Abstract
Retroviruses have developed immunmodulatory mechanisms to avoid being attacked by the immune system. The mechanisms of this retrovirus-associated immune suppression are far from clarified. Dendritic cells (DCs) have been attributed a decisive role in these pathogenic processes. We have used the Friend retrovirus (FV) mouse model in order to acquire further knowledge about the role of infection of DCs in virus-induced immunosuppression. About 20% of the myeloid DCs that were generated from the bone marrow of FV-infected mice carried FV proteins. The infection was productive, and infected DCs transmitted the virus in cell culture and in vivo. FV infection of DCs led to a defect in DC maturation, as infected cells expressed very little costimulatory molecules. Live imaging analysis of the cell contact between DCs and T cells revealed prolonged contacts of T cells with infected DCs compared with uninfected DCs. Although naive T cells were still activated by FV-infected DCs, this activation did not result in antigen-specific T-cell proliferation. Interestingly, infected DCs expanded a population of Foxp3+ regulatory T cells with immunosuppressive potential, suggesting that the contact between naive T cells and retrovirus-infected DCs results in tolerance rather than immunity. Thus, retroviral infection of DCs leads to an expansion of regulatory T cells, which might serve as an immune escape mechanism of the virus.
Introduction
Dendritic cells (DCs) are the most potent antigen-presenting cells (APCs) of the immune system, and they are pivotal in the initiation of immune responses against viruses.1 However, a number of viruses are able to infect DCs, and several recent studies have investigated the effect of such infections on the biology of DCs. Although only very few studies report enhanced or unchanged functions of DCs after viral infection, most viruses seem to impair functional properties of DCs (for review, see Pollara et al2 ). These data imply that viruses infect DCs as a strategy of immune escape. In most reports that demonstrate the impairment of DCs by viruses, the morphology, phenotype, viability, and the ability to secrete cytokines of infected DCs was found to be altered, suggesting that viruses might have a significant impact on the DC-mediated initiation of an immune response in an infected host.2 In fact, there is 1 report that directly shows that measles virus infection of DCs can result in unstable DC–T-cell contacts and, as a consequence, in impaired T-cell activation.3 Retroviruses, such as HIV, are also known to infect DCs and interfere with their maturation.4 Maturation of DCs can easily be measured by analyzing the expression of costimulatory molecules such as CD40, CD80, and CD86, or maturation markers like the molecule CD83.5 However, the biological consequences of retrovirus infection of DCs for antigen presentation to T cells has not been investigated in detail so far. The most important functional properties of DCs are T-cell engagement and subsequent activation, which is the critical step in inducing adaptive immunity after infection. Since the interaction of DCs and naive T cells requires physical cell-cell contact, we used a 3D collagen matrix model6 to investigate the contact duration and kinetics of virus-infected DCs with naive T cells. Friend virus (FV) is a retroviral complex comprised of 2 components: a replication-competent helper virus called Friend murine leukemia virus (F-MuLV), which is nonpathogenic in adult mice; and a replication-defective but pathogenic component called spleen focus-forming virus (SFFV).7 Coinfection of cells by the 2 viruses allows SFFV to spread by being packaged into F-MuLV–encoded virus particles. FV infection of susceptible adult mice induces polyclonal proliferation of erythroid precursor cells, causing severe splenomegaly. This proliferation is caused by the binding of the SFFV envelope glycoprotein to the erythropoietin receptors of nucleated erythroid cells.8 In susceptible mice, FV subsequently transforms erythroid precursor cells, leading to fully malignant erythroleukemias.9 However, beside erythroid precursor cells, FV can also infect a variety of other cells types, including B cells, monocytes, and granulocytes.10 In addition, FV induces a severe generalized immunosuppression during acute infection9,10 ; however, it is unknown whether this is the result of a functional impairment of APCs due to virus infection. Susceptibility to both FV-induced immunosuppression and erythroleukemia is strongly influenced by the genetic background of a given mouse strain. However, resistance to immunosuppression does not directly correlate with recovery from splenomegaly, since some mouse strains are resistant to immunosuppression but still die from erythroleukemia.9
Here, we show that FV productively infects myeloid DCs in vivo and in vitro and interfers with the maturation of these cells. Functional studies indicated that infected DCs had prolonged contacts with naive T cells during antigen presentation, but were able to induce antigen-specific T-cell proliferation poorly. In contrast, T cells that emerged from coculture with antigen-presenting FV-infected DCs expressed markers of regulatory T (Treg) cells and suppressed the proliferation of T cells in vitro. Thus, virus-induced alteration of DCs is a likely cause of the immunosuppression found in FV infection.
Materials and methods
Mice
BALB/c mice were obtained from Harlan Winkelman laboratories (Borchen, Germany). DO11.10 mice on a BALB/c background expressing a transgenic T-cell receptor (TCR), which recognizes ovalbumin peptide 323-339 (ISQAVHAAHAEINEAGR) in the context of I-Ad,11 were from Dr Blankenstein (Max-Delbrück-Centrum, Berlin, Germany). All mice were housed under specific pathogen–free conditions according to the guidelines of the regional animal care committee and used at 8 to 12 weeks of age.
Generation and culture of myeloid DCs from bone marrow cells
Bone marrow (BM) DCs were generated as described by Inaba et al12 with some modifications. In brief, BM cells were collected from murine tibias and femurs and suspended, and 3 × 106 cells were placed in 6-well plates (BD Pharmingen, Heidelberg, Germany) containing 5 mL DC media (RPMI supplemented with 5% FCS, 1 × nonessential amino acids, 2 mM l-glutamine, 500 nM 2-ME, 100 U/mL penicillin/streptomycin, and 20 μg/mL gentamycin), 5 ng/mL granulocyte-macrophage colony stimulating factor (GM-CSF; R&D Systems, Wiesbaden, Germany), and 1 ng/mL IL-4 (BD Pharmingen). On day 6, 2 × 106 nonadherent cells were transferred into a new 6-well plate containing 3 mL DC media/well. After a total of 7 to 9 days of culture, DCs were harvested and used in subsequent experiments. For coculture experiments with T cells, DCs were stimulated on day 7 for 48 hours with anti-CD40 (clone 1C10; a kind gift from S. Amigorena, Paris, France).
Detection of FV-infected DCs
To detect FV-infected cells, either BM cells or cultured BM DCs from FV-infected mice were stained with tissue culture supernatant containing monoclonal antibody (mAb) 34, which is specific for F-MuLV glycosylated Gag protein that is expressed on the surface of infected cells.13 mAb 34 binding was detected with a goat anti-mouse IgG2b-PE antiserum (BD Pharmingen).
Infectious center
For infectious center assays, infected DCs were cocultivated with Mus dunni cells at 10-fold dilutions. Cultures were incubated for 5 days, fixed with ethanol, stained with F-MuLV envelope-specific mAb 720, and developed with peroxidase-conjugated goat anti-mouse and aminoethylcarbazol to detect foci.
Electron microscopy
DCs were pelleted by centrifugation, and cell pellets were fixed in glutaraldehyde and embedded in 2% agarose. After fixation in osmiumtetroxide, these cell blocks were dehydrated in graded ethanols and embedded in Epon. Thin sections were stained with uranyl acetate and lead citrate and evaluated on a Zeiss EM 902A transmission electron microscope (Zeiss, Oberkochen, Germany).
Enrichment of FV-infected DCs
For most experiments, FV-infected DCs were enriched from BM DC cultures. Two different techniques were used to generate DC cultures in which more than 90% of the cells were infected with FV. In the first technique, BM cells from FV-infected mice were isolated on day 11 after infection as described and stained with anti-mAb 34 and PE-conjugated secondary antibody. Infected cells were then isolated using anti-PE microbeads and magnetic-activated cell sorter (MACS) sorting (Milteny, Bergisch-Gladbach Germany). Cells were cultured to generate BM DCs as described, and were used in indicated experiments. In the second technique, BM cells were isolated from FV-infected mice and cultured as described. After 7 to 9 days of culture, infected myeloid DCs were isolated by CD11c+, CD11b+, and mAb 34 staining. Positive cells were separated by either flow cytometry or MACS technology.
