Abstract
Recent studies have demonstrated that cell populations intended for therapeutic purposes that are cultured in heterologous animal products can acquire xenoantigens, potentially limiting their utility. In investigations of the immune response to murine embryonic stem cells, we found that a strong antibody response was generated after the second infusion. Both polyclonal and monoclonal antibody responses, derived from immunized mice, were found to be specific for bovine apolipoprotein B-100, which binds to abundant low-density lipoprotein receptors on the cell surface and is internalized. Here we show that in the majority of patients administered 3 different types of cell-based therapies using cells grown in fetal calf serum-containing media, an antibody response to bovine apolipoprotein B-100 develops after the second infusion and is the dominant specificity. The known and potential clinical effects of such antibodies are discussed.
Introduction
Cellular therapies are of increasing interest for repair or replacement of damaged or destroyed tissues. While the pluripotentiality of embryonic stem (ES) cells is considered very attractive for tissue replacement,1,2 gene-modified autologous or major histocompatibility complex (MHC)–matched lymphocytes have already been transplanted for restoration of immunity in various settings. A major concern for all such therapies is the potential for immune-based rejection caused by xenoresponses involving media components of animal origin.3-5 Immune responses to fetal calf serum (FCS) from patients treated with cultured lymphocytes have been observed in several clinical trials,6-9 although only N-glycoylneuraminic acid (Neu5Gc), originating from FCS, has been identified as an immune target to date.10 In this work we conclusively demonstrate in both mice and humans that the predominant immunogen in FCS-cultured cells is bovine apolipoprotein B-100 (apoB-100). Furthermore, our study indicates that such antibody responses induce hypersensitivity-type reactions in treated patients and can potentially adversely impact engraftment and therapeutic efficacy
Materials and methods
Mice
Female FVB/N mice were purchased from Taconic Farms (Germantown, NY). Female C57BL/6 mice and BALB/c mice were obtained from the Jackson Laboratory (Bar Harbor, ME). The 6- to 12-week-old mice were used for the experiments. Animal care was in accordance with the guidelines of the National Institute of Health Animal Research Advisory Committee.
Cell lines, cell culture, serum, and antibodies
The BL6.9 cell line was derived in the Transgenic Mouse Facility of the Johns Hopkins School of Medicine from a C57BL/6 blastocyst by culture on mitotically inactivated (mitomycin C-treated) primary mouse embryonic fibroblasts (Specialty Media, Phillipsburg, NJ) in high-glucose Dulbecco modified Eagle medium (DMEM) containing 15% fetal calf serum (Hyclone, Logan UT), MEM nonessential amino acids (100 μM), sodium pyruvate (1 mM), glutamine (2 mM), penicillin (100 U/mL), streptomycin (100 μg/mL), 2-mercaptoethanol (5 μM), and 1000 U/mL leukemia inhibitory factor (ESGRO; Chemicon, Temecula, CA). For immunoprecipitation, ES cells were cultured on 0.1% gelatin-coated flasks without primary mouse embryonic fibroblasts.11 Undifferentiated cultures were characterized by expression of SSEA-1 (Chemicon)12 and confirmed by teratoma generation in C57BL/6 mice. CEM, EL4, P815, BW5147.3, K562, HeLa, Sultan, and MDA-MB-231 cell lines were purchased from American Type Culture Collection (Manassas, VA) and cultured with DMEM containing 10% FCS (Hyclone). Lipoprotein-deficient serum was purchased from Sigma (St. Louis, MO). FITC-conjugated antimouse IgG1, IgG2a, IgG2b, IgG3, and IgM, antibodies, control mouse isotype IgG1, control mouse isotype IgG2a, and antirabbit Ig were purchased from BD Pharmingen (San Diego, CA). Rabbit antihuman apolipoprotein B polyclonal antibody was purchased from Cortex Biochem (San Leandro, CA). Normal rabbit immunoglobulin fraction was purchased from DAKO (Carpinteria, CA). An affinity-purified monospecific chicken anti-Neu5Gc antibody was prepared as previously described.13
Generation of monoclonal antibody against ES cells
The BL6.9 cells cultured on primary mouse embryonic fibroblasts were trypsinized and resuspended in the ES cell culture media. Trypsinized cells were maintained in suspension and allowed to recover for 1.5 hours. The suspension was collected to avoid precipitated feeder cells, and was washed 3 times with phosphate-buffered saline (PBS). Then 107 cells were injected intraperitoneally into each FVB/N mouse, once per week for 4 weeks. Spleen cells were harvested and fused with SP2/0-Ag14 myeloma cells.
Immunoprecipitation and Western blot analysis
Cells and serum were lysed with buffer containing 1% triton-X100, 0.5% sodium deoxycholate, 150 mM NaCl, 50 mM Tris (tris[hydroxymethyl]aminomethane)-HCl, pH 7.5, 1.5 mM CaCl2, 1.5 mM MgCl2, 100 pg/mL aprotinin, and 100 pg/mL phenylmethylsulfonyl fluoride (PMSF). The lysate was precleared with ProteinG Sepharose 4Fast Flow (Amersham Bioscience, Piscataway, NJ) and immunoprecipitated by 3E8.1 or isotype-matched control antibody. Proteins were separated by SDS-PAGE with 4% to 12% Bis-Tris gels and 3% to 8% Tris-Acetate gels (Invitrogen, Carlsbad, CA). For Western blotting, the cell surface proteins were biotinylated using Sulfo-NHS-Biotin (Pierce, Rockford, IL) and washed 3 times with 4°C PBS. ES cells were biotinylated in the flask without trypsinization. Immunoprecipitates were transferred (60 V, 4 hours in 25 mM Tris, 192 mM glycine, 10% methanol) to PVDF membranes (Immobilon-P; Millipore, Billerica, MA). Membranes were incubated for 1 hour in PBS containing 5% membrane blocking agent (Amersham Bioscience). Proteins were detected by using streptavidin horseradish peroxidase-conjugate and developed with ECL Western blotting reagents (Amersham Bioscience) by autoradiography.
