In childhood acute lymphoblastic leukemia (ALL), a rapid decline of circulating leukemic blasts in response to induction chemotherapy or prednisone is one of the most important prognostic factors, not only for achieving remission but also for relapse-free survival (RFS). However, in acute myeloid leukemia (AML) parameters of chemosensitivity have been restricted mainly to the rapidity of achievement of complete remission (CR) or the assessment of residual leukemic bone marrow blasts during aplasia. We hypothesized that the time to circulating peripheral blood blast clearance, as a potential surrogate for in vivo chemosensitivity, would have prognostic relevance in AML also. In a retrospective analysis of a cohort of 86 adult patients with AML receiving uniform induction and consolidation chemotherapy, we demonstrate that the time to clearance of circulating blasts during induction chemotherapy is an independent prognostic marker of RFS, superseding other known or established risk factors, including karyotype and number of inductions to achieve CR.
Introduction
Although the majority of patients with acute myeloid leukemia (AML) achieve complete remission (CR) following standard induction chemotherapy with an anthracycline and a cytarabine (“3+7 regimen”), most will relapse. The risk of relapse is determined by several patient- and disease-related prognostic factors. Karyotype has the most important independent impact and allows patients to be categorized into risk groups that help define those patients for whom standard consolidation chemotherapy (high-dose cytarabine) is appropriate and those for whom the greater risk of nonrelapse mortality of allogeneic stem cell transplantation in first CR is justified.1 Despite the use of additional prognostic factors such as age and residual bone marrow blasts following the first induction for stratification models, the prognosis of an individual patient cannot yet be estimated accurately.2 There is great promise in identifying molecular mutations such as NUCLEOPHOSMIN-1 that will further refine prognostic assessment, but these techniques are not widely available, or prospectively validated3
In childhood acute lymphoblastic leukemia (ALL), a rapid decline of circulating peripheral blood blasts (PBBs) in response to induction chemotherapy or prednisone has been identified as one of the most important prognostic factors, not only for achieving remission, but also for RFS4,5 In AML, however, parameters of responsiveness to chemotherapy identified have been restricted mainly to the rapidity of achievement of CR or the assessment of residual leukemic bone marrow blasts during aplasia, the latter being an independent prognostic parameter even in patients achieving CR.2,6,7
We hypothesized that the time to PBB clearance, as a potential surrogate for in vivo chemosensitivity, would have prognostic relevance with respect to relapse-free survival (RFS) and overall survival (OS). Therefore, we undertook a retrospective analysis of the prognostic impact of time to PBB clearance on RFS in a cohort of 86 adult AML patients receiving uniform induction and consolidation chemotherapy.
Patients and methods
From 1994 to 2006, outcomes of 86 adult patients with previously untreated AML (excluding acute promyelocytic leukemia) achieving CR and receiving high-dose cytarabine-based consolidation have been included in this retrospective analysis approved by the Institutional Review Board of the Mayo Clinic, Rochester, MN. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki. The characteristics of the study population are outlined in Table 1. The complete blood count (CBC) and initial differential counts were routinely performed on an automated hematology analyzer (Coulter LH750; Beckman Coulter, Fullerton, CA). One-hundred-cell manual slide differential counts were done daily in all cases with circulating blasts (n = 73). No differential counts were performed if the white blood cell (WBC) count was less than 0.5 × 109/L.
Standard remission induction consisted of idarubicin at 12 mg/m2 per day (n = 70) or daunorubicin at 45 mg/m2 per day (n = 16) on days 1 to 3 in conjunction with a continuous infusion of cytarabine at 100 mg/m2 per day on days 1 to 7 (“3+7 regimen”). In those with persistent bone marrow blasts on day 14 (n = 17), a repeat course usually of the same drugs in the same doses, or in some cases as an abbreviated course (“2 + 5 regimen”) were administered. The first consolidation was generally the same as that used to achieve CR. Thereafter, patients were scheduled to receive 3 courses of high-dose cytarabine at 3g/m2 (1.5 g/m2 for those over 60 years old) every 12 hours on days 1, 3, and 5. Those who required 2 courses of the 7 + 3 regimen to achieve remission, and some patients in whom cardiac issues precluded further anthracycline exposure, proceeded directly to high dose cytarabine consolidation (n = 26).