DC isolation from the spleen
Splenic DCs were obtained using a variation of the method described by Vremec et al.14 Briefly, spleens from infected and uninfected mice were perfused with RPMI-FCS supplemented with 0.5 M ETDA, 5 mg/mL collagenase (Typ III; Worthington Biochemical, CellSystems, St. Katharinen, Germany), and DNase I (1 mg/mL; AppliChem, Darmstadt, Germany). Spleens were digested for 20 minutes at 37°C. After centrifugation by 1200 U/min for 10 minutes, the red cells were removed with lysis buffer. Then, DCs were further enriched using anti-CD11c microbeads (Miltenyi Biotech).
Cell-surface marker staining on FV-infected DCs
Expression of cell-surface molecules on DCs was quantified using following mAbs: αCD11c-APC (HL-3), αCD54-FITC (3E2), αH-2Dd-FITC (34–2-12S), αI-A/I-E-FITC (2G9), αCD80-FITC (16–10A1), αCD86-FITC (GL1), and αCD40-FITC (3/23) (BD Pharmingen). For flow cytometry, 2 × 105 DCs were incubated with 1 to 5μg/mL mAb for 30 minutes at room temperature, washed twice in PBS/1% FCS, and analyzed with a FACS Calibur (BD Pharmingen). Data were analyzed using Cell Quest Pro software (BD Pharmingen).
For measuring antigen uptake and processing by FV-infected DCs, DCs were incubated for 2 hours with 0.5 μg/mL DQ Ovalbumin (Molecular Probes, Eugene, OR) and analyzed by flow cytometry and immunofluorescence microscopy.
Cytokine measurement
The cytokine production by BM DCs was determined by measuring IL-12 in cell culture supernatants from CD11c+ DCs using a cytometric bead array (CBA Inflammation; BD Pharmingen) and enzyme-linked immunosorbent assay (ELISA; eBioscience, San Diego, CA) according to the manufacturer's instructions.
T-lymphocyte isolation and staining
Naive CD4+ T cells from spleens of DO11.10 mice were enriched to a purity of greater than 95% as described by Gunzer et al.15 Purified CD4+ T cells were cultured in RPMI supplemented with 5% FCS, 1 × nonessential amino acids, 2 mM l-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 500 nM 2-ME, and 100U/mL penicillin/streptomycin.
CD25+ CD4+ T cells were enriched to a greater than 96% purity by magnetic cell sorting.
Analysis of cell-cell interactions within 3D collagen gels
DC–T-cell interactions within 3D collagen gels were analyzed as described.6 Cell migration was monitored simultaneously by time-lapse microscopy using an Olympus BX61 microscope with a UAPO lens (Olympus, Frankenthal, Germany) (20×/0.75 NA, total magnification 340×) and an FView camera with AnalySIS software (SIS, Muenster, Germany). The same equipment was used to take the pictures in Figure 4C,D. The imaging medium was 33% T-cell medium plus 66% collagen. In some experiments, 24 hours after starting the coculture, 3D collagen matrices were digested by type VII collagenase (30 U/100 mL gel; Sigma, Deisenhofen, Germany) for 30 minutes at 37°C, and T cells were analyzed by flow cytometry. Expression of activation markers on T cells was quantified using the following mAbs: αCD62-FITC (MEL-12), αCD69- PE (H1.2F3), αCD25-PE (PC-61), αCD44-FITC (IM7), and αCD54-FITC (3E2) (BD Pharmingen). In addition, Treg cells were quantified by fluorescence-activated cell sorter (FACS) staining with mAbs: αCD4-PerCp (RM4-5), αCD25-PE (PC-61) (BD Pharmingen), and αFoxp3-FITC (NatuTec, Frankfurt, Germany)
Antigen-specific T-cell proliferation
BM DCs from uninfected and FV-infected BALB/c mice were left untreated or were loaded with 0.1 μg/mL OVA-antigen (Ag) (peptide 323-339) and titrated in triplicates 1- to 3-fold within 3D collagen matrices. Afterwards, 5 × 104 antigen-specific T cells (DO11.10) were added to obtain DC–T-cell ratios of 1:10, 1:30, 1:90, and 1:270. After 3 days, proliferating T cells were labeled by incorporation of [3H] thymidine (37 k Bq/well [1 μCi/well]) for 12 hours. The next day, gels were digested by type VII collagenase (30 U/100 mL gel; Sigma), and thymidine incorporation was measured by liquid scintillation counting.
T-cell suppression assay
For the proliferation assay, 2 × 105 naive T cells derived from Balb/c mice were labeled with CFSE, stimulated with anti-CD3 and anti-CD28, and analyzed 3 days later by flow cytometry. For the suppression assay, 2 × 105 CD4+ cells from T-cell–DC cultures were simultaneously isolated and added to the proliferation assay. These CD4+ T cells were isolated from 3-day cultures (containing 250 μL AIM-V 5%, FCS, and IL-2 [100 U]) of FACS-sorted FV-infected or uninfected DCs that had been loaded with OVA peptides (0.1 μg/mL) and MACS-enriched transgenic CD4+ T cells from DO11.10 mice (1:1 ratio).
Statistical analysis
Differences between mean values were calculated by the Student t test for unpaired data or by 1-way analysis of variance (ANOVA) test, which was corrected by Dunnett multiple comparison correction if more than 2 groups were compared. P values less than .05 were considered statistically significant.
Results
Generation of FV-infected myeloid DCs in vitro and detection of infected DCs ex vivo
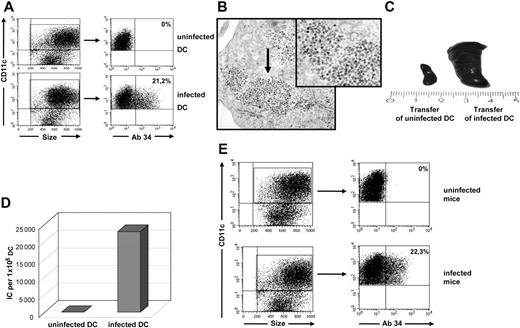
To study the functional properties of infected DCs, we generated DCs from mouse BM using GM-CSF and IL-4.12 However, unlike many other viruses,2 FV did not efficiently infect DCs in vitro (data not shown). To solve this problem, we took advantage of the ability of FV to infect BM cells in vivo. It had previously been shown that about 20% of the BM cells of C57BL/6 mice were infected at 2 weeks after FV inoculation.16 Thus, we infected BALB/c mice with FV and used their BM cells to generate infected DCs (Figure 1A). To visualize infected DCs, electron microscopy analyses of DC cultures were performed. Figure 1B clearly shows the accumulation of virus particles in the cytoplasm of CD11c+ and CD11b+ (data not shown) myeloid DCs after 9 days of in vitro culture. Up to 20% of the DCs from BALB/c mice were infected with FV after 9 days of culture with GM-CSF and IL-4 (Figure 1A). Viral antigen on DCs was detected using mAb 34, which recognizes the glycosylated form of Gag, expressed on the cell surface of both F-MuLV– and SFFV-infected cells.13 Synthesis of Gag protein, which is essential for virus particle formation, indicated that the DCs were productively infected by FV. In order to show that infected DCs can transmit virus in vitro and in vivo, sorted CD11c+ DCs from infected mice were adoptively transferred into naive mice or plated on FV-susceptible M dunni cells. Transfer of these DCs induced severe erythroleukemia in susceptible BALB/c mice, indicating that infected DCs were able to established a productive FV infection in vivo (Figure 1C). In addition, FV-infected DCs also transferred infectious virus to M dunni cells in vitro. In this assay, up to 30% of the DCs from cultures of FV-infected mice were found to be infectious (Figure 1D).
