55 Otherwise, PODXL gene expression is not well studied, and Stat5 has not previously been implicated as a PODXL regulator. Correlations between elevated Stat5 activity and increased PODXL expression, however, do exist in the contexts of blood, breast, and prostate cancers.56-64 Among leukemias, abnormally elevated PODXL expression has been described in blasts from 77% of acute myelogenous leukemia (AML) patients with t(8;21)(q22;q22), inv(16)(p13q22), or t(15;17)(q22;q21) events and in 81% of blasts from acute lymphoblastic leukemia (ALL) patients with t(9;22)(q34;q11.2) translocations.62 In AML and ALL, evidence also exists to indicate that chronic Stat5 activation is of causative importance.56,57 Direct correlations between Stat5 and PODXL in AML and ALL progression, however, remain to be tested. In invasive breast carcinoma, increased PODXL expression is an indicator of poor outcomes,63 and Stat5 deregulation may also affect tumorigenesis.58,59 While controversial, outcomes of certain breast cancer trials with Epo have raised questions concerning possible disease progression effects.65,66 Finally, Epo has recently been demonstrated to stimulate Stat5 expression in prostate cancer cells,67 and PODXL germ line variants within a 7q32-q33 region of allelic imbalance are also associated with prostate tumor risk.64
Within erythropoietic contexts, the discovered stage specificity of Epo-induced PODXL expression, as well as PODXL's proposed functions, are next considered. Previously, and at embryonic day 10, predominant PODXL expression was described for the majority of hematopoietic progenitors and for nucleated RBCs.26 At subsequent neonatal stages, however, PODXL expression was progressively restricted to early Kit+ progenitors. By day 7 postpartum, PODXL expression appeared to be restricted further to early Lin−Sca-1+Kit+ progenitors. These analyses used largely unfractionated bone marrow cells (in which erythroid progenitor cell representation is limited) and gated on overall PODXL+ cells. By comparison, the present ex vivo system provided for sensitive analyses of PODXL expression at discrete substages of erythroid cell development and revealed substantial frequencies of PODXL+ Kit+CD71high, Kit−CD71high, and CD71highTer119+ erythroblasts. Moreover, levels of PODXL expression were discovered to depend sharply on Epo levels. In previous analyses, PODXL expression in adult bone marrow erythroblasts was probably not detected due to this apparent need for increased Epo levels. This finding therefore is consistent with a select role for increased PODXL expression during anemia. Induction of anemia with phenylhydrazine, in fact, has recently been shown to lead to increased frequency of PODXL+Ter119+ erythroblasts.26 The observed dependence of efficient PODXL induction on an intact EpoR PY343 Stat5 binding site likewise implicates prime roles for PODXL selectively during stress erythropoiesis.12
Somewhat unexpectedly, Epo's effects on PODXL expression also proved to be exerted in late-stage erythroblasts and perhaps within reticulocyte populations. In stage R4 and R5 reticulocytes, increases in PODXL expression levels were rapidly affected by Epo (ie, within 12 hours). Epo is thought to act primarily on late CFUe stage erythroblasts, but apparent effects in derived reticulocytes could involve (for example) a stabilization of PODXL transcripts and/or protein. In addition, Epo appeared to induce a release of mature R4 reticulocytes from marrow in a parallel time course. The notion that immature reticulocytes might comprise a direct target for this (and possibly additional) Epo response(s) therefore merits consideration. The overall impact of this released red cell population on the erythron, however, is presently undetermined. Finally, in mice expressing a minimal PY-null EpoR allele (EpoR-HM), Epo failed to efficiently stimulate circulating reticulocyte production. In addition, EpoR-HM reticulocytes exhibited only low-level PODXL expression and were abnormally represented in bone marrow as apparently unreleased late Draq5− Ter119+ cells. At limiting Epo concentrations or during anemia, EpoR-HM erythroblasts exhibit defects in survival and/or growth.12,33,68 In the present experiments, however, these potentially complicating features should not be major factors, in that anemia was not induced and high-dose Epo was administered (1500 U/kg). This result, together with discovered effects of Epo on a select set of adhesion factors (and cytokines) in bone marrow erythroblasts, supports the case that a novel action mode exists via which Epo acts dynamically (especially during anemia) to modulate (pro)erythroblast surfaces within a hypothesized stromal niche and to promote their migration (possibly to blood islands; Figure 7D model). Whether Epo's actions in other tissues might involve similar responses is of significant interest, as is the prospect that PODXL or its expression might be altered in the contexts of myelodysplasia, resistance to Epo therapy, and/or diseases involving altered red cell adhesion (eg, sickle cell disease).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Fiser and K. Miller for expert assistance in manuscript assembly.
This work was supported by National Institutes of Health (NIH) R01 HL044491 and core facilities sponsored by NIH NCRR COBRE grants P20-RR18789 and P20-RR15555.
National Institutes of Health
Authorship
Contribution: P.S., M.P.M., O. Bogacheva, O. Bogachev, and D.M.W. each contributed in major ways to the design and execution of essentially all experiments. W.S.K., E.H., and J.F. assisted in the development and use of our SP34-EX expansion system and in most primary culture experiments. W.S.K. also performed most RT and quantitative PCR analyses. All authors, including J.F. and K.N., contributed to figure construction, data analyses, and interpretations, as well as manuscript writing and assembly.
Conflict-of-interest disclosure: K.N. is employed by Pfizer Inc, which has no commercial products discussed in this article. All other authors declare no competing financial interests.