Statistical analyses were performed using StatView (SAS Institute, Cary, NC). A P value less than .05 was considered statistically significant. Actuarial survival curves were plotted using the product-limit estimate according to Kaplan and Meier and compared using the Mantel-Cox test. OS was defined as the time from day 1 of induction to death or last contact. RFS was the time from CR to relapse, death, or last contact. Univariate analysis identified parameters significantly associated with prognosis. These were then combined in a step-wise fashion for a multivariate analysis using a Cox proportional hazard model.
Results and discussion
The median time to blast clearance was 5 days (range: 2-10). The majority (83%) completed at least 3 cycles of the planned consolidation chemotherapy (median: 4 cycles; range 1-5). At the time of analyses, 37 (43%) had died, primarily of relapsed leukemia (97%). The median follow-up for surviving patients was 42.5 months (range: 8-133). The median OS and RFS were 30.2 months (range: 5.5-133) and 14 months (range: 2-131.5), respectively. The median RFS was similar for those with and without PBB at initiation of induction, 14 months (2.5-131.5) and 15 months (2-125.5), respectively.
Prognostic impact of PBB clearance on RFS univariate analysis
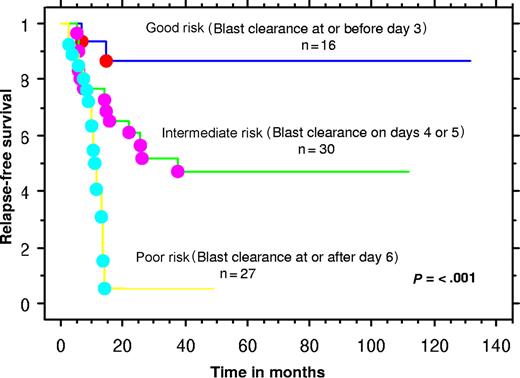
We hypothesized that the time to PBB clearance would have prognostic relevance with respect to RFS. For this analysis, only those with PBBs at initiation of induction chemotherapy (n = 73) were included. We defined the day of blast clearance as the first day after commencing induction chemotherapy that PBBs were no longer present. Separation of patients into 2 groups according to the day to blast clearance was performed for cutoff values for days 2, 3, 4, 5, 6, 7, 8, 9, and 10. Separation according to a cutoff of blast clearance on or before day 5 resulted in the most balanced distribution and the strongest significant difference between each subgroup of 45 and 28 patients, with significantly different rates of relapse of 33% and 79%, respectively (P < .001). Significant differences in RFS and OS were seen between those patients clearing PBB by day 5 or before, versus those requiring longer. We also defined 3 “blast risk groups” as good, intermediate, and poor risk, according to the rate of blast clearance on or before day 3, on days 4 or 5, or on day 6 or beyond, respectively. This provided separation into 3 well-balanced groups (good, intermediate, and poor risk) of 16, 30, and 27 patients with significantly different and escalating rates of relapse of 12.5%, 47%, and 78%, respectively (Figure 1).
Kaplan-Meier plot of “blast risk group”–defined relapse-free survival.
In addition, we calculated the initial absolute blast count (ABC) from the leukocyte count on day one of induction times the blast percent on that day and examined that variable in the analysis. We calculated the median and range of the ABC for the entire cohort and the individual blast risk groups. For the entire cohort (n = 73), the median ABC was 1.26 × 109/L (range: 0.26-78.476). When analyzed according to “blast-risk groups,” a marked difference in the ABC was seen: good (n = 16), 0.65 × 109/L (range: 0.26-5.198); intermediate (n = 30), 0.893 × 109/L (0.26-78.476); and poor (n = 27), 5.208 × 109/L (0.154-46.4691). However, the ABC was not found to be a significant predictor of RFS on univariate analysis (P = .412). The results were the same when the ABC was included in multivariable analysis (P = .823) along with “blast risk group” (P < .001) as a covariate. This analysis further strengthened the significance of the time to circulating blast clearance as an independent prognostic factor.
