Abstract
Calcium is a second messenger for many signaling pathways in B cells, but its role as a receptor ligand has not been well characterized. However, pulses of free calcium were found to cause the rapid release of internal calcium stores in normal human B cells. This response appeared to be mediated by a cell surface protein with receptor properties as it could be blocked by pretreatment with trypsin and with kinase and phospholipase Cγ inhibitors. The calcium receptor on B cells was not the conventional calcium-sensing receptor (CaSR) since B cells did not express CaSR and calcium-induced responses could not be blocked by specific CaSR inhibitors. B-cell responses to extracellular calcium activated phosphoinositide-3 kinase/AKT, calcineurin, extracellular signal regulated kinase, p38 mitogen-activated protein kinase, protein kinase C, Ca2+/calmodulin kinase II, and nuclear factor-κB signaling pathways, and resulted in transcription of the early response gene, CD83. This extracellular calcium sensor enhanced B-cell responses to Toll-like receptor, B-cell receptor, and cytokine receptor agonists. These findings suggest a means by which B cells prepare to engage in immune responses by responding to calcium fluctuations in their environment.
Introduction
Increased intracellular calcium (Cai2+) characterizes many cellular responses to external stimuli,1-3 such as mitogens and receptor ligands.4,5 These changes in Cai2+ propagate primary signals from a receptor and arise from internal Ca2+ stores or an influx of extracellular calcium (Cao2+) through voltage-gated or Ca2+ release–activated Ca2+ channels.6-8 Along with its role as a second messenger, Ca2+ has recently been found to be a receptor ligand. For example, the 36pS cation channel, on hippocampal neurons, elicits action potentials in response to small changes in Cao2+.9 Many cell types respond to Cao2+ via the G-protein–linked calcium-sensing receptor (CaSR),10 including cells from endocrine organs,11 bone (osteoblasts and osteoclasts),10 brain,10 intestine,10 kidney,10 skin,12 ovary,13 as well as fibroblasts.14 CaSR is also expressed by normal hematopoietic cells (including red cell and platelet precursors, monocytes, macrophages,15 and hematopoietic stem cells16 ) and myeloma cells and cell lines.17 Despite the evidence for a CaSR on malignant B cells, normal lymphocytes are not known to express CaSR,15,17 and it is not clear if B cells can respond directly to Cao2+
In the course of studying human B-cell responses to immunomodulatory agents, we observed that Ca2+-containing culture medium was sufficient to cause the release of Cai2+ stores in B cells. Subsequent experiments suggested that this response to changes in extracellular calcium was mediated by a receptor that is not the previously identified CaSR.
Materials and methods
Reagents and antibodies
Phosphate-buffered solution (PBS), Hanks balanced salt solution (HBSS), α- and D-modified Eagle medium (αMEM, DMEM), DMEM/F12, RPMI-1640, and fetal bovine serum (FBS), as well as essential and nonessential amino acids, 0.25% trypsin and glutamine, were purchased from Wisent Bioproducts (St-Bruno, QC). AIM-V medium and the calcium indicator, Indo-1 acetoxymethyl (AM), were obtained from Invitrogen Life Technologies (Burlington, ON). Recombinant human albumin (rHA) was from GTC Biotherapeutics (Framingham, MA). Twenty-five percent human serum albumin (HSA) and immunoglobulin (Ig) (Bayer, Toronto, ON), thrombin (Instrumentation Laboratory, Lexington, MA), heparin (Leo Pharma, Thornhill, ON), human insulin (Eli Lilly Canada, Toronto, ON), chlorpromazine (Bioniche Pharma, Pointe Claire, QC), and cyclosporine A (CsA; Sandoz Canada, Dorval, QC), as well as interleukin-2 (IL-2; Chiron, Emeryville, CA), were purchased from the hospital pharmacy. 2-Mercaptoethanol, CaCl2, MgCl2, ZnCl2, NiSO4, CoCl2, BaCl2, thapsigargin, pyruvate, vitronectin, superfibronectin, chondroitin sulfate, hyaluronic acid, insulin growth factor I, actinomycin D, cycloheximide, NPS2390, verapamil, suramin, guanosine 5′-[β-thio]diphosphate, phorbol 12-myristate 13-acetate (PMA), dichlorofluorescein diacetate (DCFH), and β-actin antibodies were purchased from Sigma-Aldrich (St Louis, MO). Caffeic acid phenethyl ester (CAPE), Bay 11–7082, wedelolactone, PD98059, Ly294002, Gö6976, KN-93, KN-92, SB203580, 1,2-bis(o-aminophenoxy)ethane tetraacetate/acetoxymethyl (BAPTA/AM), PP2, SKF96365, econazole, Syk inhibitor, U73122, and U73343 were from Calbiochem (San Diego, CA). F(ab′)2 goat anti–human IgM was from Jackson Immunoresearch (West Grove, PA). Ethylenediaminetetraacetic acid (EDTA) and genistein were purchased from BioShop (Burlington, ON). Rat brain C6 cells, the Toll-like receptor 7 (TLR-7) agonist, S28690,18,19 and serum amyloid protein were kindly provided by R. Kerbel (University of Toronto, Toronto, ON), R. Miller (3M Pharmaceuticals, St Paul, MN), and R. Gomer (Rice University, Houston, TX), respectively.
Blood samples and cell purification
Heparinized blood (30 mL) was collected from consenting healthy volunteers with approval from the Research Ethics Board, Sunnybrook Health Sciences Center. B cells and monocytes were isolated by negative selection (RosetteSep Human B Cell Enrichment Cocktail and RosetteSep Human Monocyte Enrichment Cocktail; StemCell Technologies, Vancouver, BC) as described previously.20
Cell culture
Isolated peripheral blood mononuclear cells (PBMCs) or B cells (1.5 × 106 cells/mL) were cultured in the indicated serum-free medium containing 2-mercaptoethanol (5 × 10−5 M) in 24-well plates or 5-mL round-bottom tubes (BD Labware, Franklin Lakes, NJ) at 37°C in 5% CO2 for 4 hours, unless indicated otherwise in the figure legends. Signal transduction inhibitors, plasma, albumin, amino acids, pyruvate, glucose, inorganic salts, PMA, and S28690 were added at the indicated concentrations at the beginning of the culture period. Cells were trypsinized by incubation with 0.25% trypsin at 37°C for 30 minutes prior to being washed in PBS and analyzed.
Flow cytometry
Cells were blocked with human intravenous gamma globulin (Bayer) for 5 minutes prior to being stained for surface immunophenotyping with preoptimized volumes of CD54, CD62L, CD69, CD80, CD83, CD86, 41BBL, and CD19, CD14, or CD3 (or matched isotype controls) (BD Pharmingen, Missisauga, ON) for 20 minutes at room temperature. Cells were then washed and resuspended in PBS and 0.25 μg/sample 7-amino-actinomycin D (7AAD) (BD Pharmingen) prior to flow cytometric analysis. Staining of viable nucleated cells was determined by gating on forward scatter properties and 7AAD− cells. Reactive oxygen species (ROSs) in B cells were analyzed by staining with 5 μM DCFH21 and CD19 antibodies for 20 minutes at 37°C, followed by 2 washings with PBS. Ten thousand events were collected per sample with a FACSCalibur (Becton Dickinson, San Jose, CA) and analyzed using FlowJo software (Tree Star, Ashland, OR). Standardization of the flow cytometer was performed before each experiment using Sphero Calibration Particles (BD Pharmingen).
Isolation of total RNA
Purified B cells and monocytes were cultured in AIM-V and rat brain C6 cells were cultured in RPMI with 10% FBS, with or without the indicated inhibitors for 1 hour. Cells were then harvested and washed in PBS. Total RNA was extracted using the RNeasy kit (Qiagen, Missisauga, ON) according to the manufacturer's instructions. To remove contaminating genomic DNA, 10 μg of the total RNA preparation was incubated with 10 U RNase-free DNase I (Promega, Madison, WI) for 30 minutes at 37°C. Total RNA concentration was determined in a spectrophotometer at 260 nm.