For the removal of Neu5Gc, 10 milliunits of recombinant Arthrobacter ureafaciens neuraminidase, purchased from Sigma, were added with the reaction buffer, incubated for 6 hours, washed 3 times with deionized water (dH2O), and then eluted. Samples were analyzed with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting using an affinity-purified monospecific chicken anti-Neu5Gc antibody and horseradish peroxidase-conjugated antichicken IgY (Chemicon) as a secondary antibody.10,13
Peptide preparation and mass spectrometric analysis
Gel bands were destained in 50% acetonitrile (ACN) solution in 25 mM NH4HCO3 and lyophilized. Dried gel bands were reduced and alkylated with 10 mM dithiothreitol (DTT) and 55 mM iodoacetamide, incubated at 4°C for 1 hour in porcine modified trypsin (20 ng/uL; Promega, Madison, WI), and allowed to digest overnight at 37°C in 25 mM NH4HCO3. Peptides were extracted from the gel with a 70% ACN/5% formic acid solution, lyophilized to near dryness, and reconstituted in 6.5 μL of HPLC buffer A (95% H2O/5% ACN/0.1% formic acid). Microcapillary reverse phase liquid chromatography (LC)/mass spectrometry (MS)/MS analysis was performed with LC Packings liquid chromatography system (Dionex, Sunnyvale, CA) coupled online to a ThermoFinnigan LCQ Classic ion trap mass spectrometer (San Jose, CA) with a modified nanospray source. Reverse-phase separations were performed with an in-house–manufactured, slurry-packed capillary column. Full MS scans were followed by 4 MS/MS scans of the most abundant peptide ions (in a data-dependent mode) and collision-induced dissociation was performed at a collision energy of 38%.
Data analysis of LC/MS/MS
Data analysis was performed by searching MS/MS spectra in the European Bioinformatics Institute's nonredundant proteome set of Swiss-Prot, TrEMBL, and Ensembl entries through the Sequest Bioworks Browser (ThermoFinnigan). Peptides were considered legitimate hits after filtering the correlation scores and manual inspection of the MS/MS data.
Flow cytometry and confocal scanning laser microscopy
For adherent cell staining, cells were washed with PBS, trypsinized with 0.05% trysin/0.53 mM EDTA (ethylenediamine tetraacetic acid), and resuspended in media. Trypsinized adherent cells and tumor cell lines were washed 3 times and stained with the indicated antibody in staining buffer. For intracellular staining, cells were washed 3 times with PBS, treated with 0.05% trysin/0.53 mM EDTA for more than 8 minutes to achieve complete trypsinization, fixed with 2% formaldehyde in PBS for 20 minutes at 25°C, washed with PBS, and then preincubated with 0.5% saponin (Sigma) PBS for 10 minutes at room temperature. Cells were then incubated with fluorescein isothiocyanate (FITC) conjugated 3E8.1 at 5 μg/mL or FITC-conjugated isotype-matched control mouse IgG1 (BD Pharmingen) at 5 μg/mL for 30 minutes. After 2 washes with 0.5% saponin PBS, cells were washed with PBS. Bovine serum albumin was not used because of contamination by low levels of bovine apoB-100. For fluorescence activated cell sorting (FACS) analysis of live cells, the cells were resuspended in PBS containing 10 μg/mL propidium iodide and immediately run on a FACS Caliber (Becton Dickinson, Mountain View, CA). Data were analyzed using the CellQuest software program (Becton Dickinson). Alexa Fluor 488 conjugated goat antimouse IgG (Invitrogen) was used as a second antibody for confocal scanning laser microscopic analysis. Confocal images were obtained with the Zeiss LSM 5 PASCAL confocal laser scanning microscope installed on a Zeiss Axioskop2 MOT microscope (Carl Zeiss MicroImaging, Thornwood, NY). To visualize cells, the Zeiss Plan Neofluar 1.3 (100×) lens was used. Images were acquired using the Zeiss LSM 5 PASCAL system and Zeiss LSM 5 PASCAL version 3.2 SP2 software.
Patient sera/plasma from clinical trials and healthy donor materials
Human serum and peripheral blood mononuclear cells obtained from healthy volunteers were provided by the National Institutes of Health blood bank. Peripheral blood mononuclear cells were obtained from buffy coats collected from de-identified healthy donors as byproducts of blood donated for patient care that has received an Institutional Review Boards exemption. Human lymphocytes were elutriated in our facility.
Serum or plasma of the patients was obtained as described.8,14,15 Adenosine deaminase (ADA)-deficient patients receiving gene-modified T cells were previously described.8 The clinical research protocol and procedures involving human subjects were approved by the National Cancer Institute, National Heart, Lung, and Blood Institute, and National Human Genome Research Institute Institutional Review Boards. Each patient received 11 or 12 infusions of autologous T cells cultured in RPMI1640–10% FCS and transduced with LASN (a retroviral vector expressing both human ADA cDNA and the neor gene). The number of cells in each infusion ranged from 6 × 109 to 20 × 109 cells.
The clinical study involving adoptive transfer of genetically modified lymphocytes from HIV-discordant identical twins has been described previously.14 Twelve pairs of identical twins discordant for HIV-1 infection were enrolled in a study of syngeneic lymphocyte transfers at the National Institute of Allergy and Infectious Disease. The study was approved by the National Institute of Allergy and Infectious Disease Institutional Review Board. Mononuclear cells were purified by Ficoll-Hypaque gradient centrifugation, and 106 cells were placed into AIM V media (Invitrogen) supplemented with 5% FCS, anti-CD3 (OKT-3; 10 μg/105cells; Ortho, Raritan, NJ), and recombinant IL-2 (100 I.U/mL; Chiron, Emeryville, CA). After 3 days of activation in culture, cells were transduced with LNL6 or G1NA (a retroviral vector expressing the neor gene). The cells were expanded ex vivo for an additional 6 to 8 days before harvest.