FV infection of myeloid DCs in vitro and in vivo. Myeloid DCs were generated from the BM of FV-infected BALB/c mice and analyzed for viral infection. (A) CD11c-gated DCs were stained for FV glycosylated Gag protein using mAb 34. A flow cytometric analysis representative for more than 20 independent DC cultures is shown (mean, 21.2% ± 0.8%). The mean percentage of positive cells (FV-infected cells) is given in the top right quadrant. (B) Electron microscopy picture of virus particles in an infected DC. Virus particles are visible as numerous small electron-dense bodies at the margin of the cytoplasm ( ). Transmission electron microscopy; original magnification, ×28 000. See “Analysis of cell-cell interactions within 3D collagen gels” for complete image acquisition information. (C) FV-induced splenomegaly in BALB/c mice at 2 weeks after transfer of 3.5 × 105 infected DCs (right). (D) Quantification of infected DCs using M dunni cells. A total of 1 × 105 DCs were cocultivated with the indicator cells, which were subsequently stained for infectious centers (ICs). Representative results for more than 5 independent experiments are shown. The uninfected DCs were generated from naive mice. (E) Myeloid DCs were isolated from the spleen of FV-infected BALB/c mice and analyzed for viral infection directly ex vivo. CD11c-gated DCs were stained for FV glycosylated Gag protein using mAb 34. A flow cytometric analysis representative for 5 individual mice is shown (mean, 22.3% ± 1.5%). The mean percentage of positive cells (FV-infected cells) is given in the top right quadrant.
). Transmission electron microscopy; original magnification, ×28 000. See “Analysis of cell-cell interactions within 3D collagen gels” for complete image acquisition information. (C) FV-induced splenomegaly in BALB/c mice at 2 weeks after transfer of 3.5 × 105 infected DCs (right). (D) Quantification of infected DCs using M dunni cells. A total of 1 × 105 DCs were cocultivated with the indicator cells, which were subsequently stained for infectious centers (ICs). Representative results for more than 5 independent experiments are shown. The uninfected DCs were generated from naive mice. (E) Myeloid DCs were isolated from the spleen of FV-infected BALB/c mice and analyzed for viral infection directly ex vivo. CD11c-gated DCs were stained for FV glycosylated Gag protein using mAb 34. A flow cytometric analysis representative for 5 individual mice is shown (mean, 22.3% ± 1.5%). The mean percentage of positive cells (FV-infected cells) is given in the top right quadrant.
FV infection of myeloid DCs in vitro and in vivo. Myeloid DCs were generated from the BM of FV-infected BALB/c mice and analyzed for viral infection. (A) CD11c-gated DCs were stained for FV glycosylated Gag protein using mAb 34. A flow cytometric analysis representative for more than 20 independent DC cultures is shown (mean, 21.2% ± 0.8%). The mean percentage of positive cells (FV-infected cells) is given in the top right quadrant. (B) Electron microscopy picture of virus particles in an infected DC. Virus particles are visible as numerous small electron-dense bodies at the margin of the cytoplasm ( ). Transmission electron microscopy; original magnification, ×28 000. See “Analysis of cell-cell interactions within 3D collagen gels” for complete image acquisition information. (C) FV-induced splenomegaly in BALB/c mice at 2 weeks after transfer of 3.5 × 105 infected DCs (right). (D) Quantification of infected DCs using M dunni cells. A total of 1 × 105 DCs were cocultivated with the indicator cells, which were subsequently stained for infectious centers (ICs). Representative results for more than 5 independent experiments are shown. The uninfected DCs were generated from naive mice. (E) Myeloid DCs were isolated from the spleen of FV-infected BALB/c mice and analyzed for viral infection directly ex vivo. CD11c-gated DCs were stained for FV glycosylated Gag protein using mAb 34. A flow cytometric analysis representative for 5 individual mice is shown (mean, 22.3% ± 1.5%). The mean percentage of positive cells (FV-infected cells) is given in the top right quadrant.
). Transmission electron microscopy; original magnification, ×28 000. See “Analysis of cell-cell interactions within 3D collagen gels” for complete image acquisition information. (C) FV-induced splenomegaly in BALB/c mice at 2 weeks after transfer of 3.5 × 105 infected DCs (right). (D) Quantification of infected DCs using M dunni cells. A total of 1 × 105 DCs were cocultivated with the indicator cells, which were subsequently stained for infectious centers (ICs). Representative results for more than 5 independent experiments are shown. The uninfected DCs were generated from naive mice. (E) Myeloid DCs were isolated from the spleen of FV-infected BALB/c mice and analyzed for viral infection directly ex vivo. CD11c-gated DCs were stained for FV glycosylated Gag protein using mAb 34. A flow cytometric analysis representative for 5 individual mice is shown (mean, 22.3% ± 1.5%). The mean percentage of positive cells (FV-infected cells) is given in the top right quadrant.
To determine whether the infection of DCs in cell culture reflects the in vivo situation, we stained spleen-derived DCs from acutely infected mice directly ex vivo for expression of FV-glycosylated Gag. In susceptible BALB/c mice, around 20% of the CD11c+ DCs were positive for viral antigen at 10 days after infection (Figure 1E). All FV-infected DCs found in the spleen were of myeloid (CD11c+ CD11b+) or lymphoid (CD11c+ CD8+) origin, but no infected plasmacytoid DCs (CD11clow CD11b− B220+ or anti-mPDCA sorted cells) could be detected (data not shown). Thus, the infection rate of myeloid DCs in vitro resembled the in vivo situation, and therefore in vitro–generated FV-infected myeloid DCs were used to study the biological features of retrovirus-infected DCs. Since there was no evidence of in vivo infection of plasmacytoid DCs, this cell subset was excluded from the subsequent phenotypic and functional analysis. Up to 15% of the lymphoid DCs that were analyzed ex vivo from the spleens of infected mice were positive for FV (data not shown). However, since FV-infected lymphoid DCs could not be generated in culture, the numbers of infected cells were too low to functionally characterize this DC subset.
FV infection interfered with maturation of DCs but not with cytokine production
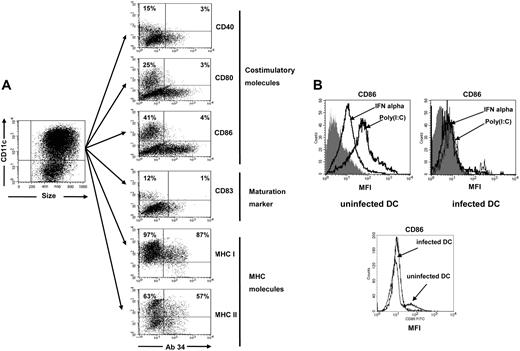
To analyze the consequences of FV infection of DCs on the phenotype of these cells, the expression of cell-surface markers was compared between infected and uninfected DCs. We investigated costimulatory molecules, major histocompatibility complex (MHC) molecules, and maturation markers expressed on murine CD11c+ DCs17 in order to characterize the phenotypical consequences of FV infection. About 20% of the DCs were infected in cell culture, with the rest of the cells remaining uninfected (Figure 1A). Thus, both subsets were directly compared within the same DC culture from infected mice. After 9 days of culture with GM-CSF and IL-4, the CD11c-gated, uninfected DCs generated from infected (Figure 2A) as well as uninfected mice (data not shown) were semimature, and about 12% expressed the maturation marker CD83. In addition, 15% to 40% of the uninfected DCs also expressed the costimulatory molecules CD40, CD80, or CD86 (Figure 2A). In contrast, only very few FV-infected DCs from the same culture expressed costimulatory molecules or the maturation marker CD83, indicating that FV infection interfered with maturation of myeloid DCs. Interestingly, no differences in the expression of MHC class I or II molecules were found between infected and uninfected DCs (Figure 2A).