Semiquantitative reverse-transcription–polymerase chain reaction
cDNA was synthesized as described previously20 or using a one-step reverse-transcription–polymerase chain reaction (RT-PCR) system (Invitrogen Life Technologies) according to the manufacturer's instructions. The following primers were used: CD83 forward, 5′-GTTATTGGAGGGTGGTGAAGAGAGG-3′; CD83 reverse, 5′-GTGAGGAGTCACTAGCCCTAAATGC-3′; CaSR forward, 5′-GGCTCCATCGTGTTTAAGGA-3′; CaSR reverse, 5′-CTCCGTCCACGACAGAAACT-3′; hypoxanthine-guanine phosphoribosyltransferase (HPRT) forward, 5′-GAGGATTTGGAAAGGGTGTT-3′; HPRT reverse, 5′-ACAATAGCTCTTCAGTCTGA-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward, 5′-GAAGGTGAAGGTCGGAGTC-3′; and GAPDH reverse, 5′-GAAGATGGTGATGGGATTTC-3′. These primer sets yield RT-PCR products of 448 bp, 352 bp, 241 bp, and 227 bp for CD83, CaSR, HPRT, and GAPDH, respectively. PCR reactions for CD83 and GAPDH were performed using 3 μL cDNA in the presence of 50 mM KCl, 20 mM Tris-HCl (pH 8.4), 1.5 mM MgCl2, 0.2 mM dNTP, 1 μM of each primer, and 1 U Taq DNA polymerase (Invitrogen Life Technologies) in a total volume of 50 μL using a Flexigene Thermal Cycler (Techne, Princeton, NJ). Reactions were cycled 35 times after initial denaturation (95°C, 15 minutes) with the following parameters: denaturation at 90°C for 30 seconds, annealing of primers at 60°C for 30 seconds, and extension at 72°C for 30 seconds. Using the one-step RT-PCR kit, reverse transcription of 2 μg CaSR or HPRT RNA was performed at 50°C for 30 minutes and PCR reactions were cycled 40 times with an annealing temperature of 55°C. Final PCR products were electrophoresed on a 1.5% agarose gel.
Calcium flux assays
PBMCs or C6 cells were incubated with Indo-1 AM (5 μM) in serum-free, Ca2+-free PBS (unless indicated otherwise) for 30 minutes at 37°C. CD19 antibodies were then added to PBMCs and the cells were incubated for an additional 15 minutes at room temperature. Cells were then washed and resuspended at 1 × 106 cells/mL in Ca2+-free PBS.
In response to the indicated stimulus, changes in the concentrations of free Cai2+ in viable Indo-1 AM–labeled cells maintained at 37°C (using a water bath and tube jacket) were analyzed at rates of approximately 500 events/second using a fixed alignment, 18-color, 4-laser LSRII flow cytometer (Becton Dickinson). The baseline ratio of free Ca2+/bound Ca2+ was established over the first 45 seconds, after which the tube was removed, the stimulant added, and the tube replaced. Recording continued for up to 400 seconds. The data acquired were analyzed using FlowJo.
Immunoblotting
Proteins were extracted and immunoblotting with antibodies against phosphorylated signal transducer and activator of transcription 1 (STAT1), STAT3, inhibitor of nuclear factor κB (NF-κB) (IκB), AKT, extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (MAPK), Ca2+/calmodulin (CaM) kinase II (CaMKII), serine-phosphorylated PKC substrates, phosphorylated tyrosine, and total IκB (Cell Signaling Technology, Danvers, MA), as well as CaSR (Abcam, Cambridge, MA), was performed as previously described.20 Supersignal West Pico Luminal Enhancer and Stable Peroxide Solution (Pierce, Rockford, IL) created the chemiluminescent signal that was detected using a Syngene InGenius system (Syngene, Cambridge, United Kingdom).
Cytometric bead array
Isolated B cells were cultured in PBS with or without 250 μM or 500 μM CaCl2 for 4 hours prior to being washed and resuspended in AIM-V with or without IL-2 and S28690 for 24 hours. Supernatants were then collected and tumor necrosis factor α (TNF-α) secretion was analyzed by Cytometric Bead Array (BD Pharmingen) according to the manufacturer's instructions.
Statistical analysis
Paired Student t tests were used to determine P values for differences between sample means.
Results
Cai2+ release by B cells responding to Cao2+
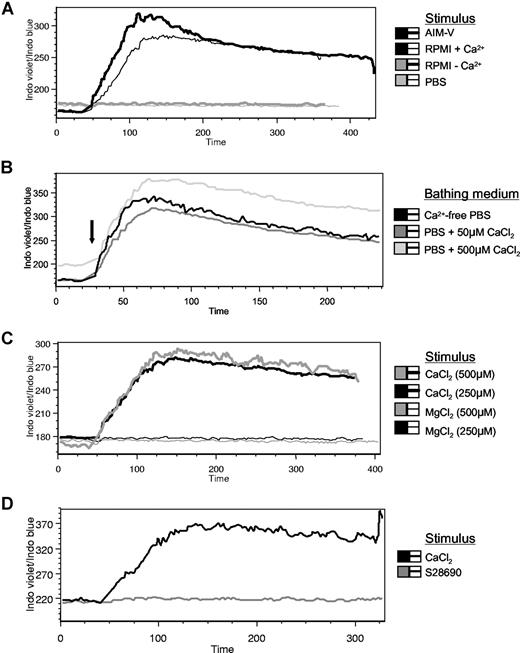
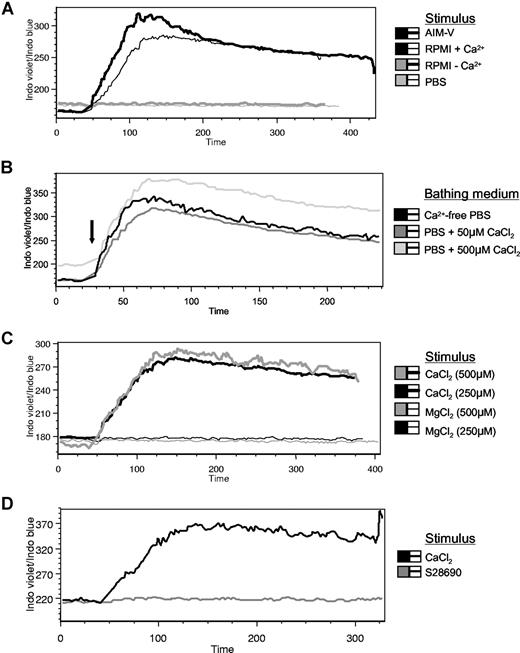
While measuring Cai2+ levels in B cells exposed to a number of immunomodulatory agents, we observed that Ca2+-containing media alone caused rapid increases in Cai2+ (Figure 1A). Addition of CaCl2 to cells in Ca2+-free PBS, or to PBS containing various amounts of CaCl2, produced similar increases in free Cai2+ concentrations (Figure 1B) (which could not be replicated by other cations, such as Mg2+ [Figure 1C], Zn2+, Ni2+, Co2+, or Ba2+ [data not shown], or by stimuli known to signal in a Ca2+-independent fashion, such as the TLR7 agonist S2869022 [Figure 1D]). Of concern was that these observations were due simply to toxicity of Cao2+23,24 causing a calcium leak across damaged plasma membranes or that internal calcium stores had been depleted resulting in open Ca2+ release–activated Ca2+ channels.25 However, very little cell death was seen when cells were cultured in Ca2+-containing media, such as AIM-V, for several days.20 Moreover, stimulation of B cells bathed in Ca2+-free or Ca2+-containing medium for short periods of time (30-45 minutes) with thapsigargin resulted in Cai2+ release (4.2 ± 0.6-fold increases in the Indo violet/Indo blue ratio upon thapsigargin treatment [n = 20] compared with 2.3 ± 0.5-fold increases upon CaCl2 treatment [n = 3]), indicating that internal calcium stores were intact. In addition, the patterns of Cai2+ changes in Figure 1A-D were more consistent with the rapid, biphasic changes caused by activation of a cell surface receptor, rather than influx through open channels on the plasma membrane.26 Note that addition of 500 μM CaCl2 to cells bathed in nontoxic levels of calcium (50 or 500 μM) caused the release of Cai2+ stores (Figure 1B), suggesting that B cells sensed changes in Cao2+ and not merely the presence or absence of the ion.
Effects of Cao2+ on Cai2+ release in human B cells. AIM-V, RPMI with or without calcium, or PBS (500 μL) was added to Indo-1 AM–labeled PBMCs bathed in 750 μL Ca2+-free PBS (A), or 500 μM CaCl2 was added to cells bathed in PBS containing various levels of calcium (B-D). Alternatively, MgCl2 (C) or a TLR-7 agonist, S28690 (1 μg/mL), (D) was added to other Indo-1–loaded cells in Ca2+-free PBS.  indicates addition of the stimulus during the 6-minute collection period of calcium flux data. Using Indo-1 AM, bound Ca2+ is detected at approximately 400 nM (blue) and free Ca2+ is detected at approximately 475 nm (violet). The data are presented as the ratio of Indo violet/Indo blue as a function of time for the B-cell population (gated on CD19+ cells) and represent 8 independent experiments.
indicates addition of the stimulus during the 6-minute collection period of calcium flux data. Using Indo-1 AM, bound Ca2+ is detected at approximately 400 nM (blue) and free Ca2+ is detected at approximately 475 nm (violet). The data are presented as the ratio of Indo violet/Indo blue as a function of time for the B-cell population (gated on CD19+ cells) and represent 8 independent experiments.