The breast cancer vaccine study using costimulatory gene (CD80)-modified, HLA-A2–matched, allogeneic breast cancer cells has been described previously.15 HLA-A2 + women with metastatic breast cancer were enrolled in the study at Providence Portland Medical Center. The study was approved by the Providence Portland Medical Center Institutional Review Board. MDA-MB-231 cells were cultured in RPMI1640 with 10% FCS and transduced with CMV-B7 vector expressing human CD80 cDNA and the neor gene. On the day of vaccination, MDA-MB-231-CD80 cells were lethally irradiated (10 000 cGy), washed, and injected subcutaneously. The number of cells given in each infusion ranged from 107 to 108 cells, with recombinant granulocyte macrophage colony-stimulating factor or Bacille Calmette Guerin (BCG) as an adjuvant.
In all clinical trials, written informed consent was obtained from all participants after the nature and possible consequence of these treatments were explained; 10 μL of each patient's serum were used for immunoprecipitation. Immunoprecipitants were analyzed by SDS-PAGE. The median intensity of each band was measured and calculated in comparison to the intensity of the band from patient 2 in the ADA-SCID trial, defined as 100%. The definitions of “+” or “−” markers are as follows: 0% or below 0% is −; 0% to 20% is plus/minus; 20% to 60% is plus; 60% to 100% is 2 pluses; 100% to 140% is 3 pluses; and over 140% is 4 pluses.
Results
The immune response to cultured murine ES cells is directed to bovine apoB
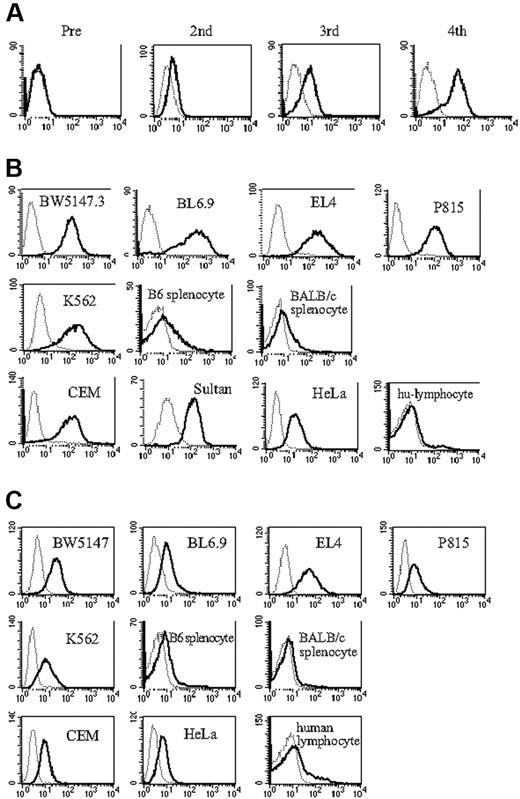
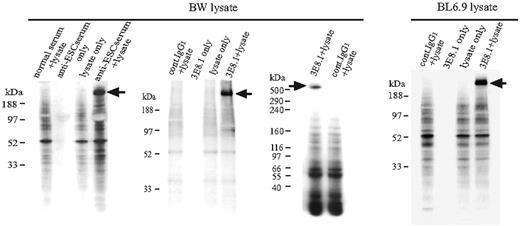
To generate antibodies to determinants expressed on ES cells, we immunized B6 and FVB/N female mice with BL6.9, a C57BL/6-derived mouse embryonic stem cell line. An early IgM antibody response was detected in FVB mice after the second immunization (N.S. and A.S.R. unpublished data, April 3, 2005) and a prominent IgG response, most notably IgG1, was detected after the third immunization (Figure 1A). The antiserum was broadly cross-reactive, binding to most cultured mouse and human tumor cell lines, but minimally or not at all to human and murine primary lymphocytes (Figure 1B). B6 mice failed to generate antibody, despite multiple immunizations. However, given that murine ES cells do not express MHC antigens16 (K.T., N.S., and A.S.R. unpublished data, April 7, 2003), and the diverse MHC phenotypes of the cell populations stained with the antiserum, we suspected that the antiserum was not specific for MHC antigens, but rather that it might be specific for markers expressed on rapidly dividing cell populations. To better facilitate identification of the target ligand, we generated monoclonal antibodies from the immunized FVB/N mice. Three monoclonal antibodies (mAbs) were cloned, one of which was of IgG isotype (3E8.1, IgG1; see “Materials and methods”). This mAb exhibited a binding profile similar to the antiserum with prominent staining on BL6.9 ES cells and tumor cell lines, but minimal staining of primary lymphocyte populations (Figure 1C). To define the specificity of the polyclonal response, we immunoprecipitated cell surface-biotinylated BW5147.3, a mouse T-cell lymphoma cell line, with the antiserum to ES cells. One unique band was precipitated (Figure 2, BW lysate left panel). Comparably, on immunoprecipitation of BW5147.3 and BL6.9 cells with 3E8.1 mAb, we observed the same unique high MW band from both cell lines (Figure 2, BW lysate middle panel and BL6.9 lysate), which proved to be approximately 520 kDa on a sizing gel (Figure 2, BW lysate right panel). The binding of the ES cell antiserum was partially blocked with 3E8.1 but not with control anti-H-2Kk mAb (data not shown), indicating that the major component of the antiserum was directed to the same determinant as the 3E8.1 mAb.
Mice immunized with embryonic stem cells produce antibodies. (A) FACS analysis of antiserum from FVB/N mice immunized with BL6.9 ES cells. Sera from mice before and after the second, third, and fourth immunizations were analyzed (solid line); secondary antibody only (dotted line). (B) Indicated tumor cell lines, C57BL/6 and BALB/c splenocytes, and human peripheral blood lymphocytes were stained with serum from ES cell immunized FVB/N mice (solid line), with serum from nonimmunized FVB/N mice (dotted line), and (C) with monoclonal antibody 3E8.1 (solid line) and isotype-matched antibody (dotted line).