Correspondence: Don M. Wojchowski, Maine Medical Center Research Institute, 81 Research Dr, Scarborough, ME 04074; e-mail: wojchd@mmc.org.

![Figure 1. Gene array–based discovery of Epo-modulated cytokines and cell-surface adhesion factors in murine bone marrow–derived erythroblasts. (A) Illustrated are steps used to expand and isolate Kit+CD71highTer119− erythroblasts together with a representative flow cytometric profile. (B) For erythroblasts prepared from independent bone marrow preparations (n = 4), hematopoietic cytokines were withdrawn for 6 hours and cells were then exposed to Epo (± 5 U/mL). At 90 minutes, RNA was prepared and used in transcriptome analyses. As a control, levels of Epo-induced Cis1 transcript levels (right panel) were analyzed by quantitative RT-PCR. (C) For Epo-regulated genes, genome-wide outcomes are illustrated by relative difference analyses. Cel files were analyzed using ChipInspector. Significantly changed probes were then defined by SAM analysis (left panel) at a false discovery rate of 0.0% (and corresponding significantly changed transcripts needed to be covered by at least 3 such probes). The SAM analysis method is described by Tusher et al69 and Storey and Tibshirani.70 The false discovery rate is a measure for discrimination of significant features. (D) Affymetrix 430–2.0 array-based analyses of Epo-regulated cytokine and cell-surface adhesion factors. Values are mean fold-modulation by Epo (± a standard error [SE], n = 4). (E) Quantitative RT-PCR analyses of Epo regulation of OncoM, Gdf3, Cxcr4, Itga4, and PODXL. Values are means (± SE) and are normalized to beta-actin. (F) For PODXL, Cxcr4, and Itga4, cell-surface levels were also assayed (by flow cytometry) among Kit+CD71high erythroblasts following their isolation and subsequent 24-hour culture in Epo at 0.1, 0.4, and 1.6 U/mL in the presence or absence of SCF (50 ng/mL).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/2/10.1182_blood-2006-11-056465/4/m_zh80130704510001.jpeg?Expires=1765907140&Signature=20cLF~-zX7VJlJbCpcyN~PlzQGfnKKG29rNedW-r8IN3s205JB1n05P3o87oVb0JEkcFUokTcDY1yv~dg8rWkbV3~f9mj70ywoBTgWTSdkrzwDhaOLUvMHo2RBTcKCVENH~XR6CWNiXJSHJ62yiL753Pz9rd1L-~AN9ZpxAQ36yp7wPodC1WNIa7i7ivIY9q96zkA7KpbCJQaq36Qs72WAAqgNHN4m2eel9yr5SDdoaW4biH~DLGCKrX0TbAC~rYG72zNtDoimihhtrpWwszQKRxCo5i-~an~wh-cjqOuJGoQQx-kiLFfRLJW-hd1Ik8TpBXw~iQvJ81FcEDo6aiHg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 1. Gene array–based discovery of Epo-modulated cytokines and cell-surface adhesion factors in murine bone marrow–derived erythroblasts. (A) Illustrated are steps used to expand and isolate Kit+CD71highTer119− erythroblasts together with a representative flow cytometric profile. (B) For erythroblasts prepared from independent bone marrow preparations (n = 4), hematopoietic cytokines were withdrawn for 6 hours and cells were then exposed to Epo (± 5 U/mL). At 90 minutes, RNA was prepared and used in transcriptome analyses. As a control, levels of Epo-induced Cis1 transcript levels (right panel) were analyzed by quantitative RT-PCR. (C) For Epo-regulated genes, genome-wide outcomes are illustrated by relative difference analyses. Cel files were analyzed using ChipInspector. Significantly changed probes were then defined by SAM analysis (left panel) at a false discovery rate of 0.0% (and corresponding significantly changed transcripts needed to be covered by at least 3 such probes). The SAM analysis method is described by Tusher et al69 and Storey and Tibshirani.70 The false discovery rate is a measure for discrimination of significant features. (D) Affymetrix 430–2.0 array-based analyses of Epo-regulated cytokine and cell-surface adhesion factors. Values are mean fold-modulation by Epo (± a standard error [SE], n = 4). (E) Quantitative RT-PCR analyses of Epo regulation of OncoM, Gdf3, Cxcr4, Itga4, and PODXL. Values are means (± SE) and are normalized to beta-actin. (F) For PODXL, Cxcr4, and Itga4, cell-surface levels were also assayed (by flow cytometry) among Kit+CD71high erythroblasts following their isolation and subsequent 24-hour culture in Epo at 0.1, 0.4, and 1.6 U/mL in the presence or absence of SCF (50 ng/mL).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/2/10.1182_blood-2006-11-056465/4/m_zh80130704510001.jpeg?Expires=1765994126&Signature=4zjR6Dkm~jVXWdfZvEJ30OzV9~jJb6aXpGlEZ22jiFZ8ynQbDGCETavuSccfvmnCMKqgx4edaGoKdyljglBg8lJ8jpUc71zqqi24-e-votvQmK-Gh29UyToCgq-aiaQPiUu4oaSwaaSD~85xS9ZetHCjphAbSHQeP1XyI-hG90DF7WvQNkfVxgBEo7abB7EkyrCRS7aZQkBFK736wfNerMFvDm4D3pv9rOulw5FN68Dfp1hKvvvEZ5qCN7wzD3BAJRUt-4N-224S7iYmMEJb6ZSvqwTgN2NU4uuIU5nqxEhXRF8evLpsWBHPXZHIOEuSvtWU3Nxbp5oiQgh~xqwCoQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)