Expression of costimulatory and MHC molecules on FV-infected DCs. (A) Myeloid DCs were generated from the bone marrow of FV-infected BALB/c mice and analyzed for the expression of cell-surface molecules. CD11c-gated DCs were stained for FV glycosylated Gag protein using mAb 34 and for costimulatory molecules, maturation maker CD83, or MHC molecules. The infected cells are in the right quadrants, whereas uninfected cells are shown in the left quadrants. The different populations were from the same culture of DCs generated from an infected mouse. The percentage of cells positive for the different DC markers in both the infected and uninfected populations of DCs is given in the top quadrants. A flow cytometric analysis representative for more than 10 independent DC cultures is shown. (B) Myeloid DCs were generated from the bone marrow of FV-infected or uninfected BALB/c mice and stimulated in vitro with IFNα (1000 U/mL) or poly(I:C) (100 μg/mL). Afterward, CD11c+-gated DCs were stained for the expression of the costimulatory molecule CD86 as a surrogate marker for DC maturation. The histogram on the left shows the mean fluorescence intensity (MFI) of uninfected cultures, whereas the right histogram shows the results for FV-infected DC cultures. The gray curves represent the isotype control for the CD86 staining. The lower histogram shows the CD86 staining of uninfected or infected DCs that were not treated (no IFN or poly(I:C)). A flow cytometric analysis representative for 6 independent DC cultures is shown.
Expression of costimulatory and MHC molecules on FV-infected DCs. (A) Myeloid DCs were generated from the bone marrow of FV-infected BALB/c mice and analyzed for the expression of cell-surface molecules. CD11c-gated DCs were stained for FV glycosylated Gag protein using mAb 34 and for costimulatory molecules, maturation maker CD83, or MHC molecules. The infected cells are in the right quadrants, whereas uninfected cells are shown in the left quadrants. The different populations were from the same culture of DCs generated from an infected mouse. The percentage of cells positive for the different DC markers in both the infected and uninfected populations of DCs is given in the top quadrants. A flow cytometric analysis representative for more than 10 independent DC cultures is shown. (B) Myeloid DCs were generated from the bone marrow of FV-infected or uninfected BALB/c mice and stimulated in vitro with IFNα (1000 U/mL) or poly(I:C) (100 μg/mL). Afterward, CD11c+-gated DCs were stained for the expression of the costimulatory molecule CD86 as a surrogate marker for DC maturation. The histogram on the left shows the mean fluorescence intensity (MFI) of uninfected cultures, whereas the right histogram shows the results for FV-infected DC cultures. The gray curves represent the isotype control for the CD86 staining. The lower histogram shows the CD86 staining of uninfected or infected DCs that were not treated (no IFN or poly(I:C)). A flow cytometric analysis representative for 6 independent DC cultures is shown.
Immature DCs can be matured, and their antigen-presentation capacity can be enhanced in vivo and in vitro by several different stimuli.17 Some of these stimuli, like type I interferons18 or murine retrovirus long terminal repeat, which has been shown to activate immune cells via Toll-like receptor-3 (TLR-3),19 are present in vivo during an acute FV infection. We have investigated whether or not these stimuli can overcome the impairment in maturation of infected DCs. Whereas IFNα and the TLR-3 ligand poly(I:C) both enhanced CD86 expression on uninfected DCs, no significant enhancement of the marginal CD86 expression was found after IFNα or poly(I:C) stimulation of FV-infected DCs, indicating that these stimuli could not overcome the FV-induced block in DC maturation (Figure 2B). Comparable findings were made for the other 2 costimulatory molecules, CD40 and CD80, and the maturation marker CD83 (data not shown).
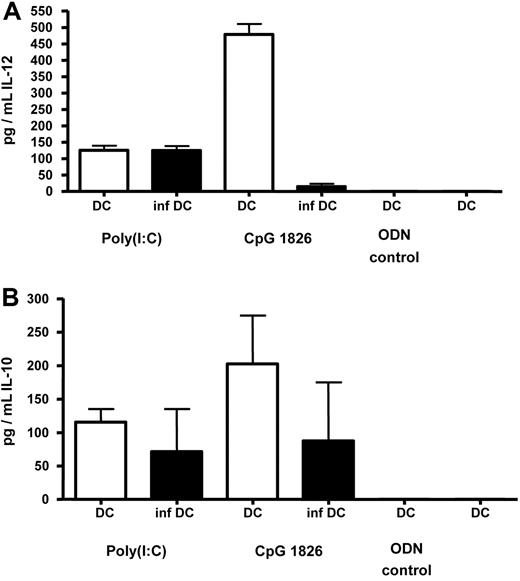
In addition to the phenotypic characterization of FV-infected DCs, we studied their ability to produce cytokines. IL-12 and IL-10 are the most important cytokines produced by DCs for activation and differentiation of T cells upon DC–T-cell contact.20 After stimulation with poly(I:C), infected and uninfected DCs produced similar amounts of IL-12 and IL-10 (Figure 3), and no influence of the infection on cytokine production by DCs was found. Stimulation of DCs with IFNα resulted in only marginal production of cytokines independent of the infection status (data not shown). Interestingly, stimulation of DCs with the TLR-9 agonist CpG1826 induced high concentrations of IL-12 and IL-10 in uninfected DCs, but not in FV-infected DCs (Figure 3). However, the biological relevance of this finding remains unclear, since CpG are present in bacterial DNA or in some viruses with a DNA genome, but not in RNA viruses such as the murine retroviruses.
Production of IL-12 and IL-10 by FV-infected DCs. Myeloid DCs were generated from the bone marrow of FV-infected (inf DC) or uninfected (DC) BALB/c mice and stimulated in vitro with CpG 1826 ODNs (6 μg/mL) or poly(I:C) (100 μg/mL). Infected DCs were enriched by FACS-based cell sorting. As negative controls, DCs were stimulated with an ODN without CpG motif (ODN-control) or left nonstimulated. After 24 hours, supernatants from stimulated cultures were analyzed by ELISA for the concentration of IL-12 (A) or IL-10 (B). Cumulative data from 3 independent experiments are shown. The error bars represent standard errors of the means.
Production of IL-12 and IL-10 by FV-infected DCs. Myeloid DCs were generated from the bone marrow of FV-infected (inf DC) or uninfected (DC) BALB/c mice and stimulated in vitro with CpG 1826 ODNs (6 μg/mL) or poly(I:C) (100 μg/mL). Infected DCs were enriched by FACS-based cell sorting. As negative controls, DCs were stimulated with an ODN without CpG motif (ODN-control) or left nonstimulated. After 24 hours, supernatants from stimulated cultures were analyzed by ELISA for the concentration of IL-12 (A) or IL-10 (B). Cumulative data from 3 independent experiments are shown. The error bars represent standard errors of the means.
FV infection of DCs changed the interaction of DCs and naive T cells
In order to become activated by APCs, T cells need to physically interact with APCs and form a highly organized contact plane (immunologic synapse).21,22 However, synapse formation is a dynamic process and is affected by the type and maturation state of APCs.23 It is currently unclear whether the contact duration to APCs determines the type of T-cell activation, but at least some evidence suggests that prolonged APC–T-cell contacts are required for the full activation of T cells.23,24
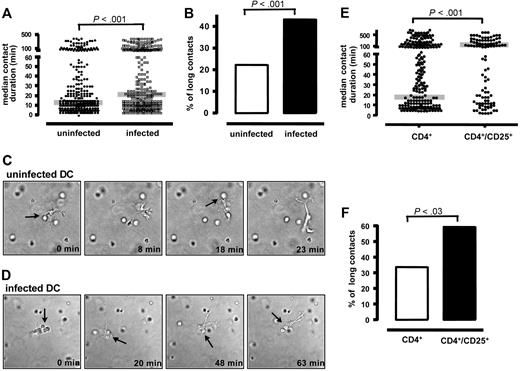
To form an immunologic synapse with T cells, DCs need to take up and process antigens. In order to show that FV-infected DCs were not impaired in these functions, they were incubated with DQ ovalbumin, a self-quenched conjugate of OVA that exhibits bright green fluorescence upon proteolytic degradation. No difference in fluorescence labeling was found between infected and uninfected DCs (data not shown), indicating that FV infection did not interfere with antigen uptake or processing. Next, the ability of FV-infected DCs to establish effective DC–T-cell contacts was analyzed by measuring the contact duration between infected DCs and naive CD4+ T cells. OVA-specific transgenic CD4+ T cells were isolated from DO11.10 mice and mixed in 3D collagen gels with uninfected or FV-infected DCs that had been loaded with the OVA antigen. Within the next 8 hours, cell movement and contact duration between T cells and DCs were documented by video microscopy. Surprisingly, the contact duration between FV-infected DCs and naive CD4+ T cells was significantly prolonged (median contact duration of 20 minutes) compared with uninfected DCs (median contact duration of 12 minutes) (Figure 4A). This significant difference was the result of an increased number of very long contacts between infected DCs and T cells. Significantly more contacts between FV-infected DCs and T cells were longer than 38 minutes (3 times the median contact duration between uninfected DCs and T cells; Figure 4B) than contacts between uninfected DCs and T cells. Examples of DC–T-cell interactions with uninfected versus infected DCs are shown in Figures 4C and 4D. From these results we conclude that FV infection of DCs can alter their interaction with CD4+ T cells.