Mice immunized with embryonic stem cells produce antibodies. (A) FACS analysis of antiserum from FVB/N mice immunized with BL6.9 ES cells. Sera from mice before and after the second, third, and fourth immunizations were analyzed (solid line); secondary antibody only (dotted line). (B) Indicated tumor cell lines, C57BL/6 and BALB/c splenocytes, and human peripheral blood lymphocytes were stained with serum from ES cell immunized FVB/N mice (solid line), with serum from nonimmunized FVB/N mice (dotted line), and (C) with monoclonal antibody 3E8.1 (solid line) and isotype-matched antibody (dotted line).
Identification of the ligand detected by 3E8.1 mAb and antiserum from BL6.9 immunized mice. Western blot analysis of ES cell antiserum identified a unique band (BW lysate, left). The same molecular-weight band was precipitated from both surface-biotinylated BW cell (BW lysate, middle) and BL6.9 cell lysates (right panel) with the 3E8.1 mAb. The band is approximately 520 kDa (BW lysate, right).
Identification of the ligand detected by 3E8.1 mAb and antiserum from BL6.9 immunized mice. Western blot analysis of ES cell antiserum identified a unique band (BW lysate, left). The same molecular-weight band was precipitated from both surface-biotinylated BW cell (BW lysate, middle) and BL6.9 cell lysates (right panel) with the 3E8.1 mAb. The band is approximately 520 kDa (BW lysate, right).
The 3E8.1 ligand is bovine apolipoprotein B
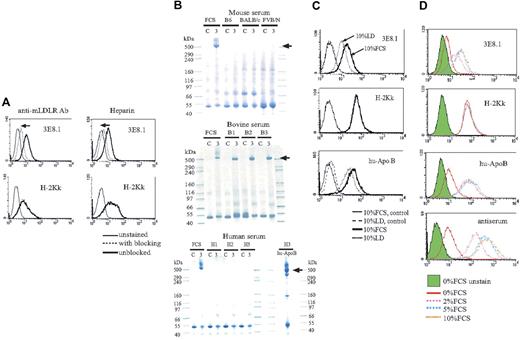
The target ligand of the 3E8.1 mAb was identified by in-gel trypsin digestion and mass spectrometry-based protein microsequencing of the immunoprecipitated protein band. Two independent tryptic peptides in the mouse proteome and 4 independent tryptic peptides in the human proteome identified apolipoprotein B (apoB) as the protein of origin (Table 1). Because murine ES cells and tumor cell lines were unlikely to have been the sources of the apoB, we reasoned that the apoB probably originated from the FCS added as a media component. The molecular weight of the unique band precipitated by 3E8.1 was approximately 520 kDa (Figure 2), indicating that it was apoB-100, the complete form of apoB, and not the truncated apoB-48, which is approximately 250 kDa. Further support for bovine apoB-100 as the ligand of 3E8.1 arose from 4 studies. First, we found that agents known to block the binding of apoB to the low-density lipoprotein (LDL) receptor, antimouse LDL receptor antibody, and heparin cross-blocked binding of 3E8.1 to apoB (Figure 3A). Second, we demonstrated that 3E8.1 specifically immunoprecipitated bovine apoB-100, but not human or murine apoB (Figure 3B). The slightly higher molecular weight of apoB of fetal as opposed to adult bovine origin is consistent with its origin from the placenta.17 Third, the binding of 3E8.1 to cells cultured in lipoprotein-deficient bovine serum, which contains less than 0.5% lipoprotein, was clearly reduced (Figure 3C). However, an appreciable amount of bovine apoB-100 cell surface staining remained, indicating that only minimal amounts of apoB in the medium are required to fully saturate cell surface receptors (Figure 3C). More severe reduction of FCS in the medium demonstrated a dose-dependent decrease in staining (Figure 3D). Finally, although bovine apoB has not itself been sequenced, the relatively high sequence conservation between mouse and human (70%-80%), suggests that bovine apoB is likely to be highly homologous to both mouse and human proteins, a supposition supported by the cross reactivity of antihuman apoB on bovine apoB-100 (Figure 3C,D). Taken together, these data indicate that 3E8.1 is specific for bovine apoB-100, further indicate the high conservation of apoB across species, both by sequence and antigenicity, and, finally, suggest that ordinary culture conditions contain sufficient apoB-100 in the medium to fully saturate LDL receptors on the cell surface.
ApoB peptides identified by mass spectrometry analysis
| Position . | MH+ . | Sequence . |
|---|---|---|
| Mouse | ||
| 696-707 | 1309.49 | GFEPTLEALFGK |
| 935-949 | 1605.82 | LFSGSNTLHLVSTTK |
| Human | ||
| 51-71 | 2283.35 | KYTYNYEAESSSGVPGTADSR |
| 696-707 | 1309.49 | GFEPTLEALFGK |
| 950-960 | 1281.48 | TEVIPPLIENR |
| 1770-1781 | 1493.64 | LDNIYSSDKFYK |
| Position . | MH+ . | Sequence . |
|---|---|---|
| Mouse | ||
| 696-707 | 1309.49 | GFEPTLEALFGK |
| 935-949 | 1605.82 | LFSGSNTLHLVSTTK |
| Human | ||
| 51-71 | 2283.35 | KYTYNYEAESSSGVPGTADSR |
| 696-707 | 1309.49 | GFEPTLEALFGK |
| 950-960 | 1281.48 | TEVIPPLIENR |
| 1770-1781 | 1493.64 | LDNIYSSDKFYK |
The target of antibody responses is bovine apoB. (A) 3E8.1 binding to BW cells (thick solid lines) is blocked with antimouse LDL receptor antibody and heparin (dotted lines) but the binding of H-2Kk antibody is not blocked. (B) Sera from mice, adult cows, and human donors were immunoprecipitated by 3E8.1 or isotype-matched control. Human serum was immunoprecipitated by antihuman apoB antibody (bottom, right). “C” refers to immunoprecipitation using isotype matched control antibody and “3” to immunoprecipitation with 3E8.1 mAb. (C) BW cells cultured with 10% FCS (dark solid line) or 10% lipoprotein deficient (LD) medium (dotted line) were stained with indicated antibodies. Isotype-matched antibody (thin solid line) and normal rabbit Ig (dashed line) are negative controls. (D) BW cells incubated for 1 hour in PBS containing various concentrations of FCS were stained with indicated reagents.