Effects of Cao2+ on Cai2+ release in human B cells. AIM-V, RPMI with or without calcium, or PBS (500 μL) was added to Indo-1 AM–labeled PBMCs bathed in 750 μL Ca2+-free PBS (A), or 500 μM CaCl2 was added to cells bathed in PBS containing various levels of calcium (B-D). Alternatively, MgCl2 (C) or a TLR-7 agonist, S28690 (1 μg/mL), (D) was added to other Indo-1–loaded cells in Ca2+-free PBS.  indicates addition of the stimulus during the 6-minute collection period of calcium flux data. Using Indo-1 AM, bound Ca2+ is detected at approximately 400 nM (blue) and free Ca2+ is detected at approximately 475 nm (violet). The data are presented as the ratio of Indo violet/Indo blue as a function of time for the B-cell population (gated on CD19+ cells) and represent 8 independent experiments.
indicates addition of the stimulus during the 6-minute collection period of calcium flux data. Using Indo-1 AM, bound Ca2+ is detected at approximately 400 nM (blue) and free Ca2+ is detected at approximately 475 nm (violet). The data are presented as the ratio of Indo violet/Indo blue as a function of time for the B-cell population (gated on CD19+ cells) and represent 8 independent experiments.
Receptor-mediated sensing of Cao2+ changes by B cells
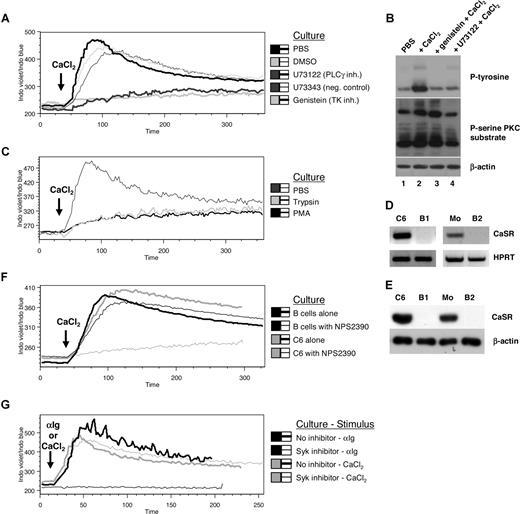
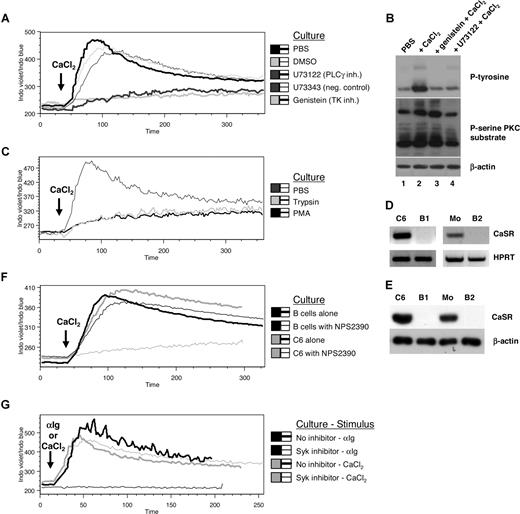
Since calcium channel inhibitors such as SKF96365,27 econazole,27 and verapamil28 could not block release of Cai2+ stores caused by Cao2+ (data not shown), other signal transduction inhibitors (including G-protein, tyrosine kinase, and phospholipase Cγ [PLCγ] inhibitors) were tested for their ability to block responses to Cao2+. Strikingly, tyrosine kinase (genistein) and PLCγ (U73122) inhibitors abrogated Cai2+ release (Figure 2A), suggesting that the response to Cao2+ may involve a signaling cascade from a Ca2+-activated extracellular receptor or receptors. Consistent with this, Cao2+-induced tyrosine phosphorylation was inhibited by genistein (Figure 2B lanes 2-3 top panel), and PKC substrate phosphorylation (which correlates with PLC activity) was abrogated by U73122 (Figure 2B lanes 2,4 middle panel). Cao2+-induced Cai2+ release was also inhibited when cells were pretreated with trypsin (further suggesting that the response was mediated by a cell surface protein or proteins) (Figure 2C). In addition, PMA inhibited Cao2+-induced Cai2+ release, suggesting negative regulation by activated PKC29-31 (Figure 2C).
Effects of CaSR, signal transduction inhibitors, and trypsinization on Cao2+-mediated Cai2+ changes. (A) PBMCs were treated with U73122 (10 μM) and its negative control U73343 (10 μM), genistein (50 μg/mL), or DMSO (2 μL/mL) for 30 minutes prior to adding Indo-1 AM and CD19 PE antibodies. Calcium flux data were obtained upon the addition of 500 μM CaCl2 ( ) in B cells over a 6-minute period. (B) Isolated B cells were cultured in PBS with or without genistein or U73122 for 30 minutes prior to adding CaCl2 (500 μM) for 2 minutes followed by cell lysis. Samples were then electrophoresed and immunoblotted for phosphorylated (P)–tyrosine, P-serine PKC substrate, and β-actin (loading control). (C) B cells were treated with trypsin or PMA (16 nM) for 30 minutes and 4 hours, respectively, in PBS prior to calcium flux analysis. (D,E) RNA and protein were extracted from C6 cells, isolated monocytes (Mo's), and B cells from 2 different donors (B1, B2) prior to one-step RT-PCR (D) or immunoblotting (E) for CaSR expression. (F) PBMCs or rat brain C6 cells were treated with NPS2390 (20 μM) for 30 minutes prior to calcium flux analysis. (G) B cells were treated with Syk inhibitor (20 μM) for 30 minutes prior to calcium flux analysis in response to 10 μg/mL anti-Ig or 500 μM CaCl2. Data are representative of 3 different samples.
) in B cells over a 6-minute period. (B) Isolated B cells were cultured in PBS with or without genistein or U73122 for 30 minutes prior to adding CaCl2 (500 μM) for 2 minutes followed by cell lysis. Samples were then electrophoresed and immunoblotted for phosphorylated (P)–tyrosine, P-serine PKC substrate, and β-actin (loading control). (C) B cells were treated with trypsin or PMA (16 nM) for 30 minutes and 4 hours, respectively, in PBS prior to calcium flux analysis. (D,E) RNA and protein were extracted from C6 cells, isolated monocytes (Mo's), and B cells from 2 different donors (B1, B2) prior to one-step RT-PCR (D) or immunoblotting (E) for CaSR expression. (F) PBMCs or rat brain C6 cells were treated with NPS2390 (20 μM) for 30 minutes prior to calcium flux analysis. (G) B cells were treated with Syk inhibitor (20 μM) for 30 minutes prior to calcium flux analysis in response to 10 μg/mL anti-Ig or 500 μM CaCl2. Data are representative of 3 different samples.
Effects of CaSR, signal transduction inhibitors, and trypsinization on Cao2+-mediated Cai2+ changes. (A) PBMCs were treated with U73122 (10 μM) and its negative control U73343 (10 μM), genistein (50 μg/mL), or DMSO (2 μL/mL) for 30 minutes prior to adding Indo-1 AM and CD19 PE antibodies. Calcium flux data were obtained upon the addition of 500 μM CaCl2 ( ) in B cells over a 6-minute period. (B) Isolated B cells were cultured in PBS with or without genistein or U73122 for 30 minutes prior to adding CaCl2 (500 μM) for 2 minutes followed by cell lysis. Samples were then electrophoresed and immunoblotted for phosphorylated (P)–tyrosine, P-serine PKC substrate, and β-actin (loading control). (C) B cells were treated with trypsin or PMA (16 nM) for 30 minutes and 4 hours, respectively, in PBS prior to calcium flux analysis. (D,E) RNA and protein were extracted from C6 cells, isolated monocytes (Mo's), and B cells from 2 different donors (B1, B2) prior to one-step RT-PCR (D) or immunoblotting (E) for CaSR expression. (F) PBMCs or rat brain C6 cells were treated with NPS2390 (20 μM) for 30 minutes prior to calcium flux analysis. (G) B cells were treated with Syk inhibitor (20 μM) for 30 minutes prior to calcium flux analysis in response to 10 μg/mL anti-Ig or 500 μM CaCl2. Data are representative of 3 different samples.