Contact duration between FV-infected DCs and naive T cells. DC–T-cell interactions were analyzed within a 3D collagen gel. Transgenic CD4+ T cells from DO11.10 mice were isolated and mixed with OVA-antigen loaded DCs in a 10:1 ratio. (A) Contact duration times (minutes) between uninfected DCs (●) or FV-infected DCs (■) and CD4+ T cells are shown. The median for both groups (gray line) was statistically significantly different (P < .001). Shown are the cumulative results from 4 independent experiments with similar outcomes. The total number of measured contacts were 330 in uninfected DCs and 329 in FV-infected DCs. (B) In addition, cell contacts between uninfected (□) and FV-infected (■) DCs and CD4+ T cells that lasted longer than 38 minutes (3 times the median of the contact times between T cells and uninfected DCs) were counted. Cumulative results from 4 independent experiments are shown. The difference between both groups was statistically significant (P < .001). (C) Representative example of short cell contacts between 1 uninfected DC and 2 naive CD4+ T cells. The time point when the picture was taken after initiation of the culture is given. (D) Representative example of a long contact between 1 FV-infected DC and 2 naive CD4+ T cells. Arrows indicate cell-to-cell contacts between DC and T cells. See “Analysis of cell-cell interactions within 3D collagen gels” for complete image acquisition information. (E) Contact duration between DCs and natural Treg cells. DC–T-cell interactions were analyzed within a 3D collagen gel. Naive CD25+ (Foxp3+ but CD69− and CD44−) or CD25− transgenic CD4+ T cells from DO11.10 mice were isolated and mixed with OVA-antigen–loaded uninfected DCs in a 10:1 ratio. Contact duration times (minutes) between DCs and CD4+CD25− (left) or CD4+CD25+ (right) are shown. The median for both groups (gray line) was statistically significantly different (P < .001). Shown are the cumulative results from 3 independent experiments with similar outcomes. The numbers of measured contacts were 167 for CD4+ and 106 for CD4+CD25+. (F) In addition, cell contacts between CD4+CD25− (□) or CD4+CD25+ (■) T cells and DCs that lasted longer than 3 times the median of the contact times between the total CD4+ T cells and uninfected DCs were counted. Cumulative results from 3 independent experiments are shown.
Contact duration between FV-infected DCs and naive T cells. DC–T-cell interactions were analyzed within a 3D collagen gel. Transgenic CD4+ T cells from DO11.10 mice were isolated and mixed with OVA-antigen loaded DCs in a 10:1 ratio. (A) Contact duration times (minutes) between uninfected DCs (●) or FV-infected DCs (■) and CD4+ T cells are shown. The median for both groups (gray line) was statistically significantly different (P < .001). Shown are the cumulative results from 4 independent experiments with similar outcomes. The total number of measured contacts were 330 in uninfected DCs and 329 in FV-infected DCs. (B) In addition, cell contacts between uninfected (□) and FV-infected (■) DCs and CD4+ T cells that lasted longer than 38 minutes (3 times the median of the contact times between T cells and uninfected DCs) were counted. Cumulative results from 4 independent experiments are shown. The difference between both groups was statistically significant (P < .001). (C) Representative example of short cell contacts between 1 uninfected DC and 2 naive CD4+ T cells. The time point when the picture was taken after initiation of the culture is given. (D) Representative example of a long contact between 1 FV-infected DC and 2 naive CD4+ T cells. Arrows indicate cell-to-cell contacts between DC and T cells. See “Analysis of cell-cell interactions within 3D collagen gels” for complete image acquisition information. (E) Contact duration between DCs and natural Treg cells. DC–T-cell interactions were analyzed within a 3D collagen gel. Naive CD25+ (Foxp3+ but CD69− and CD44−) or CD25− transgenic CD4+ T cells from DO11.10 mice were isolated and mixed with OVA-antigen–loaded uninfected DCs in a 10:1 ratio. Contact duration times (minutes) between DCs and CD4+CD25− (left) or CD4+CD25+ (right) are shown. The median for both groups (gray line) was statistically significantly different (P < .001). Shown are the cumulative results from 3 independent experiments with similar outcomes. The numbers of measured contacts were 167 for CD4+ and 106 for CD4+CD25+. (F) In addition, cell contacts between CD4+CD25− (□) or CD4+CD25+ (■) T cells and DCs that lasted longer than 3 times the median of the contact times between the total CD4+ T cells and uninfected DCs were counted. Cumulative results from 3 independent experiments are shown.
FV-infected DCs preferentially expanded Treg cells
Since FV infection prolonged the contact duration of DCs with T cells, it was important to determine whether this altered interaction resulted in sufficient T-cell activation and proliferation. To this end, T cells were isolated from the 3D collagen gels after 24 hours of coculture with DCs and analyzed for their expression of activation markers. As negative controls, OVA-specific T cells were cultured in the gel without DCs or with DCs that had not been loaded with the OVA peptide. In these control cultures, T cells were not activated as shown by high expression of CD62L, but low expression of the activation markers CD69, CD25, CD54, and CD44 (Figure 5A). In contrast, if T cells were re-isolated from gels containing uninfected or infected DCs loaded with the OVA peptide, CD62L expression was reduced and the expression of activation markers was strongly enhanced. The overall activation of the T cells was slightly lower after incubation with infected DCs than with uninfected DCs, but the difference was not statistically significant. This result indicated that the OVA-loaded uninfected and FV-infected DCs activated naive T cells in an antigen-dependent manner.
Activation and proliferation of T cells after cocultivation with FV-infected DCs. (A) Naive TCR transgenic CD4+ T cells from DO11.10 mice were incubated with infected or uninfected DCs in a 3D collagen gel. After 24 hours, gels were digested and T cells were analyzed for activation marker CD62L, CD69, CD25, CD54, or CD44 surface expression by flow cytometry. T cells were mixed with uninfected (□) or FV-infected DCs (■), which were loaded (+Ova) or not (−Ova) with OVA peptide. T cells without DCs served as negative control (▩). The MFI or the percentage of positive stained cells is shown. Cumulative data from 5 independent experiments are being presented. The error bars represent standard errors of the means. (B) FV-infected (■) and uninfected (●) DCs were loaded with 0.1μg/mL OVA peptide and titrated onto naive TCR transgenic CD4+ T cells from DO11.10 mice in a 3D collagen gel. Cells were incubated for 3 days and then pulsed with [3H] thymidine overnight to determine T-cell proliferation. All assays were performed in triplicate. Cultures with DCs that were not loaded with peptide served as negative controls (open symbols). *Statistically significant differences (P < .001) between cultures with FV-infected versus uninfected DCs loaded with OVA antigen. Representative results from 4 independent experiments are shown.