The target of antibody responses is bovine apoB. (A) 3E8.1 binding to BW cells (thick solid lines) is blocked with antimouse LDL receptor antibody and heparin (dotted lines) but the binding of H-2Kk antibody is not blocked. (B) Sera from mice, adult cows, and human donors were immunoprecipitated by 3E8.1 or isotype-matched control. Human serum was immunoprecipitated by antihuman apoB antibody (bottom, right). “C” refers to immunoprecipitation using isotype matched control antibody and “3” to immunoprecipitation with 3E8.1 mAb. (C) BW cells cultured with 10% FCS (dark solid line) or 10% lipoprotein deficient (LD) medium (dotted line) were stained with indicated antibodies. Isotype-matched antibody (thin solid line) and normal rabbit Ig (dashed line) are negative controls. (D) BW cells incubated for 1 hour in PBS containing various concentrations of FCS were stained with indicated reagents.
Bovine apoB-100 is retained on the cell surface and internalized into the cell
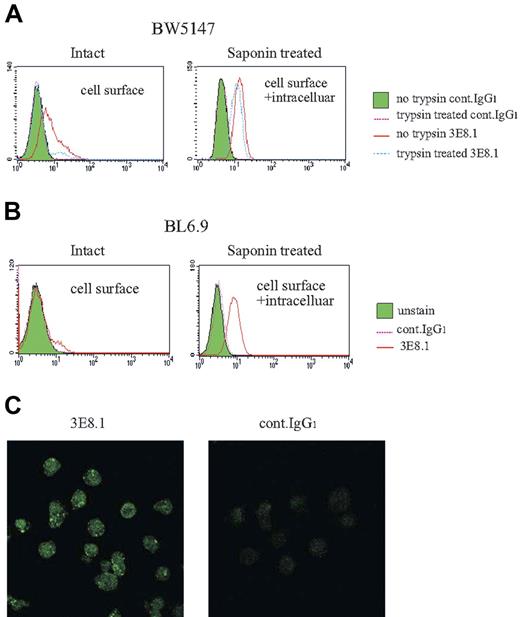
ApoB-100 is known to bind to cell surface receptors and to be internalized into the cell via LDL receptors and proteoglycans.18-21 We next examined the intracellular transport and accumulation of bovine apoB-100 by murine cells. Complete trypsinization of both BW5147.3 and BL6.9 ES cells reduced 3E8.1 binding to background levels, attributable to the removal of cell surface LDL receptors and proteoglycans, known to be trypsin-sensitive. Trypsinization revealed prominent intracellular staining of bovine apoB in both BW5147.3 (Figure 4A) and BL6.9 ES cells (Figure 4B), indicating that the endocytosed apoB-100 is stored intracellularly. Confocal laser microscopy confirmed the abundant intracellular stores of bovine apoB within cultured cells (Figure 4C). These data demonstrate that cells cultured in FCS bind bovine apoB-100 via surface receptors, and then internalize and store it.
Bovine apoB-100 is present on the cell surface and is found intracellularly. (A) Trypsin-treated BW and (B) BL6.9 ES cells were stained by 3E8.1-FITC with or without saponin permeabilization. The staining medium contained PBS only. (C) Permeabilized BW cells were stained with 3E8.1-FITC or isotype-matched control antibodies and then stained with Alexa Fluor 488 conjugated goat antimouse IgG.
Bovine apoB-100 is present on the cell surface and is found intracellularly. (A) Trypsin-treated BW and (B) BL6.9 ES cells were stained by 3E8.1-FITC with or without saponin permeabilization. The staining medium contained PBS only. (C) Permeabilized BW cells were stained with 3E8.1-FITC or isotype-matched control antibodies and then stained with Alexa Fluor 488 conjugated goat antimouse IgG.
Antibodies to bovine apoB-100 dominate the human immune response to cells cultured in FCS
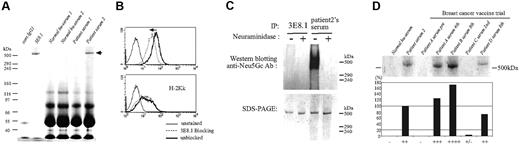
Multiple reports in the literature indicated that patients treated with diverse cell populations that had been cultured in FCS generated antibodies to FCS.6-9 In one study, such responses were monospecific.6 We tested the specificity of antibodies to FCS from patients treated with cell populations cultured in FCS, including ADA-SCID patients treated with gene-modified autologous cells, HIV patients treated with identical twin-donated and cultured T cells, and patients with breast cancer administered cultured allogeneic breast cancer cells as a vaccine. Using the one positive ADA-SCID patient's serum to which we had access, a unique band was precipitated from FCS that was identical to the precipitant of 3E8.1, indicating that the antiserum of patient 2 was directed predominantly to bovine apoB-100 (Figure 5A and Table 2). 3E8.1 was able to partially block the binding of the serum of patient 2 to cell-bound bovine apoB (Figure 5B). Both antimouse LDL receptor antibody and heparin also blocked the binding of the serum of patient 2 (data not shown). These studies demonstrated that the serum of patient 2 contained substantial antibody against bovine apoB-100. Particularly surprising was the fact that this response had persisted for 13 years after the last therapeutic exposure, possibly because of ongoing exposure via an oral route.