In addition to number of days to blast clearance, “blast risk group,” and the ABC, we also investigated the potential impact at diagnosis of PBB, age, gender, FAB type, de novo or secondary AML, leukocyte count, platelet count, bone marrow blast percent, LDH, cytogenetic risk group, number of inductions to CR, and weeks from induction to first consolidation on RFS. With univariate analysis, only day to blast clearance (P < .001), “blast risk group” (P < .001), 2 cycles of induction (P = .004), and cytogenetic risk group (P = .028) were significantly associated with RFS. The prognostic effect of each parameter is illustrated by the strength of the chi-square (22.7, 22.7, 8.17, and 7.1 for each, respectively).
Multivariable analysis
For the multivariate analysis, only variables with a P value of .05 or less were considered. Accordingly, a Cox proportional hazards regression model was performed based on either day to blast clearance or “blast risk group” (both reflecting the same parameter), 2 inductions to achieve CR, and cytogenetic risk group. Parameters were added serially to the model based on the strength of their chi-square obtained in univariate analysis. Results demonstrated that only one of the 3 variables (day of blast clearance or “blast-risk group”) was independent of the others and retained an impact on prognosis with respect to RFS (P < .001).
Effect of age
We repeated the entire analysis for all patients aged less than 60 years of age (n = 50). With univariate analysis the following variables were once again confirmed to be of prognostic significance for RFS: “blast risk group” (P = < .001), 2 inductions to CR (P = .02), and cytogenetic risk group (P = .05). Once again, the ABC did not have any prognostic significance (P = .84). On multivariable analysis, only “blast risk group” retained significance (P = .0041). Furthermore, despite the smaller number of patients aged 60 years and above (n = 23), the prognostic value of early blast clearance for RFS was validated also in the advanced age cohort, using either day of blast clearance as a continuous variable (P = .005) or comparing good risk versus bad risk blast groups (P = .05).
Overall survival
Similar univariate analysis was performed for OS in which only “blast-risk group” (P = .024) and number of induction cycles to achieve CR (P = .007), proved to be parameters that had prognostic impact. On multivariable analysis, the “blast-risk group” retained a trend to greater prognostic impact (P = .058) over the number of induction cycles (P = .07).
In summary, we have shown that in AML patients who achieve CR using standard chemotherapy, the time to clearance of PBB is the strongest independent predictor of both RFS and OS. This early assessment of response to therapy provides an in vivo assessment of chemosensitivity and may be a useful means to define the prognosis in individual patients according to treatment administered. Early work by Preisler demonstrated that in vitro chemosensitivity to daunorubicin and cytarabine of clonogenic leukemic cells harvested from marrow specimens of AML patients correlated with outcome of remission induction therapy with these 2 agents.8 Time to PBB clearance, as in childhood ALL, may also provide a potentially powerful tool to predict treatment outcome in AML.4,5 It is a prognostic marker that is readily available to all AML patients with PBB (present in the majority at presentation) and does not require more sophisticated technology than a routine peripheral blood count with manual white cell differential. At this time, decisions regarding postremission therapy for AML patients in CR1 remain a challenge. Our assessment indicates that PBB clearance may provide another means of risk stratification in addition to (and possibly more powerful than) well established prognostic factors such as age, karyotype, and residual bone marrow blasts during aplasia.1,2,9 The strength of the data presented here is compromised by the small numbers included. Therefore, a confirmatory study with a larger number of patients is required to insure that the current findings are generally applicable. The statistical significance of time to blast clearance on RFS, the corroboration of an assessment of chemosensitivity already proven in childhood ALL, and the intuitive implication of measured responsiveness to chemotherapy administered underscore the need for further validation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.A.E. contributed to the design of the study, provided patients, performed the research, contributed to the analysis of the data, and wrote the manuscript. A.T. came up the concept, participated in the design of the study and analysis of the data, provided patients, and reviewed the manuscript. M.S.T. came up the concept, participated in the design of the study, and reviewed the manuscript. C.A.H. reviewed the hematopathology and reviewed the manuscript. R.C.W. participated in the pharmacy aspects of the research and reviewed the manuscript. L.L. and M.R.L. provided patients and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: M. A. Elliott, MD, Division of Hematology/Department of Internal Medicine, Mayo Clinic, 200 First Street, SW, Rochester, MN 55905; e-mail:elliott.michelle@mayo.edu.