) in B cells over a 6-minute period. (B) Isolated B cells were cultured in PBS with or without genistein or U73122 for 30 minutes prior to adding CaCl2 (500 μM) for 2 minutes followed by cell lysis. Samples were then electrophoresed and immunoblotted for phosphorylated (P)–tyrosine, P-serine PKC substrate, and β-actin (loading control). (C) B cells were treated with trypsin or PMA (16 nM) for 30 minutes and 4 hours, respectively, in PBS prior to calcium flux analysis. (D,E) RNA and protein were extracted from C6 cells, isolated monocytes (Mo's), and B cells from 2 different donors (B1, B2) prior to one-step RT-PCR (D) or immunoblotting (E) for CaSR expression. (F) PBMCs or rat brain C6 cells were treated with NPS2390 (20 μM) for 30 minutes prior to calcium flux analysis. (G) B cells were treated with Syk inhibitor (20 μM) for 30 minutes prior to calcium flux analysis in response to 10 μg/mL anti-Ig or 500 μM CaCl2. Data are representative of 3 different samples.
The Cao2+-sensing mechanism in B cells was found to be independent of CaSR or other G-protein–coupled receptors (GPCRs). In contrast to rat brain C6 cells or monocytes, B cells did not express CaSR mRNA (Figure 2D) or protein (Figure 2E). The CaSR inhibitor, NPS2390,11 did not inhibit Cao2+-induced Cai2+ release in B cells, whereas it was able to block Cao2+-induced Cai2+ release in C6 cells that expressed the CaSR (Figure 2F). In addition, Cao2+-induced Cai2+ release was not abrogated by the G-protein inhibitors suramin and guanosine 5′-[β-thio]diphosphate (data not shown), further arguing against a role for the CaSR (which is a GPCR) in B-cell Cao2+ sensing.
An alternative explanation for the apparent activation of a specific calcium-sensing receptor on B cells could be that the 3-dimensional conformations of other cell surface receptors or proteins were altered by binding calcium, either causing signal transduction directly or disrupting the binding of negative regulatory molecules and producing signal transduction and Cai2+ release. Candidate receptors on B cells that might cause Cai2+ release in this manner upon binding Cao2+ include the B-cell receptor (BCR),32 TLRs,33 and integrins.34 As shown in Figure 1D, the activation of at least one TLR (TLR7) on B cells did not lead to the release of Cai2+ stores, suggesting that TLR modification by Cao2+ was not causing Cao2+-induced Cai2+ release. Similarly, BCR cross-linking is known to induce the release of Cai2+ stores (Figure 2G) via the activation of PLCγ through Syk.32 However, a Syk inhibitor,35 which is otherwise capable of inhibiting BCR cross-linking–induced Cai2+ release, failed to prevent Cao2+-induced Cai2+ release (Figure 2G), suggesting that the binding of Cao2+ did not simply increase tonic BCR signaling.36 Other candidate receptors, such as integrins, which signal through Src,34 were excluded, since the Src inhibitor PP2 did not affect Cao2+-induced Cai2+ release (data not shown). It remains possible that other receptors on B cells are conformationally altered and activated by binding Cao2+, but the results are consistent with the presence of a specific calcium receptor on B cells, which is not the CaSR.
Induction of CD83 expression by Cao2+ pulses
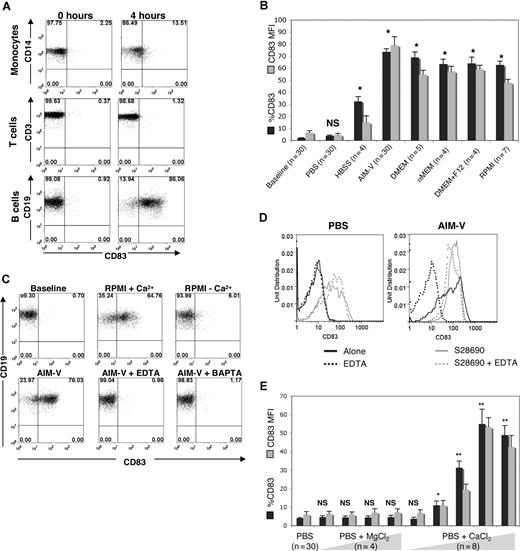
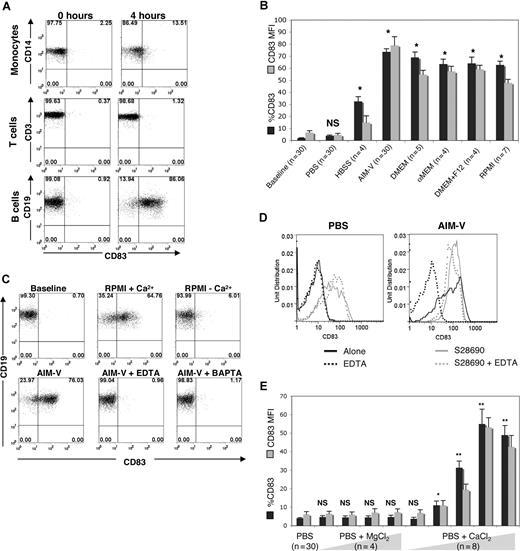
To determine whether Cao2+-induced Cai2+ release caused phenotypic changes in B cells, the expression of a number of surface molecules was studied by flow cytometry. Immediately after isolation, B cells expressed low surface levels of CD54, CD62L, CD69, CD80, CD83, and CD86. Upon exposure to Cao2+, high CD83 surface expression was noted within 4 hours (Figure 3A bottom panels). The percentage of B cells exhibiting CD83 at 4 hours ranged from 57.5% to 93.8% (mean of 74.2%; n = 30). In contrast to B cells, CD83 did not increase on CD3+ T cells or CD14+ monocytes cultured under similar conditions (Figure 3A top and middle panels). Expression of the activation marker CD69 also increased on B cells, while CD54, CD62L, CD80, or CD86 did not change (data not shown).
Effects of Cao2+ on CD83 expression on human B cells. (A) Flow cytometric analysis of CD83 in monocytes, T cells, and B cells (indicated by costaining with CD14, CD3, and CD19 antibodies, respectively) immediately after isolation (0 h) and 4 hours after culture in AIM-V. The example shown is representative of 8 different experiments. (B) PBMCs were cultured in different media (PBS without Ca2+ or Mg2+, HBSS with Ca2+ and Mg2+, AIM-V, DMEM, αMEM, DMEM + F12, or RPMI) for 4 hours and then analyzed by flow cytometry. Statistical analysis was performed on percentage CD83 expression of samples cultured in media compared with baseline. *P < .001. (C) PBMCs were cultured in RPMI with and without calcium, with AIM-V alone, or with AIM-V and EDTA (10 μM) or BAPTA (10 μM) for 4 hours prior to flow cytometric analysis of the B cells (gated on CD19+ cells). The results were similar for 6 additional samples. (D) CD83 on gated B cells from PBMCs cultured in calcium-free PBS (left panel) or AIM-V (right panel) and treated with EDTA, S28690, or EDTA + S28690 for 4 hours prior to analysis by flow cytometry. (E) PBMCs were cultured in PBS alone, with MgCl2 (50, 250, 500, or 1000 μM) or with CaCl2 (5, 50, 250, 500, 1000 μM) for 4 hours, and then B cells were analyzed by flow cytometry. The averages and standard errors of the percentage of cells expressing CD83, and the mean fluorescent intensity (MFI) of expression, from the indicated numbers of samples, are shown. The statistical significance of the differences between percentage CD83 expression of MgCl2- or CaCl2-treated compared with untreated samples is indicated. *P < .05; **P < .001; n = 5.
Effects of Cao2+ on CD83 expression on human B cells. (A) Flow cytometric analysis of CD83 in monocytes, T cells, and B cells (indicated by costaining with CD14, CD3, and CD19 antibodies, respectively) immediately after isolation (0 h) and 4 hours after culture in AIM-V. The example shown is representative of 8 different experiments. (B) PBMCs were cultured in different media (PBS without Ca2+ or Mg2+, HBSS with Ca2+ and Mg2+, AIM-V, DMEM, αMEM, DMEM + F12, or RPMI) for 4 hours and then analyzed by flow cytometry. Statistical analysis was performed on percentage CD83 expression of samples cultured in media compared with baseline. *P < .001. (C) PBMCs were cultured in RPMI with and without calcium, with AIM-V alone, or with AIM-V and EDTA (10 μM) or BAPTA (10 μM) for 4 hours prior to flow cytometric analysis of the B cells (gated on CD19+ cells). The results were similar for 6 additional samples. (D) CD83 on gated B cells from PBMCs cultured in calcium-free PBS (left panel) or AIM-V (right panel) and treated with EDTA, S28690, or EDTA + S28690 for 4 hours prior to analysis by flow cytometry. (E) PBMCs were cultured in PBS alone, with MgCl2 (50, 250, 500, or 1000 μM) or with CaCl2 (5, 50, 250, 500, 1000 μM) for 4 hours, and then B cells were analyzed by flow cytometry. The averages and standard errors of the percentage of cells expressing CD83, and the mean fluorescent intensity (MFI) of expression, from the indicated numbers of samples, are shown. The statistical significance of the differences between percentage CD83 expression of MgCl2- or CaCl2-treated compared with untreated samples is indicated. *P < .05; **P < .001; n = 5.