Bovine apoB-100 is the predominant target of the antibody response in patients treated with FCS cultured cells. (A) FCS was immunoprecipitated by isotype-matched control IgG1, 3E8.1 mAb, serum samples from 2 healthy human volunteers, or 2 serum samples from ADA-SCID patients in a gene therapy trial. Samples were analyzed by SDS-PAGE. (B) BW cells were incubated with the FITC-conjugated IgG fraction of serum from patient 2 with (dotted light line) or without (thick solid line) 3E8.1 mAb. (C) FCS treated with ( + ) or without (−) neuraminidase was immunoprecipitated by 3E8.1 or by serum from patient 2 and analyzed by SDS-PAGE (lower panel) or by Western blotting with anti-Neu5Gc antibody (upper panel). (D) FCS immunoprecipitated by serum from a healthy human volunteer, by serum from patient 2, or by serum samples from 4 patients in the breast cancer vaccine trial, were analyzed by SDS-PAGE. The definitions of + and − are in “Materials and methods, Patient sera/plasma from clinical trials and healthy donor cells.”
Bovine apoB-100 is the predominant target of the antibody response in patients treated with FCS cultured cells. (A) FCS was immunoprecipitated by isotype-matched control IgG1, 3E8.1 mAb, serum samples from 2 healthy human volunteers, or 2 serum samples from ADA-SCID patients in a gene therapy trial. Samples were analyzed by SDS-PAGE. (B) BW cells were incubated with the FITC-conjugated IgG fraction of serum from patient 2 with (dotted light line) or without (thick solid line) 3E8.1 mAb. (C) FCS treated with ( + ) or without (−) neuraminidase was immunoprecipitated by 3E8.1 or by serum from patient 2 and analyzed by SDS-PAGE (lower panel) or by Western blotting with anti-Neu5Gc antibody (upper panel). (D) FCS immunoprecipitated by serum from a healthy human volunteer, by serum from patient 2, or by serum samples from 4 patients in the breast cancer vaccine trial, were analyzed by SDS-PAGE. The definitions of + and − are in “Materials and methods, Patient sera/plasma from clinical trials and healthy donor cells.”
Presence of antibody to bovine apoB-100 in patients receiving therapeutic cell populations cultured in FCS
| Infusion no. . | Time after infusion . | Presence of bovine* ApoB-100 antibody . |
|---|---|---|
| GT trial | ||
| Patient 1: 11 | 13 y | − |
| Patient 2: 12 | 13 y | ++ |
| BCV trial | ||
| Patient A | ||
| Preinfusion | NA | − |
| 4 | 4 wk | +++ |
| Patient B | ||
| Preinfusion | NA | SNA |
| 8 | 4 wk | ++++ |
| Patient C | ||
| Preinfusion | NA | SNA |
| 2 | 20 wk | +/− |
| Patient D | ||
| Preinfusion | NA | SNA |
| 8 | 4 wk | ++ |
| Patient E | ||
| Preinfusion | NA | − |
| 4 | 4 wk | + |
| 5 | 4 wk | ++ |
| Patient F | ||
| Preinfusion | NA | SNA |
| 2 | 2 wk | − |
| Patient G | ||
| Preinfusion | NA | SNA |
| 2 | 3 wk | + |
| Infusion no. . | Time after infusion . | Presence of bovine* ApoB-100 antibody . |
|---|---|---|
| GT trial | ||
| Patient 1: 11 | 13 y | − |
| Patient 2: 12 | 13 y | ++ |
| BCV trial | ||
| Patient A | ||
| Preinfusion | NA | − |
| 4 | 4 wk | +++ |
| Patient B | ||
| Preinfusion | NA | SNA |
| 8 | 4 wk | ++++ |
| Patient C | ||
| Preinfusion | NA | SNA |
| 2 | 20 wk | +/− |
| Patient D | ||
| Preinfusion | NA | SNA |
| 8 | 4 wk | ++ |
| Patient E | ||
| Preinfusion | NA | − |
| 4 | 4 wk | + |
| 5 | 4 wk | ++ |
| Patient F | ||
| Preinfusion | NA | SNA |
| 2 | 2 wk | − |
| Patient G | ||
| Preinfusion | NA | SNA |
| 2 | 3 wk | + |
The FCS lysate was immunoprecipitated with 10 μL of each serum and the precipitants analyzed by SDS-PAGE.
GT trial indicates gene therapy trial for adenosine deaminase (ADA) deficiency; BCV trial, breast cancer vaccine trial; NA, not applicable; SNA, sample not available.
Reference band intensity: — indicates ≤ 0%; +/−, 0%-20%; +, 20%-60%; ++, 60%-100%; +++, 100%-140%; ++++, >140%.
Because of the existence in humans of preexisting antibody to N-glycoylneuraminic acid (Neu5Gc), present as terminal sugar residues on many proteins of all animals studied to date, except for humans and chickens,10,13 we investigated whether the antibody response to bovine apoB in humans was directed to Neu5Gc determinants, potentially present on the glycoprotein apoB-100. As expected,13 Western blot revealed the presence of Neu5Gc antibodies in patient serum (Figure 5C upper panel). While neuraminidase pretreatment abrogated the reactivity of anti-Neu5Gc antibodies (Figure 5C upper panel), it failed to abrogate binding of patient serum or 3E8.1 mAb to bovine apoB-100, indicating that the antibody response to bovine apoB-100 is not directed to Neu5Gc determinants, but rather to protein determinants (Figure 5C lower panel).
We next examined the sera of patients immunized with a cultured cellular vaccine optimized to produce an immune response to tumor antigens by transgenically induced expression of a costimulatory molecule.15,22 Five of 7 patients were strongly positive for antibodies to bovine apoB (Figure 5D and Table 2), perhaps reflecting their extensive exposure in circumstances optimized for immunity. Finally, we examined the sera from HIV-infected patients administered enriched T-cell populations from their HIV-negative twin. Such cells were harvested, stimulated in vitro with anti-CD3 antibody and IL-2, transduced with LNL6 or G1NA, and cultured in FCS-containing medium.6,14 Nine of 17 HIV patients receiving identical twin lymphocytes had a response to bovine apoB (2 were weak responses; Table 3). As in all other patient populations examined, generation of antibodies required, at minimum, a second exposure to cultured cell populations.