Activation and proliferation of T cells after cocultivation with FV-infected DCs. (A) Naive TCR transgenic CD4+ T cells from DO11.10 mice were incubated with infected or uninfected DCs in a 3D collagen gel. After 24 hours, gels were digested and T cells were analyzed for activation marker CD62L, CD69, CD25, CD54, or CD44 surface expression by flow cytometry. T cells were mixed with uninfected (□) or FV-infected DCs (■), which were loaded (+Ova) or not (−Ova) with OVA peptide. T cells without DCs served as negative control (▩). The MFI or the percentage of positive stained cells is shown. Cumulative data from 5 independent experiments are being presented. The error bars represent standard errors of the means. (B) FV-infected (■) and uninfected (●) DCs were loaded with 0.1μg/mL OVA peptide and titrated onto naive TCR transgenic CD4+ T cells from DO11.10 mice in a 3D collagen gel. Cells were incubated for 3 days and then pulsed with [3H] thymidine overnight to determine T-cell proliferation. All assays were performed in triplicate. Cultures with DCs that were not loaded with peptide served as negative controls (open symbols). *Statistically significant differences (P < .001) between cultures with FV-infected versus uninfected DCs loaded with OVA antigen. Representative results from 4 independent experiments are shown.
Since naive T cells were activated by FV-infected DCs, it was of interest whether or not infected DCs could stimulate antigen-driven T-cell proliferation. To test this, we performed T-cell proliferation assays. Uninfected DCs loaded with the OVA peptide stimulated proliferation of OVA-specific CD4+ T cells (Figure 5B). At a DC–T-cell ratio as low as 1:270, a significant increase in counts per minute (cpm) was found when cultures containing uninfected DCs loaded with OVA were compared with nonloaded DCs (P < .05). The magnitude of T-cell proliferation was influenced by the number of DCs added to the cultures. In contrast, proliferation was significantly reduced when FV-infected DCs were used to stimulate the T cells. Only in the presence of numerous DCs (DC–T-cell ratios of 1:30 to 1:10) was a weak T-cell proliferation detected with infected DCs, whereas much fewer DCs (ratios of 1:270 to 1:10) could induce proliferation when uninfected DCs were used for antigen presentation.
Thus, although FV-infected DCs were able to activate naive T cells, this activation did not lead to efficient proliferation of the cells in vitro. This phenomenon might contribute to the virus-induced immunosuppression observed in acute FV-infected mice. To test this hypothesis, we analyzed whether FV-infected DCs induced or expanded regulatory CD4+ T cells, which have been shown to be associated with immunosuppression in FV infection.25 As a marker for Treg cells, we used the transcription factor Foxp3.26 After 24 hours of coculture with uninfected or FV-infected DCs, T cells were isolated from the 3D collagen gels and analyzed for Foxp3 expression. Less than 14% of the T cells that were mixed with OVA-loaded uninfected DCs expressed Foxp3, which was indistinguishable from CD4+ T cells prior to coculture (Figure 6A). However, a significant increase of Foxp3+ T cells was found when antigen-loaded, FV-infected DCs were used for antigen presentation to T cells (more than 26% of the total CD4+ T cells were positive; Figure 6A). Almost all of the Foxp3+ T cells that were expanded by the infected DCs were also positive for the Treg cell markers CD25 and GITR (more than 97%), and about 30% were positive for CD103 (data not shown). The expansion of T cells with a phenotype of Treg cells correlated with the ability of such cells to suppress the proliferation of effector T cells. Total CD4+ T cells that were activated by FV-infected DCs and contained about 26% Foxp3+ cells partly inhibited the proliferation of naive CD4+ T cells that were stimulated by antibodies against CD3 and CD28 (Figure 6B). Thus, a sufficient number of Treg cells was present in these cultures that could suppress other T-cell responses. In contrast, the CD4+ T cells from cultures with uninfected DCs showed no suppressive activity. Antigen presentation by infected DCs to the naive T cells was required for the expansion of the Treg cells, as no suppressive T cells were found without loading the DCs with the OVA peptide.
Induction of Treg cells after cocultivation with FV-infected DCs. (A) Naive TCR transgenic CD4+ T cells from DO11.10 mice were incubated with DC in a 3D collagen gel. After 24 hours, gels were digested and T cells were analyzed for the intracellular expression of the Treg cell marker Foxp3 by flow cytometry. T cells were mixed with uninfected (□) or FV-infected DCs (■), which were loaded with OVA (+Ova) or not loaded with the OVA peptide (−Ova). T cells prior to coculture served as negative control (▩). The percentage of positively stained cells in the total CD4+ T cells is shown. Cumulative data from 7 independent experiments are shown. The error bars represent standard errors of the means. Differences between cultures with FV-infected versus uninfected DCs were statistically significant (P = .006). (B) Naive TCR transgenic CD4+ T cells from DO11.10 mice were incubated with FV-infected or uninfected DCs for 3 days. After culture, T cells were isolated and added to a polyconal CD4+ T-cell proliferation assay. Left panel: as negative control, 2 × 105 nonstimulated CD4+ T cells were labeled with CFSE and analyzed by flow cytometry 3 days later. Middle panel: 2 × 105 naive CD4+ T cells were stimulated with antibodies against CD3 and CD28 and labeled with CFSE 3 days prior to analysis. At the same time, 2 × 105 total CD4+ T cells that were stimulated previously by uninfected DCs were added. However, adding nonstimulated CD4+ T cells to the culture had no influence on the proliferation of the anti-CD3, anti-CD28–stimulated T cells (data not shown). The MFI of 3 independent tests was 1155 ± 21.6. Right panel: 2 × 105 naive CD4+ T cells were stimulated with antibodies against CD3 and CD28 and labeled with CFSE 3 days prior to analysis. At the same time, a total of 2 × 105 CD4+ T cells containing about 5 × 104 Foxp3+ cells that had been stimulated previously by FV-infected DCs were added. The MFI of 3 independent tests was 1842 ± 51.7. M1 shows the percentage of nonproliferating cells in each culture. Representative data for 3 independent experiments with similar results are shown. The difference in the MFI between the groups labeled “uninfected DC” and “infected DC” was statistically significant (P < .001).
Induction of Treg cells after cocultivation with FV-infected DCs. (A) Naive TCR transgenic CD4+ T cells from DO11.10 mice were incubated with DC in a 3D collagen gel. After 24 hours, gels were digested and T cells were analyzed for the intracellular expression of the Treg cell marker Foxp3 by flow cytometry. T cells were mixed with uninfected (□) or FV-infected DCs (■), which were loaded with OVA (+Ova) or not loaded with the OVA peptide (−Ova). T cells prior to coculture served as negative control (▩). The percentage of positively stained cells in the total CD4+ T cells is shown. Cumulative data from 7 independent experiments are shown. The error bars represent standard errors of the means. Differences between cultures with FV-infected versus uninfected DCs were statistically significant (P = .006). (B) Naive TCR transgenic CD4+ T cells from DO11.10 mice were incubated with FV-infected or uninfected DCs for 3 days. After culture, T cells were isolated and added to a polyconal CD4+ T-cell proliferation assay. Left panel: as negative control, 2 × 105 nonstimulated CD4+ T cells were labeled with CFSE and analyzed by flow cytometry 3 days later. Middle panel: 2 × 105 naive CD4+ T cells were stimulated with antibodies against CD3 and CD28 and labeled with CFSE 3 days prior to analysis. At the same time, 2 × 105 total CD4+ T cells that were stimulated previously by uninfected DCs were added. However, adding nonstimulated CD4+ T cells to the culture had no influence on the proliferation of the anti-CD3, anti-CD28–stimulated T cells (data not shown). The MFI of 3 independent tests was 1155 ± 21.6. Right panel: 2 × 105 naive CD4+ T cells were stimulated with antibodies against CD3 and CD28 and labeled with CFSE 3 days prior to analysis. At the same time, a total of 2 × 105 CD4+ T cells containing about 5 × 104 Foxp3+ cells that had been stimulated previously by FV-infected DCs were added. The MFI of 3 independent tests was 1842 ± 51.7. M1 shows the percentage of nonproliferating cells in each culture. Representative data for 3 independent experiments with similar results are shown. The difference in the MFI between the groups labeled “uninfected DC” and “infected DC” was statistically significant (P < .001).