A number of commercially available tissue culture media were studied to determine whether Cao2+-mediated CD83 expression required factors other than Ca2+. Media containing various levels of Ca2+, Mg2+, Na+, K+, amino acids, vitamins, glutamine, glucose, pyruvate, and phenol red (AIM-V, DMEM, αMEM, DMEM + F12, and RPMI-1640) all induced high expression of CD83 on the cell surface, whereas media containing little or none of these components (Ca2+/Mg2+-free PBS and HBSS) did not (Figure 3B). Further testing revealed that only the presence of Ca2+ (which was supplied concomitantly with Mg2+ in the media) was associated with increased CD83 expression, whereas phenol red, amino acids, pyruvate, and glucose appeared to have little or no effect on CD83 (Table 1).
To provide further evidence for a role of Cao2+-induced Cai2+ release in changing CD83 expression, the effects of RPMI ± Ca2+, EDTA, and the Cai2+ chelator BAPTA/AM were tested. Only RPMI containing Ca2+ induced CD83 expression, whereas Ca2+ chelation with EDTA and BAPTA/AM abrogated CD83 expression (Figure 3C). As a control, the TLR7 agonist S28690 (which signals through calcium-independent pathways22 [Figures 1D and 7]) induced strong CD83 expression on B cells in the presence of EDTA, suggesting that the effects of EDTA on Ca2+-induced CD83 expression were specifically due to chelation of Cao2+ (Figure 3D). Cao2+ induced CD83 expression in a dose-dependent manner, whereas MgCl2 did not (Figure 3E). Taken together, these results suggested that exposure to increased levels of Cao2+ was responsible for the release of Cai2+ and the expression of CD83 on the B-cell surface.
Transcriptional regulation of Cao2+-induced CD83 expression
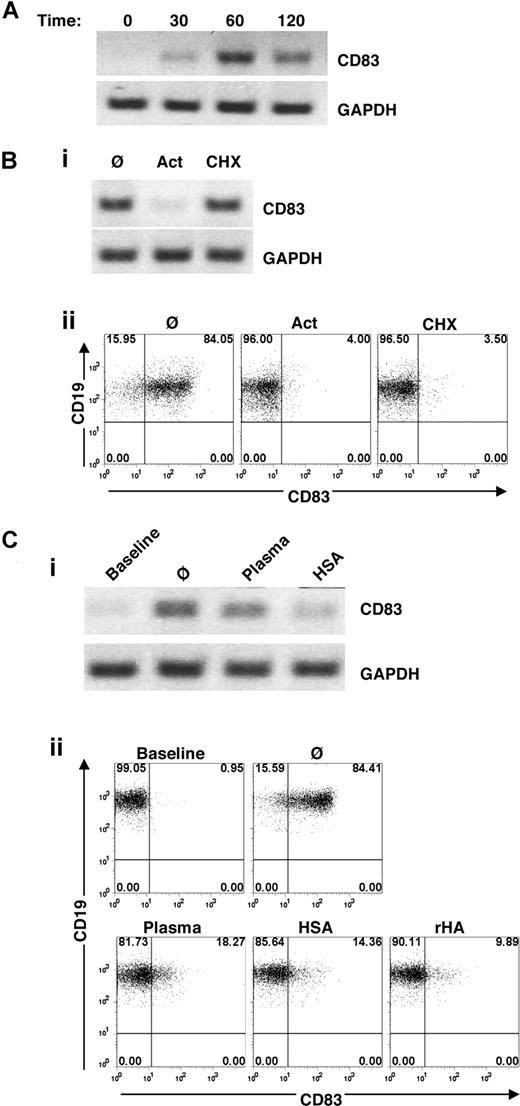
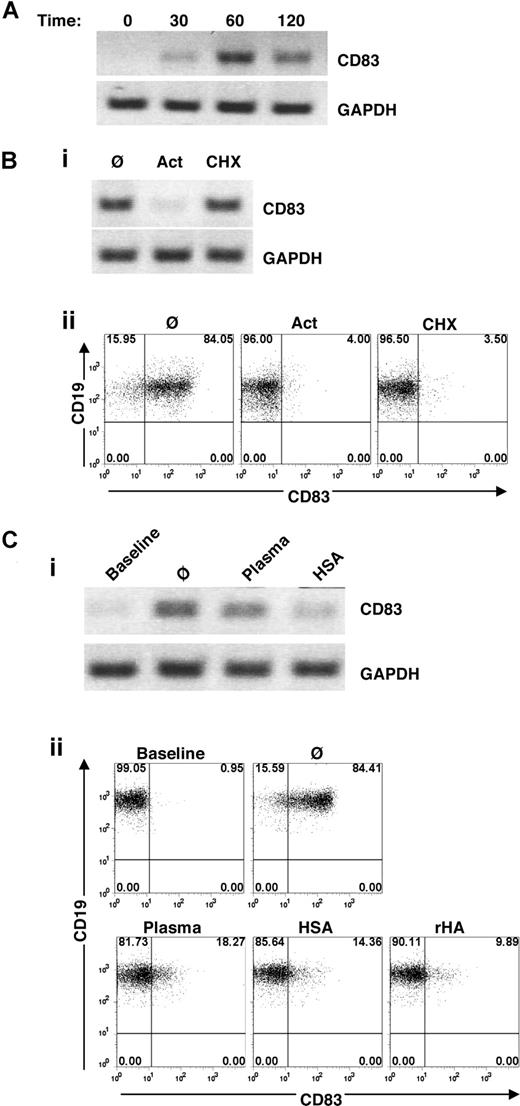
To determine whether the increased expression of CD83 on B cells caused by Cao2+ was transcriptionally regulated, CD83 mRNA levels were measured by RT-PCR. CD83 message was expressed within 30 minutes and peaked after 1 hour of exposure to Cao2+ (Figure 4A).
Transcriptional regulation of Cao2+-induced CD83 expression on human B cells. (A) Time course of CD83 gene expression (as determined by RT-PCR) in isolated B cells cultured in AIM-V for 0, 30, 60, or 120 minutes. The example is representative of 5 additional samples. (B) Effects of transcriptional and translational inhibitors on CD83 expression. Isolated B cells were cultured for 1 hour in AIM-V alone (Ø) or with actinomycin D (Act) (100 ng/mL) or cycloheximide (CHX) (100 μM) prior to total RNA extraction and RT-PCR (i) and PBMCs were cultured for 4 hours prior to flow cytometric analysis of CD83 expression in B cells (CD19+-gated PBMCs) (ii). (C) Effects of plasma and albumin on CD83 expression. (i) Purified B cells were cultured in AIM-V with plasma or HSA for 1 hour prior to RNA extraction and RT-PCR. (ii) Flow cytometric analysis of CD83 on B cells (gated on CD19+ cells in PBMC populations) cultured for 4 hours in AIM-V with 30% autologous plasma, HSA (50 mg/mL), or rHA (50 mg/mL). The data shown are representative of 7 different experiments.
Transcriptional regulation of Cao2+-induced CD83 expression on human B cells. (A) Time course of CD83 gene expression (as determined by RT-PCR) in isolated B cells cultured in AIM-V for 0, 30, 60, or 120 minutes. The example is representative of 5 additional samples. (B) Effects of transcriptional and translational inhibitors on CD83 expression. Isolated B cells were cultured for 1 hour in AIM-V alone (Ø) or with actinomycin D (Act) (100 ng/mL) or cycloheximide (CHX) (100 μM) prior to total RNA extraction and RT-PCR (i) and PBMCs were cultured for 4 hours prior to flow cytometric analysis of CD83 expression in B cells (CD19+-gated PBMCs) (ii). (C) Effects of plasma and albumin on CD83 expression. (i) Purified B cells were cultured in AIM-V with plasma or HSA for 1 hour prior to RNA extraction and RT-PCR. (ii) Flow cytometric analysis of CD83 on B cells (gated on CD19+ cells in PBMC populations) cultured for 4 hours in AIM-V with 30% autologous plasma, HSA (50 mg/mL), or rHA (50 mg/mL). The data shown are representative of 7 different experiments.
CD83 message was abrogated by the transcriptional inhibitor, actinomycin D, but not the translational inhibitor, cycloheximide (Figure 4Bi). Cell surface expression of CD83 protein was also blocked by actinomycin D (Figure 4Bii), suggesting that the spontaneous increase in CD83 was due to de novo RNA transcription.
Abrogation of Cao2+-induced CD83 expression by albumin
In striking contrast to the observations in serum-free media, CD83 expression did not increase when cells were cultured in the presence of 10% FBS (data not shown) or 30% plasma (Figure 4C). A number of serum and plasma components were tested to determine the factor responsible for preventing the Cao2+-induced increase in CD83 expression. Heparin, thrombin, vitronectin, superfibronectin, chondroitin sulfate, hyaluronic acid, insulin, insulin growth factor I, Ig, and serum amyloid protein did not inhibit Cao2+-induced CD83 expression (data not shown). However, albumin (both HSA and rHA) strongly abrogated CD83 message and surface protein expression in AIM-V (Figure 4C) and PBS containing Ca2+ (data not shown), consistent with its well-known ability to bind and buffer Cao2+.37-39
Role of CaM, PI3K, PKC, and NF-κB in Cao2+-induced CD83 expression
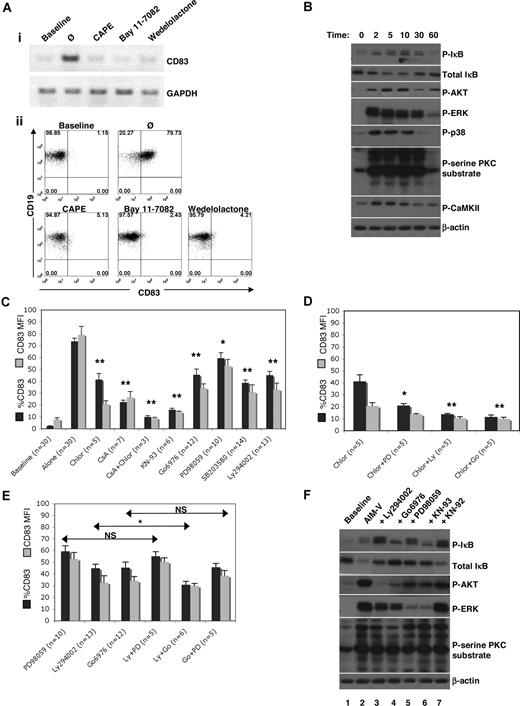
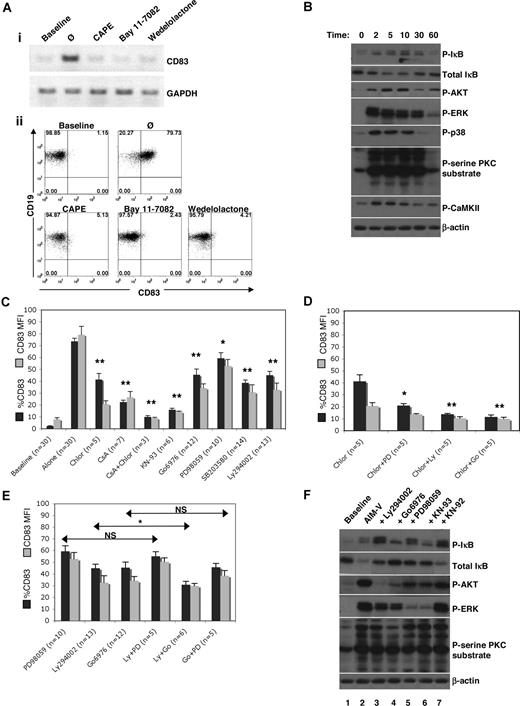
Signaling pathways connecting the putative Cao2+-sensing receptor on B cells to subsequent transcription of CD83 were then investigated. The CD83 promoter contains 4 SP1-binding sites, and one NF-κB element.40 The NF-κB inhibitors CAPE, Bay 11-7082, and wedelolactone41-43 inhibited Cao2+-induced expression of CD83 mRNA (Figure 5Ai) and protein (Figure 5Aii), in contrast to the SP1 inhibitor, mithramycin44,45 (data not shown), suggesting that Cao2+ sensing was mediated by NF-κB.
Signaling molecules involved in responses to Cao2+. (A) Effects of NF-κB inhibitors on CD83 expression. (i) Isolated B cells were cultured for 1 hour with CAPE (25 μg/mL), Bay 11–7082 (1 μM), or wedelolactone (150 μM) prior to RNA extraction and RT-PCR. (ii) PBMCs were cultured for 4 hours prior to flow cytometric analysis of CD83 expression in B cells (CD19+-gated PBMCs). Baseline CD83 expression was analyzed immediately after cell isolation. (B) Isolated B cells were cultured in AIM-V for 0, 2, 5, 10, 30, and 60 minutes prior to lysis. Samples were then electrophoresed and immunoblotted for phosphorylated (P)–IκB, total IκB, P-AKT, P-ERK, P-p38 MAPK, P-serine PKC substrate, P-CaMKII, and β-actin (loading control). Similar results were obtained using samples from 3 different donors. (C-E) Cells were incubated in AIM-V with chlorpromazine (Chlor, 2 μM), CsA (1 μg/mL), KN-93 (20 μM), Gö6976 (1 μM), PD98059 (20 μM), U0126 (20 μM), SB203580 (2 μg/mL), Ly294002 (20 μM), or wortmannin (100 nM) for 4 hours. Statistical analysis was performed on percentage CD83 expression of treated compared with untreated (“alone”) samples (C) or of samples treated with a combination of inhibitors compared with samples treated with one inhibitor (D,E). Data were obtained by gating on CD19+ cells in PBMC populations. *P < .05; **P < .001. (F) Protein extracts were obtained from isolated B cells immediately after isolation (baseline) or after 10 minutes in culture in AIM-V alone or with LY294002, Gö6976, PD98059, KN-93, or its inactive analog KN-92, and then analyzed by immunoblotting. Similar data were obtained from 2 additional donors.
Signaling molecules involved in responses to Cao2+. (A) Effects of NF-κB inhibitors on CD83 expression. (i) Isolated B cells were cultured for 1 hour with CAPE (25 μg/mL), Bay 11–7082 (1 μM), or wedelolactone (150 μM) prior to RNA extraction and RT-PCR. (ii) PBMCs were cultured for 4 hours prior to flow cytometric analysis of CD83 expression in B cells (CD19+-gated PBMCs). Baseline CD83 expression was analyzed immediately after cell isolation. (B) Isolated B cells were cultured in AIM-V for 0, 2, 5, 10, 30, and 60 minutes prior to lysis. Samples were then electrophoresed and immunoblotted for phosphorylated (P)–IκB, total IκB, P-AKT, P-ERK, P-p38 MAPK, P-serine PKC substrate, P-CaMKII, and β-actin (loading control). Similar results were obtained using samples from 3 different donors. (C-E) Cells were incubated in AIM-V with chlorpromazine (Chlor, 2 μM), CsA (1 μg/mL), KN-93 (20 μM), Gö6976 (1 μM), PD98059 (20 μM), U0126 (20 μM), SB203580 (2 μg/mL), Ly294002 (20 μM), or wortmannin (100 nM) for 4 hours. Statistical analysis was performed on percentage CD83 expression of treated compared with untreated (“alone”) samples (C) or of samples treated with a combination of inhibitors compared with samples treated with one inhibitor (D,E). Data were obtained by gating on CD19+ cells in PBMC populations. *P < .05; **P < .001. (F) Protein extracts were obtained from isolated B cells immediately after isolation (baseline) or after 10 minutes in culture in AIM-V alone or with LY294002, Gö6976, PD98059, KN-93, or its inactive analog KN-92, and then analyzed by immunoblotting. Similar data were obtained from 2 additional donors.
In the canonical NF-κB pathway,46 components of the IκB kinase (IKK) complex are phosphorylated and activated by a number of kinases. Activated IKK then phosphorylates IκB, causing it to be ubiquinated and degraded by the proteasome, releasing NF-κB dimers that then translocate to the nucleus to act as transcription factors. Analysis of several signaling pathways (by immunoblotting for phosphorylated intermediates) revealed that AKT, ERK, p38 MAPK, PKC, and CaMKII all became strongly activated prior to the phosphorylation and degradation of IκB (Figure 5B). CaM and calcineurin (CN) appeared to be especially important mediators as CD83 expression was strongly inhibited by the CaM inhibitor, chlorpromazine, and the CN inhibitor, CsA (Figure 5C). Furthermore, the inhibitory effects were additive, suggesting that CaM and CN acted in parallel. Inhibition of CaMKII by KN-93 completely abrogated CD83 expression (Figure 5C). Inhibitors of the classical PKC isozymes (Gö6976), ERK (PD98059), p38 MAPK (SB203580), and PI3K (Ly294002) also decreased Cao2+-mediated CD83 expression, although not to the same extent as chlorpromazine and CsA, or KN-93 (Figure 5C). Jun N-terminal kinase, mammalian target of rapamycin, and Bruton tyrosine kinase were found to not be significantly involved in controlling Cao2+-mediated CD83 expression (data not shown). By using these inhibitors in combination, CaM was found to signal in parallel with PI3K, ERK, and PKC, as additive effects were noted on the inhibition of CD83 expression (Figure 5D). PI3K and ERK signaled sequentially (since the effects of Ly294002 and PD98059 were the same as PD98059 alone), as did PKC and ERK (since the effects of Gö6976 and PD98059 were the same as Gö6976 alone) (Figure 5E). Furthermore, PKC was found to signal upstream of AKT (since Gö6976 inhibited phosphorylation of AKT [Figure 5F: compare lanes 2 and 4, 3rd panel from top]), PKC and AKT were upstream of ERK (since Gö6976 and Ly294002 inhibited phosphorylation of ERK [Figure 5F: compare lanes 2-4, 4th panel from top]), and CaMKII had a significant effect on ERK activation (Figure 5F: compare lanes 2 and 6, 4th panel from top).
Enhanced signaling and cytokine production by B cells exposed to Cao2+ pulses
In addition to increasing CD83 expression, B cells engage in immune responses by becoming activated through receptors (such as for antigen, cytokines, and pathogen-associated molecular patterns47 ) and altering gene expression to produce factors such as cell surface proteins and cytokines. Accordingly, the effects of exposing B cells to increased levels of Cao2+ on some of these functions were examined.
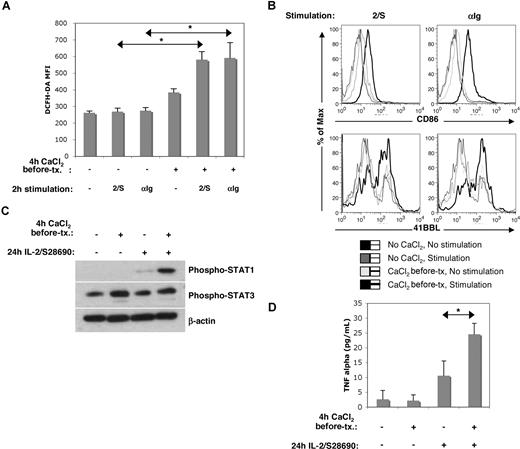
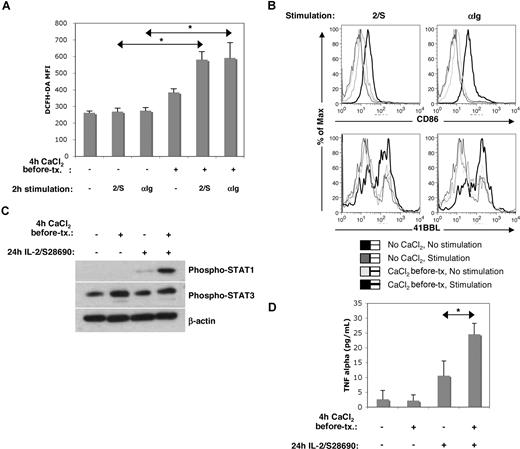
Activation of receptors on B cells leads to signal transduction that culminates in gene transcription and also mitochondrial activation to support the energy-dependent activated state.48 Activated mitochondria make ROSs, which can be measured by flow cytometry with dyes such as DCFH.21 Accordingly, the magnitude of DCFH staining early after activation of a receptor can be taken as a measure of the strength of signaling through the receptor. As shown in Figure 6A, B cells exposed to Cao2+ for 4 hours exhibited higher baseline levels of ROSs and much higher increases in ROS production in response to BCR signaling (mimicked with Ig cross-linking antibodies) or a combination of IL-2 and the TLR7 agonist, S28690 (shown previously to cause strong cytokine production by human B cells).47 These results suggested that pulses of Cao2+ may enhance subsequent responses of B cells to immunogenic signals.
Functional effects of Cao2+ on B cells. (A,B) Early activation: PBMCs were cultured in PBS plus or minus CaCl2 (500 μM) for 4 hours, prior to being washed and recultured in AIM-V with or without IL-2 (1000 U/mL) plus S28690 (1 μg/mL) (2/S) or anti-Ig (10 μg/mL). After 2 hours of stimulation, cells were incubated with DCFH-DA and CD19 antibodies for 20 minutes prior to flow cytometric analysis (A). *P < .05; n = 3. After 4 hours of stimulation, cells were stained with antibodies against CD86, 41BBL, and CD19 (B). (C,D) Cytokine production: Isolated B cells were cultured in PBS plus or minus 500 μM CaCl2 prior to being washed and recultured in AIM-V with or without IL-2 plus S28690 for 24 hours. P-STAT1, P-STAT3, and β-actin (loading control) levels were then determined by immunoblotting (similar results were obtained using samples from 3 different donors) (C). TNF-α was measured by cytometric bead array (D). *P < .005; n = 4.
Functional effects of Cao2+ on B cells. (A,B) Early activation: PBMCs were cultured in PBS plus or minus CaCl2 (500 μM) for 4 hours, prior to being washed and recultured in AIM-V with or without IL-2 (1000 U/mL) plus S28690 (1 μg/mL) (2/S) or anti-Ig (10 μg/mL). After 2 hours of stimulation, cells were incubated with DCFH-DA and CD19 antibodies for 20 minutes prior to flow cytometric analysis (A). *P < .05; n = 3. After 4 hours of stimulation, cells were stained with antibodies against CD86, 41BBL, and CD19 (B). (C,D) Cytokine production: Isolated B cells were cultured in PBS plus or minus 500 μM CaCl2 prior to being washed and recultured in AIM-V with or without IL-2 plus S28690 for 24 hours. P-STAT1, P-STAT3, and β-actin (loading control) levels were then determined by immunoblotting (similar results were obtained using samples from 3 different donors) (C). TNF-α was measured by cytometric bead array (D). *P < .005; n = 4.
Consistent with increased responsiveness to immunogenic signals, changes in expression of the costimulatory molecules CD86 and 41BBL, 4 hours after stimulation with either Ig cross-linking antibodies or IL-2 and S28690, were higher if the cells were first exposed to Cao2+ (Figure 6B). Strikingly, despite having no effect on cell numbers 24 hours later, this brief exposure to Cao2+ was sufficient to significantly enhance phosphorylated STAT1 and STAT3 levels in response to IL-2 and S28690 (Figure 6C), which has been shown to reflect the magnitude of cytokine production.19,47 Consistent with this, TNF-α levels in the culture supernatants were increased by the brief exposure to Cao2+ (Figure 6D).
Discussion
The observations in this paper suggest that increases in Cao2+ cause Ca2+ to be released from intracellular stores in human B cells (Figure 1) and activate signaling cascades that involve PLCγ, CaM, CaMKII, CN, PKC, PI3K, ERK, and NF-κB (Figure 5). This response to Cao2+ results in increased CD83 expression (Figure 3) and responsiveness to cytokine, TLR, and BCR signals as measured by ROS production, costimulatory molecule expression, and cytokine production (Figure 6). Taken together, these results suggest the presence of a receptor-mediated Cao2+-sensing mechanism on B cells that may regulate their behavior in vivo.
CD83 has been shown to be rapidly and spontaneously expressed on adherent monocytes49 cultured in serum-containing medium. However, this “spontaneous” CD83 expression on monocytes is controlled by posttranscriptional mechanisms,49 in contrast to our findings that “spontaneous” (ie, Cao2+-induced) CD83 up-regulation on B cells was transcriptionally regulated (Figure 4).
Cao2+ induced a biphasic Cai2+ response, marked by a Cai2+ transient followed by a sustained plateau (Figures 1–2), which is typical of receptor-mediated increases in Cai2+.50 The putative calcium-sensing receptor on B cells does not seem to be the well-known CaSR found on many other cell types10 since CaSR was not expressed by B cells (Figure 2D,E) and its specific inhibitor (NPS2390)11 did not block Cao2+-induced Cai2+ release or CD83 expression (Figure 2F and data not shown). Moreover, Mg2+, Zn2+, Co2+, and Ni2+ could not substitute for Cao2+ (Figures 1C, 3E and data not shown), providing further evidence that Ca2+ was not signaling through the CaSR or via calcium channels that can be activated by other cations.51 The role of calcium channels in Cao2+ sensing in B cells was further excluded as channel inhibitors, such as SKF96365, econazole, and verapamil, did not inhibit Cao2+-induced Cai2+ release or CD83 expression (data not shown). In addition, the presence of equimolar concentrations of BaCl2 in lieu of CaCl2 did not induce changes in the fluorescence of Indo-1 AM, further excluding a role for calcium channels in B-cell calcium sensing (as Ba2+ is known to enter cells via calcium channels and bind Indo-1 AM)25,52 (data not shown). However, increases in free Cai2+ concentration and CD83 expression were inhibited by tyrosine kinase and PLCγ inhibitors (Figure 2A and data not shown), and Cai2+ release was abrogated when cells were pretreated with trypsin (which would cleave extracellular portions of cell surface receptors) or with PMA (which inhibits signaling through some cell surface receptors)29-31 (Figure 2C), suggesting that a receptor on B cells was involved in sensing Cao2+.
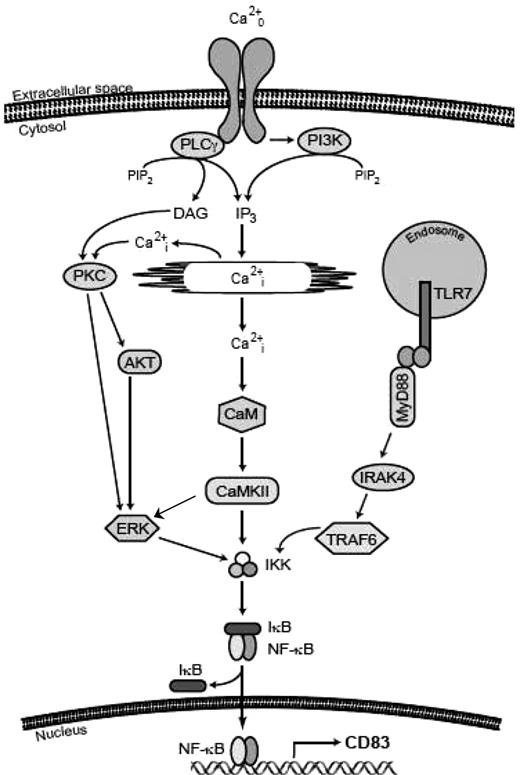
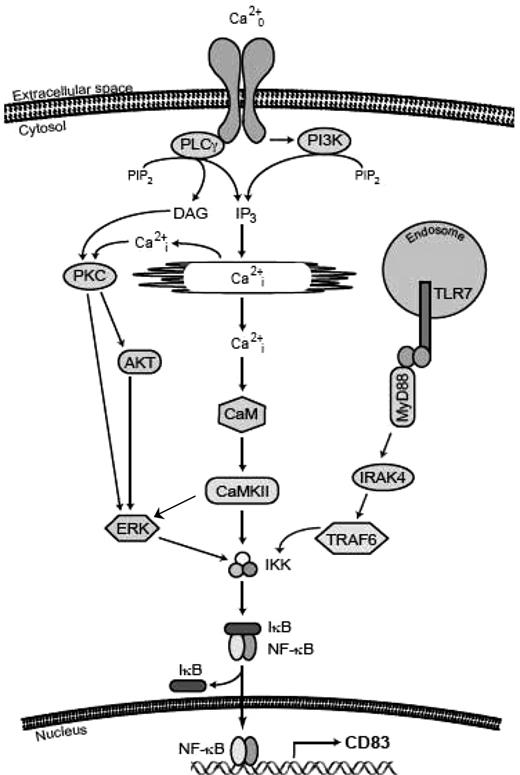
While the biochemical properties of this putative B-cell calcium receptor have not yet been characterized, we propose a model (Figure 7) whereby Cao2+ binds to a receptor, inducing the activation of PLCγ and PI3K, presumably resulting in the production of diacylglycerol and inositol triphosphate, and release of Cai2+ from internal stores. These secondary messengers then activate PKC, AKT, and CaM in parallel, resulting in the activation of CaMKII or ERK, all of which lead to phosphorylation and degradation of IκB, activation of NF-κB, and transcription of CD83. p38 MAPK also plays a role in controlling the expression of CD83 (Figure 5B,C).53 Consistent with this model, AKT has been suggested to stimulate NF-κB transcriptional activity through direct mechanisms involving IKK and NF-κB nuclear translocation,54,55 as well as by phosphorylating the transactivation domain of p65.56,57 Recent findings also show that AKT can be phosphorylated in a PKC-dependent fashion, thereby modulating its activity.58
Schema of signaling pathways from the calcium-sensing receptor on B cells to NF-κB. The binding of Cao2+ to a cell surface receptor or receptors activates PLCγ and releases Cai2+ stores, leading to the activation of 3 main signaling cascades (PKC, AKT, and CaM) that converge on IKK and result in the activation and translocation of NF-κB. The signaling pathway activated by S28690 is also shown.
Schema of signaling pathways from the calcium-sensing receptor on B cells to NF-κB. The binding of Cao2+ to a cell surface receptor or receptors activates PLCγ and releases Cai2+ stores, leading to the activation of 3 main signaling cascades (PKC, AKT, and CaM) that converge on IKK and result in the activation and translocation of NF-κB. The signaling pathway activated by S28690 is also shown.
The physiological relevance of this response of B cells to Cao2+ in vitro is not yet clear. Why should B cells have a receptor designed specifically to sense the presence of Cao2+? At present, answers to this question are necessarily speculative. Free ionized calcium levels are tightly controlled in vivo, although they vary considerably from approximately 1 mM in serum and less in interstitial fluids,59-61 to as high 10 to 15 mM in the epidermis62 and 40 mM near resorbing osteoclasts in bone.16 It may be that the absolute levels of Cao2+ are not as important to B cells as sudden changes in concentration, which may reflect tissue damage and the need to activate immune defenses. For example, calcium is known to be released by injured and/or dying cells at sites of inflammation, producing calcium concentrations 1.5- to 3-fold higher than in serum.63-65 Cao2+ levels may also rise near B cells when neighboring cells are activated (for example, T cells stimulated by antigen in a lymph node). When activated cells release Ca2+ from their internal stores, they subsequently pump cytosolic calcium into the extracellular environment to restore homeostatic Cai2+ levels (20-100 nM).59,61 These extracellular calcium fluctuations might be sensed by nearby B cells and prepare them to interact with T cells. In other words, local changes in calcium (which were mimicked in vitro by adding Ca2+ to PBS or tissue culture media [Figure 1B] in the absence of buffers such as albumin [Figure 4C]) may be interpreted as danger signals that cause B cells to increase their ability to effectively present antigen (through enhanced expression of CD83, CD86, and 41BBL)20 (Figures 3,6) and make cytokines (Figure 6).
The findings in this paper suggest that Cao2+ may have a previously uncharacterized role in B-cell responses, in addition to its well-known function as a second messenger. Further studies of this putative calcium-sensing mechanism may lead to a better understanding of B-cell physiology and pathology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Ontario Cancer Research Network (OCRN), Canadian Institutes of Health Research (CIHR), and a program project grant from the National Cancer Institute of Canada (NCIC) with funds from the Terry Fox Foundation (D.E.S.), and studentships from the Natural Sciences and Engineering Research Council of Canada (NSERC) (C.M.H.) and NCIC (J.T.).
We thank Drs R. Miller, R. Gomer, and R. Kerbel for S28690, serum amyloid protein, and C6 cells, respectively.
Authorship
Contribution: C.M.H. designed and performed the research, analyzed the data, and wrote the paper; D.W., J.T., and Y.S. performed research; D.E.S. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Spaner, Division of Molecular and Cellular Biology, Sunnybrook Research Institute, S-116A, Research Bldg, Sunnybrook Health Sciences Centre, 2075 Bayview Ave, Toronto, ON, M4N 3M5, Canada; e-mail: david.spaner@swri.ca.


 indicates addition of the stimulus during the 6-minute collection period of calcium flux data. Using Indo-1 AM, bound Ca2+ is detected at approximately 400 nM (blue) and free Ca2+ is detected at approximately 475 nm (violet). The data are presented as the ratio of Indo violet/Indo blue as a function of time for the B-cell population (gated on CD19+ cells) and represent 8 independent experiments.
indicates addition of the stimulus during the 6-minute collection period of calcium flux data. Using Indo-1 AM, bound Ca2+ is detected at approximately 400 nM (blue) and free Ca2+ is detected at approximately 475 nm (violet). The data are presented as the ratio of Indo violet/Indo blue as a function of time for the B-cell population (gated on CD19+ cells) and represent 8 independent experiments.