Presence of antibody to bovine apoB-100 in HIV patients receiving identical twin lymphocytes cultured in FCS-containing media
| Infusion no. . | Time after infusion, wk . | Presence of bovine* ApoB-100 antibody . |
|---|---|---|
| Patient A | ||
| Preinfusion | NA | − |
| 1 | 2 | − |
| 2 | 2 | − |
| 3 | 2 | ++ |
| Patient B | ||
| Preinfusion | NA | − |
| 1 | 2 | − |
| 2 | 2 | +− |
| 3 | 4 | + |
| Patient C | ||
| Preinfusion | NA | − |
| 2 | 2 | − |
| 3 | 4 | ++ |
| 4 | 4 | ++++ |
| Patient D | ||
| Preinfusion | NA | − |
| 4 | 4 | ++++ |
| Patient E | ||
| Preinfusion | NA | − |
| 1 | 1 | − |
| 1 | 3 | − |
| 2 | 1 | − |
| 2 | 3 | + |
| 3 | 2 | +++ |
| Patient F | ||
| Preinfusion | NA | − |
| 1 | 1 | − |
| 1 | 3 | − |
| 2 | 1 | − |
| 2 | 3 | + |
| 3 | 2 | ++ |
| Patient G | ||
| Preinfusion | NA | − |
| 1 | 2 | − |
| 2 | 2 | − |
| 3 | 2 | +− |
| Patient H | ||
| Preinfusion | NA | − |
| 1 | 2 | − |
| 2 | 2 | − |
| 3 | 6 | +− |
| Patient I | ||
| Preinfusion | NA | − |
| 1 | 2 | − |
| 2 | NA | − |
| 2 | 2 | + |
| Infusion no. . | Time after infusion, wk . | Presence of bovine* ApoB-100 antibody . |
|---|---|---|
| Patient A | ||
| Preinfusion | NA | − |
| 1 | 2 | − |
| 2 | 2 | − |
| 3 | 2 | ++ |
| Patient B | ||
| Preinfusion | NA | − |
| 1 | 2 | − |
| 2 | 2 | +− |
| 3 | 4 | + |
| Patient C | ||
| Preinfusion | NA | − |
| 2 | 2 | − |
| 3 | 4 | ++ |
| 4 | 4 | ++++ |
| Patient D | ||
| Preinfusion | NA | − |
| 4 | 4 | ++++ |
| Patient E | ||
| Preinfusion | NA | − |
| 1 | 1 | − |
| 1 | 3 | − |
| 2 | 1 | − |
| 2 | 3 | + |
| 3 | 2 | +++ |
| Patient F | ||
| Preinfusion | NA | − |
| 1 | 1 | − |
| 1 | 3 | − |
| 2 | 1 | − |
| 2 | 3 | + |
| 3 | 2 | ++ |
| Patient G | ||
| Preinfusion | NA | − |
| 1 | 2 | − |
| 2 | 2 | − |
| 3 | 2 | +− |
| Patient H | ||
| Preinfusion | NA | − |
| 1 | 2 | − |
| 2 | 2 | − |
| 3 | 6 | +− |
| Patient I | ||
| Preinfusion | NA | − |
| 1 | 2 | − |
| 2 | NA | − |
| 2 | 2 | + |
FCS lysate was immunoprecipitated with 10 μL of each serum and the precipitants analyzed by SDS-PAGE. Nine of 17 patients had a response to bovine apoB-100. Eight had a negative response. This table shows patients who were positive.
Reference band intensity: — indicates ≤ 0%; +/−, 0%-20%; +, 20%-60%; ++, 60%-100%; +++, 100%-140%; ++++, >140%.
Discussion
The effects of FCS on immune responses in vitro have been noted in the past to include the induction of MHC-independent cytotoxicity mediated by T cells,23 natural killer cell activation,24 induction of cytokine secretion,25 development of arthus-like reactions on cellular infusions,6 and a type I hypersensitivity reaction.7 Our studies showed that the majority of patients infused with cells cultured in FCS-containing media generated antibody responses directed predominantly to a single component of FCS, bovine apoB-100. Although responses to other xenogeneic elements, including Neu5Gc, were detected, a more sensitive technique, ie, Western blotting, was required for detection, indicating that such responses were weaker than those directed to bovine apoB-100, which were easily detected by SDS-PAGE. The response to bovine apoB appeared to be boosted by successive exposure to cell transplants or vaccines; at minimum, 2 exposures were required, both in human and mouse. In contrast, many humans have preformed antibodies to other xenogeneic antigens such as alpha-Gal and Neu5Gc. Thus, although the final antibody response to bovine apoB-100 was not glycan-specific, it is possible that the potential presence of Neu5Gc and/or α-Gal on apoB could have enhanced the immune response to the protein itself.26
The intensity of the response to bovine apoB relates to several factors: the enrichment of apoB-100 in the cellular infusate, because of the high-affinity binding to LDL receptor; the high density of such molecules on the cell surface, which are arrayed in a fashion to stimulate B-cell production of antibody via multivalent ligation of BCR; and the intracellular storage and potential processing and presentation of apoB-100 within the context of self class II. The generation of a potent antibody response by successive exposure to cultured cells engenders a situation in which antibody generated after 2 cellular infusions is able to target subsequently infused cells that transiently retain surface expression of bovine apoB. We have found that significant loss of bovine apoB from the surface of cells cultured in vitro requires several hours after placement of cells in media totally devoid of FCS (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). However, the actual kinetics of human apoB displacement of bovine apoB bound to human LDL receptor is not known and could be significantly longer. Moreover, although processing and presentation of apoB peptides in the context of self class II by host APC is inferred from the generation of isotype-switched IgG responses in mice and humans, cell-mediated responses are also generated to the cultured cells that potentially present this protein in association with self class I (L.M.M., unpublished data, May 18, 1998), raising the possibility that cytotoxic T lymphocytes (CTL) may target such cells. In contrast to the abundant binding of the polyclonal antibodies in antiserum and of 3E8.1 to cultured cells, the binding of antibodies and 3E8.1 to normal cell populations was minimal or nonexistent (Figure 1B,C), a finding undoubtedly attributable to the saturation of such cells with their own species specific LDL and lack of extended in vitro culture, as such cells were exposed to FCS-containing medium only during the FACS staining procedure in which the temperature is kept at 4°C.