Thus, FV-infected DCs were able to expand the population of Treg cells during contact to naive T cells; this activity was dependent on the presentation of the congnate antigen to specific T cells.
Treg cells that were expanded by infected DCs originated from naive CD25+ natural Treg cells
To analyze whether or not the expanded Treg cells were originated from CD25+ T cells, we depleted these cells prior to the cultivation of FV-infected DCs with naive T cells. Stimulation of the OVA-specific CD4+CD25− T cells with OVA-loaded DCs resulted in very little expression of Foxp3 in the T cells (Figure 7A), and no difference was found between cultures with infected versus uninfected DCs. In contrast, after culturing total CD4+ T cells and infected DCs, a population of Foxp3+ cells was detectable. As also shown in Figure 6, this population was expanded after the culture with FV-infected DCs in comparison with uninfected DCs. These results correlated with the results from a proliferation assay with CD25-depleted CD4+ T cells (Figure 7B). As shown before, FV-infected DCs loaded with OVA peptide induced only a very weak proliferation of OVA-specific CD4+ T cells (Figure 7B). However, if the CD25+ T cells were removed from the CD4+ T cells, the infected DCs induced an OVA-specific T-cell proliferation that was similar to that induced by uninfected DCs. Thus, naive CD25+CD4+ T cells, a cell population that has been described as natural Treg cells,27 were required for the expansion of Foxp3+ immunosuppressive Treg cells by FV-infected DCs.
Expansion of Treg cells by FV-infected DCs depends on naive CD4+CD25+ T cells. (A) Naive TCR transgenic CD4+ (purity, > 97%) or CD4+CD25− T cells (purity, > 96%) from DO11.10 mice were incubated with uninfected (thin small gray line) or FV-infected DCs (fat black line) in a 3D collagen gel. After 72 hours, gels were digested and T cells were analyzed for the intracellular expression of Foxp3 by flow cytometry. The experiments were repeated 3 times with comparable results, and the results from representative cultures are shown. (B) 5 × 103 uninfected (□) or FV-infected (■) DCs were loaded with the OVA peptide and cocultured with 5 × 104 naive TCR transgenic total CD4+ or CD4+CD25− T cells from DO11.10 mice in a 3D collagen gel. Cells were incubated for 3 days and then pulsed with [3H] thymidine overnight to determine T-cell proliferation. Assays were done in triplicates and mean counts per minute (cpm) and standard deviations are shown. Differences between cultures with CD4+ versus CD4+CD25− were statistically significant (P < .001).
Expansion of Treg cells by FV-infected DCs depends on naive CD4+CD25+ T cells. (A) Naive TCR transgenic CD4+ (purity, > 97%) or CD4+CD25− T cells (purity, > 96%) from DO11.10 mice were incubated with uninfected (thin small gray line) or FV-infected DCs (fat black line) in a 3D collagen gel. After 72 hours, gels were digested and T cells were analyzed for the intracellular expression of Foxp3 by flow cytometry. The experiments were repeated 3 times with comparable results, and the results from representative cultures are shown. (B) 5 × 103 uninfected (□) or FV-infected (■) DCs were loaded with the OVA peptide and cocultured with 5 × 104 naive TCR transgenic total CD4+ or CD4+CD25− T cells from DO11.10 mice in a 3D collagen gel. Cells were incubated for 3 days and then pulsed with [3H] thymidine overnight to determine T-cell proliferation. Assays were done in triplicates and mean counts per minute (cpm) and standard deviations are shown. Differences between cultures with CD4+ versus CD4+CD25− were statistically significant (P < .001).
To test the hypothesis that the expansion of Treg cells after interaction with infected DCs was associated with the significant increase in long contacts between naive T cells and infected DCs, we did the following control experiment in which the contact duration of natural Treg cells and DCs was determined. Enriched CD4+CD25+ natural Treg cells, which were negative for other activation markers, like CD69 and CD44, but positive for Foxp3 (data not shown), were isolated from naive OVA-specific T cells and incubated with OVA-loaded uninfected DCs. In these experiments, we found significantly prolonged contact durations between these cells compared with contacts between DCs and CD4+CD25− T cells (Figure 4E). Interestingly, the number of very long contacts was significantly increased for the natural Treg cells (Figure 4F). This was not associated with their CD25 expression because it has been previously shown that preactivated helper T cells do not form longer contacts to DCs than naive CD4+ T cells.28 The results suggest that DCs can establish long and stable contacts to Treg cells, which might be critical for their expansion and functional activation.
Discussion
In the current study, we show that Friend retrovirus productively infects myeloid DCs and changes their phenotype and interaction with T cells. Several different viruses are able to infect DCs in their host, which leads in most cases to an impairment in the induction of immune responses by DCs.2 Thus, it has been discussed that viral infections that induce severe immunosuppression in the host, like HIV or measles virus, might be associated with an infection of DCs in general.29 Infection of DCs often results in impaired maturation and cytokine production by the DCs, which directly interferes with proper antigen presentation to and activation of T cells. FV does also induce severe immunosuppression during infection,13 and we demonstrate here that FV infects DCs. The infection resulted in a large reduction in the expression of costimulatory molecules, a characteristic of immature DCs. It has been shown that immature DCs are impaired in the formation of immunologic synapses and fail to properly activate T cells.23 In contrast, we found that the mean contact times between FV-infected DCs and naive T cells were prolonged in comparison with those of uninfected DCs and CD4+ T cells, and that infected DCs were able to induce activation of T cells. It has to be pointed out that not every single contact between FV-infected DCs and T cells was prolonged, but only the number of T cells that had very long contacts was significantly increased. It remains to be determined which molecules were involved in stabilizing the long contacts of infected DCs. Since the classical costimulatory molecules were obviously not involved, other molecules that were reported to stabilize the DC–T-cell synapse, like LFA-128 or semaphorins,30 have to be investigated in the future.
Using time-lapse video microscopy of T-cell–DC interactions in collagen gels as an experimental model, one routinely observes 2 distinct modes of interaction: most T-cell–DC interactions are short-lived and transient, whereas another part of these contacts is of prolonged duration (more than 38 minutes), with only very few interactions of intermediate duration.31 It has been shown for CD8+ T cells that long-lasting T-cell–DC interactions induce T-cell activation, whereas short contacts induce tolerance24 ; however, clear experimental evidence for this scenario in CD4+ T-cell–DC interaction is still lacking. In our own experiments, prolonged T-cell–DC contacts induced by pharmacologic activation of β2 integrins on DCs resulted in impaired rather than augmented T-cell activation,32 suggesting that T-cell activation and APC–T-cell contact duration are not strictly proportional.
Interestingly, FV-infected DCs activated CD4+ T cells, but were not able to induce significant antigen-specific T-cell proliferation. This suggests that at least some of the T cells that were stimulated by the FV-infected DCs were not typical helper T cells that usually proliferate upon stimulation with their cognate antigen. In fact, it has previously been shown that Treg cells do not proliferate in vitro in the absence of exogenous IL-2, and that they efficiently suppress the proliferation of helper T cells within the same culture.27,33 We demonstrate here that FV-infected DCs induce or expand CD4+ T cells that express the Treg cell–associated marker Foxp3 in an antigen-dependent manner. Thus, FV infection might influence the mode of T-cell stimulation by DCs. It has previously been shown that immature DCs preferentially induce Treg cells that are able to suppress other T-cell responses.34-38 Interestingly, CD80/CD86 expression by the immature DCs was not required for the induction of the Treg cells,36 supporting results from our experiments. At the moment, we can only speculate whether the prolonged contacts between FV-infected DCs and T cells are directly associated with the induction or expansion of Treg cells. It is conspicuous that the percentages of T cells with significantly longer contacts to DCs as well as the percentages of Foxp3+ T cells were both about 15% higher when we compared cultures with FV-infected cells to those with uninfected DCs, suggesting that Treg cells might establish longer contacts to DCs than other T cells. This hypothesis was supported by the control experiment using CD4+CD25+ natural Treg cells. Here, we found significantly prolonged contact durations between these cells compared with contacts between DCs and CD4+CD25− T cells (Figure 4E). Again, especially the number of very long contacts was significantly increased for the natural Treg cells. Overall, our results suggest that DCs can establish long and stable contacts to Treg cells. This is in line with findings from a diabetes model in which persistent Treg cell–DC contacts were demonstrated in vivo,39 and with a recent report showing that long contacts of naive CD4+ T cells to antigen-presenting B cells resulted in Treg cell induction.40
Taken together, the infection of DCs by FV plays a substantial role in DC–T-cell interactions, the stimulation of different T-cell populations, and the induction of immunosuppression. Virus-infected, immature DCs might be responsible for the induction or expansion of Treg cells that are found during acute infection of mice with FV41 or monkeys with simian immunodeficiency virus.42 Interestingly, at least in our in vitro experiments, the Treg cells originated from the pool of natural CD25+ Treg cells. The expanded Treg cells then suppressed CD8+ T-cell responses early during infection before the virus could be completely eliminated, and consequently contribute to chronic infection.25 In fact, treatment of FV-infected mice with immunostimulatory CpG oligodeoxynucleotides (CpG-ODNs) can partly reverse the general immunosuppression observed during acute FV infection.43 In addition, we have been able to demonstrate recently that CpG-ODNs induce at least partial maturation of FV-infected DCs in vitro and increase the capacity of infected DCs to activate naive CD8+ T cells.44 These results may indicate innovative strategies for the treatment of immunosuppressive viral infections.
Along these lines, our current findings in the FV model might have relevance for human viral infections as well. For example, HIV also infects DCs and can interfere with their function and maturation.45-48 Furthermore, similar to FV-infected DCs, HIV-infected DCs are inactive in stimulating T-cell proliferation.49 It has recently been reported that semimature DCs, which stimulate T-cell tolerance rather than immunity, accumulate in the lymph nodes of patients with HIV.50 However, in this study it remains unclear whether or not these semimature DCs were infected by HIV. Several reports have clearly demonstrated that patients with HIV have expanded populations of Treg cells, which can suppress HIV-specific T-cell responses in vitro51-54 and correlate with the inability of the cellular immune response to control viral replication.53,55 Taken together, viral infection of DCs might also lead to the induction or expansion of Treg cells in humans with HIV, and subsequently results in acute immunosuppression, loss of immune control of viral replication, and the development of chronic infection. Therapeutic means to mature virus-infected DCs might be a new general strategy to successfully treat chronic retroviral infections.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank M. Steinert for excellent technical assistance and M. Krummen for help with the CBA analysis.
This work was supported by a grant to U.D., S.G., and S.B. from the Deutsche Forschungsgemeinschaft (Di 714/6-3). In addition, part of the work was also supported by grants to U.D. (Di 714/7-2 and Di 714/8-1) and S.G. (SFB629/B3).
Authorship
Contribution: S.B. designed the research, analyzed data, and wrote parts of the paper; F.K. designed the research, analyzed data, and wrote parts of the paper; K.L. designed parts of the research and analyzed data; J.U.B. designed parts of the research and analyzed data; S.G. designed the research and wrote parts of the paper; and U.D. designed the research, analyzed data, and wrote the paper.
S.B. and F.K. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ulf Dittmer, Institute for Virology, University Clinics Essen, Hufelandstr. 55, 45122 Essen, Germany; e-mail: ulf.dittmer@uni-due.de.

![Figure 5. Activation and proliferation of T cells after cocultivation with FV-infected DCs. (A) Naive TCR transgenic CD4+ T cells from DO11.10 mice were incubated with infected or uninfected DCs in a 3D collagen gel. After 24 hours, gels were digested and T cells were analyzed for activation marker CD62L, CD69, CD25, CD54, or CD44 surface expression by flow cytometry. T cells were mixed with uninfected (□) or FV-infected DCs (■), which were loaded (+Ova) or not (−Ova) with OVA peptide. T cells without DCs served as negative control (▩). The MFI or the percentage of positive stained cells is shown. Cumulative data from 5 independent experiments are being presented. The error bars represent standard errors of the means. (B) FV-infected (■) and uninfected (●) DCs were loaded with 0.1μg/mL OVA peptide and titrated onto naive TCR transgenic CD4+ T cells from DO11.10 mice in a 3D collagen gel. Cells were incubated for 3 days and then pulsed with [3H] thymidine overnight to determine T-cell proliferation. All assays were performed in triplicate. Cultures with DCs that were not loaded with peptide served as negative controls (open symbols). *Statistically significant differences (P < .001) between cultures with FV-infected versus uninfected DCs loaded with OVA antigen. Representative results from 4 independent experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/12/10.1182_blood-2007-05-092189/4/m_zh80230709890005.jpeg?Expires=1769364435&Signature=sLV3JWscJ4yeZTf0E0idrznNPir1kS8RjLNZOVUsAp3462ivdQkC4j5-lzoLnNudFL~QZfg6C-ZSjPX0yZ2IBfPj79bmdvK0eSFSSIm1jadWcH2RNjd~L0Ef7oJEFQWFHV5WdKXebFxtyQl1k8oE~0YOkdRmv-iRXFURF05C9JItEbJ~UzJvUiX2xIt-6mrii0bUrajYTymsWarxY1xGTW-Dxll33g1MPSbGXQae6B0zzobIBdue5L2LbcNZh5~a2pGd8yv1xcoi6dQi66hCvxMJAMl8eRX7PT2D-OhEpDBfTApDatPyJhVG3Ja2ab~e6R0~1onLLDXuakzmElFLkQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 7. Expansion of Treg cells by FV-infected DCs depends on naive CD4+CD25+ T cells. (A) Naive TCR transgenic CD4+ (purity, > 97%) or CD4+CD25− T cells (purity, > 96%) from DO11.10 mice were incubated with uninfected (thin small gray line) or FV-infected DCs (fat black line) in a 3D collagen gel. After 72 hours, gels were digested and T cells were analyzed for the intracellular expression of Foxp3 by flow cytometry. The experiments were repeated 3 times with comparable results, and the results from representative cultures are shown. (B) 5 × 103 uninfected (□) or FV-infected (■) DCs were loaded with the OVA peptide and cocultured with 5 × 104 naive TCR transgenic total CD4+ or CD4+CD25− T cells from DO11.10 mice in a 3D collagen gel. Cells were incubated for 3 days and then pulsed with [3H] thymidine overnight to determine T-cell proliferation. Assays were done in triplicates and mean counts per minute (cpm) and standard deviations are shown. Differences between cultures with CD4+ versus CD4+CD25− were statistically significant (P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/12/10.1182_blood-2007-05-092189/4/m_zh80230709890007.jpeg?Expires=1769364435&Signature=pFZKFY6mn6ke433YGs1k1m7RSwFRpv57ywAnhzvHVFfKT7R~GTdWRA6b2p1MXmtVpRo~4rpazcuCohnu4T7X2pzmpynFTfGcOF49UVl~TUOYttrquuKVSomynJpyyfm5G6LYXGwmShkhk0knRavl0u5ZzCg-5f8EuwlApb45ed2TbfEfEyYULXfKAIhMUdWxqbNzuZ2C4oNEOx-hYede60obtBHEeAofzZcPZi9a1atAasFpFslyfJucLn1FaKTcsYAzuLKHC3CJJXStUjqNSZbI6HkZABmZARgysz64L1zYlm64ixb~U4eaYBuHg5YClUsW94IP1t2PrH9YZ-LRBA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