The failure of B6 mice to generate antibody to bovine apoB-100 was puzzling, because multiple strains generated antibody responses in addition to FVB, including DBA/1, DBA/2, and BALB/c (N.S., and A.S.R. unpublished data, August 2005). However, it is possible that T-cell help is driven via presentation of relevant bovine apolipoprotein peptides in the context of MHC class II IE molecules, lacking in the B6 strain,27 or that the relevant peptides have only weak affinity for MHC class II IAβ. The potential requirement for IE expression in the antibody response is undergoing investigation. The diversity in generation of antibodies to bovine apoB-100, observed in mice, was also observed in the clinical trials with some patients failing to produce apoB-100 antibodies and others making robust responses. This diversity in immune response may be attributable to differences in the ability of apoB peptides to associate with various human leukocyte antigen class II molecules, differences in the extent of oral tolerance in populations with a high consumption of bovine products, diversity in presence of preexisting antibody to Neu5Gc,10,13 and, finally, differences in the immune competence of the patients in the clinical trials. Because we failed to detect bovine apoB-100 antibodies before infusion in almost all patient sera, it is apparent that the novel cellular presentation and nonoral administration of bovine apoB was necessary to generate an immune response.
Finally, the development of apoB antibodies has relevance to treatment safety and, potentially, to treatment efficacy. In the initial discordant HIV twin study, Selvaggi et al6 reported that 8 of 12 patients were positive for antibodies to a single entity of FCS. We tested the sera of these patients and found that the single component of FCS to which antibodies were generated was bovine apoB-100 (Table 3). Strikingly, 6 of 8 patients with antibodies to bovine apoB-100 had immune-mediated “arthus-type” reactions after cellular administration, with symptoms worsening with subsequent infusions, whereas the 2 antibody-positive patients who did not have such reactions had been pretreated with antipyretics and antihistamines.6 In an additional example of anti-FBS antibodies causing a clinical adverse event, 1 patient of 6 with osteogenesis imperfecta receiving gene-modified mesenchymal stem cells cultured in FCS had an antibody response to FCS and an urticarial rash after the second cellular infusion.28 More importantly, of the 6 patients in this study, the only one who failed to show evidence of cellular engraftment and to benefit from an increased growth velocity was this patient, who had an antibody response to FCS.28 It is highly probable that the anti-FCS antibody response in this patient was specific for bovine apoB-100. The potential for antibovine apoB-100 antibodies to impact on engraftment is further suggested by patient 2 in the ADA-SCID trial, who had a potent antibody response to bovine apoB and had very low levels of engraftment of autologous genetically modified cells, compared with an antibody-negative patient counterpart who manifested much higher levels of cellular engraftment.8
The importance of our findings relate to similar recent findings regarding incorporation of Neu5Gc into ES cells, potentially rendering them subject to immune attack and elimination by means of antibody-dependent cell cytotoxicity and/or opsonization,10 further highlighting the possible effect on cellular engraftment. Thus, continued use of FCS for culture of human cell products for therapeutic intent, as is currently the case for mesenchymal stem cells,29 may jeopardize engraftment, whereas its removal may afford a more favorable outcome.30 Given that even the drastic reduction of FCS components was not sufficient to prevent the robust coating of cells with foreign apoB, as evinced in our study of cells cultured in lipoprotein-deficient serum containing only 0.5% lipoproteins, alternative strategies must be considered. Although human AB serum-based medium has been used as a substitute for fetal calf serum,10,31,32 it is clear that the amounts of sera that would be needed from this rarest of blood donor groups would be prohibitive with respect to the need. Moreover, apoB has protein polymorphisms that can be recognized by human alloantibodies.33,34 Thus, autologous serum depleted of anti-Neu5Gc antibodies may be the most practical and safest means for culturing ES or other therapeutic cell populations before transplantation. Even if the patient's serum contains anti-Neu5Gc antibodies, heat inactivation could prevent complement activation until the preexisting Neu5Gc is metabolically eliminated35 and such prolonged culture may further allow sufficient time for any preexisting bovine apoB-100 to be eliminated.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mark Lowenthal, Laboratory of Pathology, National Cancer Institute, for analysis of mass spectrometry and helpful discussion, and Drs Michael Norcross, Eda Bloom, and Brenton McCright for critical reading of this manuscript.
This research was supported in part by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: N.S. was the principal investigator of the studies and primary author. K.T. contributed to the research and analysis. L.M.M. and F.C. provided patients' samples from ADA-SCID gene therapy clinical trial. A.M.L. provided the embryonic stem cells. E.F.P. contributed to the mass spectrometry analysis. J.A.M., J.A.T., and H.C.L. provided patients' samples from HIV gene-therapy clinical trials. W.J.U. and B.A.F. provided patients' samples from breast cancer vaccine trials. A.V. provided the anti-Neu5Gc antibody; J.K.L. provided critical bovine reagents. A.S.R. provided the overall project and manuscript guidance. All authors provided guidance for the studies and contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Amy S. Rosenberg, MD, Division of Therapeutic Proteins, Center for Drug Evaluation and Research, U.S. Food and Drug Administration, Bethesda, MD 20892; e-mail: amy.rosenberg@fda.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal