Abstract
HLA-G is a tolerogenic molecule whose detection in sera and within allografted tissues is associated with better graft acceptance. HLA-G mediates T-cell differentiation into suppressor cells, which are thought to promote tolerance. Here, we investigated such T cells phenotypically and functionally and assessed their clinical relevance in the peripheral blood of patients who have undergone transplantation. Our results demonstrate that HLA-G expressed by antigen-presenting cells or present as soluble protein down-regulates the expression of CD4 and CD8 on allostimulated T cells at both transcriptional and posttranslational levels. These CD3+CD4low and CD3+CD8low T-cell subsets are characterized by an increased proportion of cells expressing CD45RA and HLA-DR, and a decreased number of cells expressing CD62L. In addition, these HLA-G–induced CD3+CD4low and CD3+CD8low subpopulations are Foxp3-negative suppressor T cells whose function involves IL-10. Biologic relevance came from analysis of patients who underwent transplantation, with high HLA-G plasma concentrations associated with better graft survival. Peripheral blood from these patients contains increased levels of IL-10 concomitantly to an enhanced representation of CD3+CD4low and CD3+CD8low T cells compared with HLA-G–negative patients who underwent transplantation and healthy individuals. These data define novel immunosuppressive subpopulations of peripheral blood T cells induced by HLA-G with potent implications in peripheral tolerance.
Introduction
Although central tolerance mechanisms delete the majority of autoreactive T cells during a tightly controlled selection in the thymus, peripheral tolerance is required to avoid autoimmunity or aberrant immune responses.1 Furthermore, the induction of peripheral tolerance is a major goal in human allotransplantation where allograft rejection is mediated mainly by recipient alloreactive T cells.2 T-cell activation by antigen-presenting cells (APCs) needs at least 2 signals: the first is mediated by interaction of the T-cell receptor (TCR)–CD3 complex associated with CD4 or CD8 coreceptors with MHC class II or class I molecules on APCs, respectively. The second signal is costimulatory and originates from the engagement of CD28 with its ligands CD80/CD86 on APCs. Disrupting one of these signals may constitute a very efficient way to inhibit CD4+ and CD8+ T-cell activation, with therapeutic potential to prevent organ transplant rejection.3
HLA-G can be expressed as 7 different isoforms including 4 membrane-bound (HLA-G1 to -G4) and 3 soluble (HLA-G5 to -G7) proteins due to alternative splicing of the HLA-G primary transcript (see review in Carosella et al4 ). The HLA-G5 soluble isoform (37 kDa) differs from the membrane-anchored HLA-G1 protein (39 kDa) by the absence of both the transmembrane region and the cytoplasmic tail, which are substituted by a specific C-terminus sequence. The α1 and α2 heavy chain domains make the peptide-binding groove, while the α3 domain associated with the β2 microglobulin interacts with the CD8 T-cell coreceptor.5 HLA-G does not induce immune responses through TCR interactions but rather inhibits both natural killer (NK)– and cytotoxic T lymphocyte (CTL)–mediated cytolysis through binding to inhibitory receptors.6 To date, 3 HLA-G receptors have been identified: the killer immunoglobulin-like receptor KIR2DL4/CD158d7 and the leukocyte immunoglobulin (Ig)–like receptors LILRB1/ILT-2/CD85j and LILRB2/ILT-4/CD85d.8 KIR2DL4 expression is restricted to NK cells, and ILT-4 is myeloid lineage specific, while ILT-2 is expressed by monocytes, dendritic cells, T cells, B cells, and NK cells. With such a large distribution of HLA-G receptors, various immune functions may be altered by HLA-G, such as T-cell proliferation9 and dendritic cell (DC) maturation.10,11
In opposition to classical HLA-class I molecules, HLA-G is of low polymorphism and its expression is found in a limited number of healthy tissues.4 HLA-G was first identified in cytotrophoblast where it is involved in the maternal-fetal tolerance.12 However, HLA-G expression can be up-regulated in various tissues under “pathological” conditions such as allotransplantation.13 Indeed, an increasing number of clinical studies reported HLA-G expression in recipients who did not reject their allograft following heart transplantation,14-16 kidney transplantation,17 liver transplantation,18 and liver-kidney transplantation.19,20 Concomitantly, in vitro functional studies showed that T cells stimulated by HLA-G1–expressing APCs9 or sensitized by HLA-G521 differentiate into suppressor T cells. These HLA-G–induced T cells were found to be different from the naturally occurring CD4+CD25+ regulatory T cells.21,22 Up to now, their precise phenotype, cytokine context, mechanism of action, and in vivo relevance remained to be characterized. The aim of this work was to address these points in vitro in mixed lymphocyte reactions and in vivo in human allotransplantation.
Patients, materials, and methods
APC-HLA-G1s and HLA-G5–secreting cells
The HLA class II+ human B-lymphoblastoid cell line LCL 721.221 (ATCC, Manassas, VA) was transfected by electroporation with either pRc/RSV vector alone (Invitrogen, Frederick, MD) or the pRc/RSV vector containing the HLA-G1 cDNA, to obtain LCL-RSV and LCL-HLA-G1 cells, respectively.23 The LCL-HLA-G1 cells strongly expressed HLA-G1 at their cell surface (Figure 1A box). We used LCL-RSV and LCL-HLA-G1 as an APC-like model expressing or not HLA-G1.24 These cells will be referred in the text as APCs and APC-HLA-G1s, respectively. The HLA class I–positive, HLA-G–negative, M8 melanoma cell line was transfected with the pcDNA3.1 vector (Invitrogen) either alone to generate M8-pcDNA or containing the HLA-G5 cDNA to produce M8-HLA-G5 cells, as described previously.6
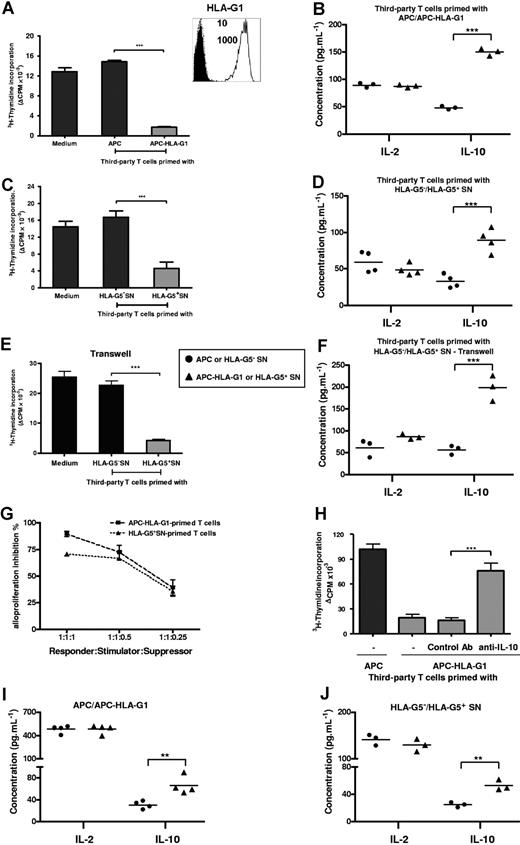
T cells primed with either APC-HLA-G1s or soluble HLA-G5 are suppressors whose generation and function occur in an IL-10 microenvironment. (A) T cells primed with either APCs or APC-HLA-G1s for 6 days were used in a suppression assay as γ-irradiated third-party cells with HLA-mismatched PBMCs as γ-irradiated stimulatory cells at a responder–stimulator–third-party cell ratio of 1:1:1. Results are expressed as the mean of thymidine incorporation (cpm) in triplicate wells plus or minus SEM from 10 allogeneic combinations and corrected for background values (Δcpm). (Box) Both APCs and APC-HLA-G1s were analyzed for cell surface expression of HLA-G1 by flow cytometry using the MEM-G/9 antibody. Dashed lines correspond to APCs and solid lines to APC-HLA-G1s. Numbers on the right correspond to the mean fluorescence intensity (MFI) observed with APCs (top) and APC-HLA-G1s (bottom). Cells were stained with an isotype-matched antibody as negative control (filled histograms). (B) Concomitantly, IL-10 and IL-2 concentrations were measured by ELISA at day 6 in suppression assay supernatants. Results from 3 independent experiments are shown. (C) Similar experiment was performed with T cells primed with supernatant from either M8-HLA-G5 (HLA-G5+ SNs) or M8-pcDNA (HLA-G5− SNs). Results are expressed as the mean of thymidine incorporation (cpm) in triplicate wells plus or minus SEM from 3 allogeneic combinations and corrected for background values (Δcpm). (D) Concomitantly, IL-10 and IL-2 concentrations were measured by ELISA at day 6 in suppression assay supernatants. Results from 4 independent experiments are shown. (E,F) Suppression assays were performed by adding HLA-G5–primed T cells at the top chamber of a transwell culture system, while MLR was at the bottom chamber. (E) One representative combination is shown. Tritiated thymidine incorporation was measured after 6 days of MLR. Results are expressed as the mean of thymidine incorporation (cpm) in triplicate wells, corrected for background values (Δ cpm). (F) IL-10 and IL-2 concentrations were measured by ELISA at day 6 in suppression assay supernatants from 3 independent experiments. (G) Suppression assays were performed at various ratios of third-party T cells primed with APC-HLA-G1s or HLA-G5+ SNs. Results are expressed as percentage of alloproliferation inhibition (%) ± SEM from 3 independent experiments. Alloproliferation inhibition (%) observed with third-party T cells primed with APC-HLA-G1s or HLA-G5+ SNs was calculated, based on the alloproliferation observed with third-party T cells primed with APCs or HLA-G5− SNs, respectively. (H) T cells primed with either APCs or APC-HLA-G1s for 6 days were used in a suppression assay at a responder–stimulator–third-party cell ratio of 1:1:1. Neutralizing IL-10 mAb (B-S10) or isotype control Ab was added on days 1 and 3 during the suppression assay. Results are expressed as the mean of thymidine incorporation (cpm) in triplicate wells plus or minus SEM from 4 allogeneic combinations and corrected for background values (Δcpm). (I,J) IL-10 and IL-2 concentrations were determined by ELISA in supernatants of MLR after 6 days of allostimulation with either (I) APCs or APC-HLA-G1s, or (J) HLA-G5− or HLA-G5+ SNs. Concentrations are given as mean plus or minus SEM of 4 (I) or 3 (J) independent experiments.
T cells primed with either APC-HLA-G1s or soluble HLA-G5 are suppressors whose generation and function occur in an IL-10 microenvironment. (A) T cells primed with either APCs or APC-HLA-G1s for 6 days were used in a suppression assay as γ-irradiated third-party cells with HLA-mismatched PBMCs as γ-irradiated stimulatory cells at a responder–stimulator–third-party cell ratio of 1:1:1. Results are expressed as the mean of thymidine incorporation (cpm) in triplicate wells plus or minus SEM from 10 allogeneic combinations and corrected for background values (Δcpm). (Box) Both APCs and APC-HLA-G1s were analyzed for cell surface expression of HLA-G1 by flow cytometry using the MEM-G/9 antibody. Dashed lines correspond to APCs and solid lines to APC-HLA-G1s. Numbers on the right correspond to the mean fluorescence intensity (MFI) observed with APCs (top) and APC-HLA-G1s (bottom). Cells were stained with an isotype-matched antibody as negative control (filled histograms). (B) Concomitantly, IL-10 and IL-2 concentrations were measured by ELISA at day 6 in suppression assay supernatants. Results from 3 independent experiments are shown. (C) Similar experiment was performed with T cells primed with supernatant from either M8-HLA-G5 (HLA-G5+ SNs) or M8-pcDNA (HLA-G5− SNs). Results are expressed as the mean of thymidine incorporation (cpm) in triplicate wells plus or minus SEM from 3 allogeneic combinations and corrected for background values (Δcpm). (D) Concomitantly, IL-10 and IL-2 concentrations were measured by ELISA at day 6 in suppression assay supernatants. Results from 4 independent experiments are shown. (E,F) Suppression assays were performed by adding HLA-G5–primed T cells at the top chamber of a transwell culture system, while MLR was at the bottom chamber. (E) One representative combination is shown. Tritiated thymidine incorporation was measured after 6 days of MLR. Results are expressed as the mean of thymidine incorporation (cpm) in triplicate wells, corrected for background values (Δ cpm). (F) IL-10 and IL-2 concentrations were measured by ELISA at day 6 in suppression assay supernatants from 3 independent experiments. (G) Suppression assays were performed at various ratios of third-party T cells primed with APC-HLA-G1s or HLA-G5+ SNs. Results are expressed as percentage of alloproliferation inhibition (%) ± SEM from 3 independent experiments. Alloproliferation inhibition (%) observed with third-party T cells primed with APC-HLA-G1s or HLA-G5+ SNs was calculated, based on the alloproliferation observed with third-party T cells primed with APCs or HLA-G5− SNs, respectively. (H) T cells primed with either APCs or APC-HLA-G1s for 6 days were used in a suppression assay at a responder–stimulator–third-party cell ratio of 1:1:1. Neutralizing IL-10 mAb (B-S10) or isotype control Ab was added on days 1 and 3 during the suppression assay. Results are expressed as the mean of thymidine incorporation (cpm) in triplicate wells plus or minus SEM from 4 allogeneic combinations and corrected for background values (Δcpm). (I,J) IL-10 and IL-2 concentrations were determined by ELISA in supernatants of MLR after 6 days of allostimulation with either (I) APCs or APC-HLA-G1s, or (J) HLA-G5− or HLA-G5+ SNs. Concentrations are given as mean plus or minus SEM of 4 (I) or 3 (J) independent experiments.
Patients who underwent transplantation and control individuals
Blood samples from patients who received a kidney transplant (KT), liver transplant (LT), or combined liver-kidney transplant (LKT) were collected during their clinical follow-up. Thirty-four KT patients (Kremlin Bicetre Hospital, Le Kremlin-Bicetre, France), 21 LT patients (Paul Brousse Hospital, Villejuif, France), and 44 LKT patients (Paul Brousse Hospital, Villejuif, France) were investigated in the present study. Within a population that underwent transplantation, patients were divided into 3 groups according to the time interval between transplantation and blood collection. Patient characteristics are described in Table 1, Table 2, and Table 3 for KT, LT, and LKT populations, respectively. All patients had stable graft function at the time of blood collection. Histologic lesions of acute or chronic graft rejection were classified according to the Banff classification. Control individuals (n = 14) were healthy volunteer blood donors (HDs) from French Blood Establishment (EFS, Saint-Louis Hospital, Paris, France). The healthy donor population was age matched (49 ± 9 years old) with that of patients who underwent transplantation. Plasma and peripheral blood mononuclear cells (PBMCs) were isolated from blood samples to investigate plasma levels of soluble HLA-G, IL-10, and soluble CD4/CD8 and phenotype of peripheral T cells. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki, and the study was approved by the local ethics committee at the Hospital Saint-Louis.
Patient characteristics: KT population
| Characteristic . | Patient groups according to follow-up period . | ||
|---|---|---|---|
| Less than 1 y . | 1 to 5 y . | More than 5 y . | |
| Patients, no. | 13 | 16 | 5 |
| Demography | |||
| Sex, no., M/F | 8/5 | 9/7 | 3/2 |
| Age, y | 48.8 ± 12.4 | 47.5 ± 12.5 | 56.2 ± 2.8 |
| Follow-up period, mo | 4.6 ± 2.7 | 24.5 ± 9.7 | 165.6 ± 104.4 |
| Medical indication for the transplantation | |||
| Chronic interstitial nephritis | 1 (7.7) | 1 (6.2) | 1 (20) |
| Diabetic nephropathy | 2 (15.4) | 3 (18.7) | 0 |
| Glomerulonephritis | 4 (30.8) | 3 (18.7) | 0 |
| IgA nephropathy | 1 (7.7) | 4 (25) | 1 (20) |
| Nephroangiosclerosis | 3 (23.1) | 2 (12.5) | 1 (20) |
| Unknown nephropathy | 2 (15.4) | 2 (12.5) | 0 |
| Other | 0 | 1 (6.2) | 2 (40) |
| Viral infection (CMV-HBV-HCV) | 1 (7.7) | 2 (12.5) | 0 |
| Immunosuppressive treatment | |||
| Cyclosporin A | 0 | 5 (31.2) | 2 (40) |
| FK506 | 10 (76.9) | 10 (62.5) | 1 (20) |
| Corticosteroid | 13 (100) | 14 (87.5) | 3 (60) |
| Mycophenolate mofetil | 13 (100) | 13 (81.2) | 2 (40) |
| Sirolimus | 2 (15.4) | 1 (6.2) | 0 |
| FTY720 | 0 | 1 (6.2) | 0 |
| Basixilimab | 1 (7.7) | 0 | 0 |
| sHLA-G | |||
| sHLA-G1 + HLA-G5, ng/mL | 10 ± 7.8 | 19.2 ± 14.7 | 23.4 ± 21.0 |
| HLA-G5, ng/mL | 1.4 ± 2.1 | 5.5 ± 7.3 | 14.4 ± 12.8 |
| Rejection | |||
| Acute rejection | 5 (38.5) | 5 (31.2) | 1 (20) |
| Chronic rejection | 1 (7.7) | 4 (25) | 0 |
| Characteristic . | Patient groups according to follow-up period . | ||
|---|---|---|---|
| Less than 1 y . | 1 to 5 y . | More than 5 y . | |
| Patients, no. | 13 | 16 | 5 |
| Demography | |||
| Sex, no., M/F | 8/5 | 9/7 | 3/2 |
| Age, y | 48.8 ± 12.4 | 47.5 ± 12.5 | 56.2 ± 2.8 |
| Follow-up period, mo | 4.6 ± 2.7 | 24.5 ± 9.7 | 165.6 ± 104.4 |
| Medical indication for the transplantation | |||
| Chronic interstitial nephritis | 1 (7.7) | 1 (6.2) | 1 (20) |
| Diabetic nephropathy | 2 (15.4) | 3 (18.7) | 0 |
| Glomerulonephritis | 4 (30.8) | 3 (18.7) | 0 |
| IgA nephropathy | 1 (7.7) | 4 (25) | 1 (20) |
| Nephroangiosclerosis | 3 (23.1) | 2 (12.5) | 1 (20) |
| Unknown nephropathy | 2 (15.4) | 2 (12.5) | 0 |
| Other | 0 | 1 (6.2) | 2 (40) |
| Viral infection (CMV-HBV-HCV) | 1 (7.7) | 2 (12.5) | 0 |
| Immunosuppressive treatment | |||
| Cyclosporin A | 0 | 5 (31.2) | 2 (40) |
| FK506 | 10 (76.9) | 10 (62.5) | 1 (20) |
| Corticosteroid | 13 (100) | 14 (87.5) | 3 (60) |
| Mycophenolate mofetil | 13 (100) | 13 (81.2) | 2 (40) |
| Sirolimus | 2 (15.4) | 1 (6.2) | 0 |
| FTY720 | 0 | 1 (6.2) | 0 |
| Basixilimab | 1 (7.7) | 0 | 0 |
| sHLA-G | |||
| sHLA-G1 + HLA-G5, ng/mL | 10 ± 7.8 | 19.2 ± 14.7 | 23.4 ± 21.0 |
| HLA-G5, ng/mL | 1.4 ± 2.1 | 5.5 ± 7.3 | 14.4 ± 12.8 |
| Rejection | |||
| Acute rejection | 5 (38.5) | 5 (31.2) | 1 (20) |
| Chronic rejection | 1 (7.7) | 4 (25) | 0 |
Patients (n = 34) were separated into 3 groups based on the time interval between the date of the transplantation and the blood sampling to measure sHLA-G plasma levels. Data are means plus or minus SD or number of patients (%). There was significant differences in sHLA-G plasma levels between groups less than 1 year and 1 to 5 years for sHLA-G1 + HLA-G5 (P< = .05) and between group less than 1 year and the others for HLA-G5 (P<.05).
M/F indicates male/female; CMV, cytomegalovirus; HBV, hepatitis B virus; and HCV, hepatitis C virus.
Patient characteristics: LT population
| Characteristic . | Patients groups according to follow-up period . | ||
|---|---|---|---|
| Less than 1 y . | 1 to 5 y . | More than 5 y . | |
| Patients, no. | 5 | 8 | 8 |
| Demography | |||
| Sex, no. M/F | 2/3 | 4/4 | 5/3 |
| Age, y | 44.8 ± 9.7 | 54.6 ± 10.6 | 56.9 ± 6.0 |
| Follow-up period, mo | 5.8 ± 4.7 | 45 ± 12.4 | 177.2 ± 58.3 |
| Medical indication for the transplantation | |||
| Viral cirrhosis | 1 (20) | 5 (62.5) | 8 (100) |
| Alcoholic cirrhosis | 2 (40) | 0 | 0 |
| Hyperoxaluria type 1/amyloidosis neuropathy | 0 | 2 (25) | 0 |
| Autoimmune cirrhosis | 0 | 1 (12.5) | 0 |
| Other | 2 (40) | 0 | 0 |
| Viral hepatic recurrence | |||
| Hepatitis B virus | 0 | 0 | 1 (12.5) |
| Hepatitis C virus | 0 | 3 (37.5) | 2 (25) |
| Immunosuppressive treatment | |||
| Cyclosporin A | 0 | 2 (25) | 7 (87.5) |
| FK506 | 5 (100) | 6 (75) | 1 (12.5) |
| Corticosteroid | 5 (100) | 7 (87.5) | 5 (62.5) |
| Mycophenolate mofetil | 5 (100) | 7 (87.5) | 5 (62.5) |
| Azathioprin | 0 | 0 | 1 (12.5) |
| sHLA-G, ng/mL | |||
| sHLA-G1 + HLA-G5 | 102.6 ± 6.2 | 116.5 ± 35.9 | 134.1 ± 50.3 |
| HLA-G5 | 97 ± 11.9 | 80.5 ± 27.3 | 110.9 ± 20.9 |
| Rejection | |||
| Acute rejection | 0 | 2 (25) | 1 (12.5) |
| Chronic rejection | 0 | 1 (12.5) | 0 |
| Characteristic . | Patients groups according to follow-up period . | ||
|---|---|---|---|
| Less than 1 y . | 1 to 5 y . | More than 5 y . | |
| Patients, no. | 5 | 8 | 8 |
| Demography | |||
| Sex, no. M/F | 2/3 | 4/4 | 5/3 |
| Age, y | 44.8 ± 9.7 | 54.6 ± 10.6 | 56.9 ± 6.0 |
| Follow-up period, mo | 5.8 ± 4.7 | 45 ± 12.4 | 177.2 ± 58.3 |
| Medical indication for the transplantation | |||
| Viral cirrhosis | 1 (20) | 5 (62.5) | 8 (100) |
| Alcoholic cirrhosis | 2 (40) | 0 | 0 |
| Hyperoxaluria type 1/amyloidosis neuropathy | 0 | 2 (25) | 0 |
| Autoimmune cirrhosis | 0 | 1 (12.5) | 0 |
| Other | 2 (40) | 0 | 0 |
| Viral hepatic recurrence | |||
| Hepatitis B virus | 0 | 0 | 1 (12.5) |
| Hepatitis C virus | 0 | 3 (37.5) | 2 (25) |
| Immunosuppressive treatment | |||
| Cyclosporin A | 0 | 2 (25) | 7 (87.5) |
| FK506 | 5 (100) | 6 (75) | 1 (12.5) |
| Corticosteroid | 5 (100) | 7 (87.5) | 5 (62.5) |
| Mycophenolate mofetil | 5 (100) | 7 (87.5) | 5 (62.5) |
| Azathioprin | 0 | 0 | 1 (12.5) |
| sHLA-G, ng/mL | |||
| sHLA-G1 + HLA-G5 | 102.6 ± 6.2 | 116.5 ± 35.9 | 134.1 ± 50.3 |
| HLA-G5 | 97 ± 11.9 | 80.5 ± 27.3 | 110.9 ± 20.9 |
| Rejection | |||
| Acute rejection | 0 | 2 (25) | 1 (12.5) |
| Chronic rejection | 0 | 1 (12.5) | 0 |
Patients (n = 21) were separated into 3 groups based on the time interval between the date of the transplantation and the blood sampling to measure sHLA-G plasma levels. Data are means plus or minus SD or number of patients (%). There was no significant difference in sHLA-G plasma levels between follow-up period groups, except between groups 1 to 5 years and more than 5 years for HLA-G5 (P=.01).
Patient characteristics: LKT population
| Characteristic . | Patients groups according to follow-up period . | ||
|---|---|---|---|
| Less than 1 y . | 1 to 5 y . | More than 5 y . | |
| Patients, no. | 14 | 18 | 12 |
| Demography | |||
| Sex, M/F | 11/3 | 10/8 | 8/4 |
| Age, y | 56.9 ± 7.8 | 56 ± 7.2 | 49.8 ± 11.1 |
| Follow-up period, mo | 4.9 ± 2.2 | 33.4 ± 14.3 | 100.2 ± 31.9 |
| Medical indication for the transplantation | |||
| Viral cirrhosis | 9 (64.3) | 7 (38.9) | 6 (50) |
| Alcoholic cirrhosis | 4 (28.6) | 3 (16.7) | 2 (16.7) |
| Hyperoxaluria type 1/amyloidosis neuropathy | 0 | 2 (11.1) | 3 (25) |
| Liver-kidney polykystosis | 1 (7.1) | 3 (16.7) | 0 |
| Other | 0 | 3 (16.7) | 1 (8.3) |
| Viral hepatic recurrence | |||
| Hepatitis B virus | 1 (7.1) | 0 | 1 (8.3) |
| Hepatitis C virus | 3 (21.4) | 4 (22.2) | 2 (16.7) |
| Immunosuppressive treatment | |||
| Cyclosporin A | 0 | 3 (16.7) | 4 (33.3) |
| FK506 | 13 (92.8) | 14 (77.8) | 4 (33.3) |
| Corticosteroid | 8 (57.1) | 7 (38.9) | 10 (83.3) |
| Mycophenolate mofetil | 6 (42.8) | 7 (38.9) | 4 (33.3) |
| Azathioprin | 0 | 0 | 3 (25) |
| Everolimus | 1 (7.1) | 1 (5.6) | 1 (8.3) |
| sHLA-G, ng/mL | |||
| sHLA-G1 + HLA-G5 | 251.4 ± 88.8 | 214.3 ± 108.9 | 238.5 ± 139.2 |
| HLA-G5 | 102.1 ± 45.3 | 95.3 ± 48.0 | 116.8 ± 61.5 |
| Rejection | |||
| Acute rejection | 0 | 1 (5.6) | 0 |
| Chronic rejection | 0 | 0 | 0 |
| Characteristic . | Patients groups according to follow-up period . | ||
|---|---|---|---|
| Less than 1 y . | 1 to 5 y . | More than 5 y . | |
| Patients, no. | 14 | 18 | 12 |
| Demography | |||
| Sex, M/F | 11/3 | 10/8 | 8/4 |
| Age, y | 56.9 ± 7.8 | 56 ± 7.2 | 49.8 ± 11.1 |
| Follow-up period, mo | 4.9 ± 2.2 | 33.4 ± 14.3 | 100.2 ± 31.9 |
| Medical indication for the transplantation | |||
| Viral cirrhosis | 9 (64.3) | 7 (38.9) | 6 (50) |
| Alcoholic cirrhosis | 4 (28.6) | 3 (16.7) | 2 (16.7) |
| Hyperoxaluria type 1/amyloidosis neuropathy | 0 | 2 (11.1) | 3 (25) |
| Liver-kidney polykystosis | 1 (7.1) | 3 (16.7) | 0 |
| Other | 0 | 3 (16.7) | 1 (8.3) |
| Viral hepatic recurrence | |||
| Hepatitis B virus | 1 (7.1) | 0 | 1 (8.3) |
| Hepatitis C virus | 3 (21.4) | 4 (22.2) | 2 (16.7) |
| Immunosuppressive treatment | |||
| Cyclosporin A | 0 | 3 (16.7) | 4 (33.3) |
| FK506 | 13 (92.8) | 14 (77.8) | 4 (33.3) |
| Corticosteroid | 8 (57.1) | 7 (38.9) | 10 (83.3) |
| Mycophenolate mofetil | 6 (42.8) | 7 (38.9) | 4 (33.3) |
| Azathioprin | 0 | 0 | 3 (25) |
| Everolimus | 1 (7.1) | 1 (5.6) | 1 (8.3) |
| sHLA-G, ng/mL | |||
| sHLA-G1 + HLA-G5 | 251.4 ± 88.8 | 214.3 ± 108.9 | 238.5 ± 139.2 |
| HLA-G5 | 102.1 ± 45.3 | 95.3 ± 48.0 | 116.8 ± 61.5 |
| Rejection | |||
| Acute rejection | 0 | 1 (5.6) | 0 |
| Chronic rejection | 0 | 0 | 0 |
Patients (n = 44) were separated into 3 groups based on the time interval between the date of the transplantation and the blood sampling to measure sHLA-G plasma levels. Data are means plus or minus SD or number of patients (%). There was no significant difference in sHLA-G plasma levels between follow-up period groups.
Cell preparation, culture procedure, MLR, and suppression assays
PBMCs from heparinized whole blood of HDs, and KT, LT, or LKT patients were obtained by density-gradient centrifugation over Ficoll-histopaque 1077 (Sigma, St Louis, MO). For allostimulation assays, PBMCs were used as responder or γ-irradiated stimulator cells (25 Gy). When lymphoblastoid cell lines (LCLs) were used as stimulator cells, a 75-Gy dose irradiation was given. For each allostimulation, responder-stimulator ratio was of 1:1 with a final concentration of responder cells of 106 cells/mL. Suppression assays used γ-irradiated third-party cells (25 Gy) added to the mixed lymphocyte reaction (MLR) at a responder–stimulator–third-party cell ratio of 1:1:1, except for dose-response assays. T-cell proliferation was measured at day 6 of MLR, as previously described.25 When indicated, suppression assays were performed using transwell (0.4 μm) culture system (Greiner bio-one, San Diego, CA). For large-scale allostimulation experiments, 40 × 106 responder cells were cocultured with γ-irradiated allogeneic stimulator cells at a ratio of 1:1 and at a final concentration of responder cells of 106 cells/mL. Each allostimulation or suppression assay was performed with PBMCs from at least 3 distinct healthy donors.
CD4+ and CD8+ T-cell isolation
After 6 days of MLR in presence or in absence of HLA-G, allostimulated CD4+ and CD8+ T cells were isolated by positive selection, respectively, using either CD4 or CD8 immunomagnetic beads according to the manufacturer's instructions (Dynal Biotech, Lake Success, NY). Positively selected CD4+ and CD8+ T-cell populations were collected after release from magnetic beads by overnight incubation at 37°C. Purity of each fraction was checked by flow cytometry showing that CD4+ and CD8+ fractions were more than 95%. In case of allostimulation using irradiated APC-HLA-G1s, we checked that none of these cells remained after 6 days of MLR, attesting that any contaminating HLA-G1+ cell was present in the CD4+ and CD8+ isolated T-cell fractions that were subsequently used in suppression assays.
Antibodies and flow cytometric analysis
The following Abs were used: MEM-G/9 anti–HLA-G1/HLA-G5 (Exbio, Vestec, Czech Republic); 5A6G7 anti–HLA-G5 (Exbio). Antibodies used for flow cytometric analyses were conjugated with either FITC, PE, ECD, or PC5 (Beckman Coulter, Hialeah, FL), except PD1-FITC (Pharmingen, San Diego, CA) and GITR-PE (R&D Systems, Minneapolis, MN). Briefly, cells were first incubated 30 minutes at 4°C in 20% human serum. Isotype control was systematically used to evaluate and compensate nonspecific signal. Cells were analyzed on EPICS XL4 cytometer using Expo32 software (Beckman Coulter).
Intracellular Foxp3 staining was performed using Alexa 488–conjugated antihuman Foxp3 Ab (clone 259D; Ozyme, Saint Quentin, France). To assess cell permeabilization, anti–α-tubulin (Sigma) was used as control. T cells activated for 48 hours with 5 μg/mL anti-CD3 Ab (OKT-3; BD Biosciences, San Jose, CA) and 1 μg/mL soluble anti-CD28 Ab (clone CD28.2; eBioscience, San Diego, CA) in the presence of 100 U/mL IL-2 (Sigma) were used as Foxp3 expression positive control.26
Anti–IL-10 blocking experiments
To neutralize IL-10, anti–IL-10 mAb (clone B-S10; Diaclone, Besançon, France) was added at a concentration of 10 μg/mL on days 1 and 3 in allostimulation or suppression assay.27 An irrelevant isotype Ab was used as a control at the same concentration.
Real-time reverse-transcription–polymerase chain reaction analysis
Total mRNA were extracted from healthy donor's peripheral blood leukocytes (PBLs; calibrator) and allostimulated T cells and reverse-transcribed to generate cDNA. Multiplex PCR amplifications were performed using commercially available primers and probe for CD4 and CD8α (Hs00181217 m1 and Hs00233520 m1, respectively; Applied Biosystems, Courtaboeuf, France) according to manufacturer's instructions. GAPDH was used as an internal standard; primer and probe were purchased from Applied Biosystems. Relative quantification was analyzed using the CT method: ΔCT = CT (CD4 or CD8) − CT (GAPDH) and ΔΔCT = ΔCT (sample) − ΔCT (calibrator). Relative expressions are given as 2−ΔΔCT.
ELISA
Soluble HLA-G concentrations were measured in plasma from KT, LT, and LKT patients, and HDs, as well as in supernatants from M8 transfectants. Two distinct enzyme-linked immunosorbent assay (ELISA) were used that detect either both shed HLA-G1 and HLA-G5 (MEM-G/9 as capture antibody and anti-β2m as detection antibody) or HLA-G5 specifically (5A6G7 as capture antibody and the Pan HLA class I W6/32 as detection antibody), as described previously.28 Plasma samples were diluted ½ or ¼ prior to measuring HLA-G.
IL-2, IFN-γ, IL-4, and IL-10 were measured by Th1/Th2 human ELISA kits (eBioscience), according to the manufacturer's instructions. ELISA was performed using either MLR or suppressor assay supernatants or undiluted plasma from KT, LT, and LKT patients. Soluble CD4 and CD8 molecules were quantified in similar samples using specific ELISA kits (US Biological, Swampscott, MA; and Bender Med System, Vienna, Austria).
Statistical analysis
Statistical analysis was performed with Student t test; ANOVA test and linear regression analyses were used to assess significance and correlation between variables. The Kaplan-Meier method was used to estimate the survival time without acute and chronic rejection. Survival differences were analyzed using the log-rank test. Statistical test, P value, and significance were given with the results assuming P values less than .05 as significant (** and *** at the top of histogram indicate P values less than .001.
Results
APC-HLA-G1s and soluble HLA-G5 induce immunosuppressive T cells
We previously showed that T cells stimulated by HLA-G1–expressing APCs9 or sensitized by soluble HLA-G521 differentiate into suppressor T cells that were different from CD4+CD25+ regulatory T cells.21 To identify and characterize these HLA-G–induced T cells, we here used HLA-G as either a membrane form presented at the cell surface of APCs or a soluble form released in biologic fluids. Previously, the direct role of HLA-G in inhibiting T-cell alloproliferation was demonstrated in these 2 expression models.24 Both HLA-G forms are biologically relevant in transplantation since infiltrating mononuclear cells expressing HLA-G were detected in vivo within grafted tissues, and since high serum levels of soluble HLA-G were measured in patients who underwent transplantation who had better graft acceptance.18,19
First, we analyzed induction of immunosuppressive T cells by HLA-G. T cells were primed with either APCs or APC-HLA-G1s and then tested as γ-irradiated third-party cells in suppression assays. APC-HLA-G1–primed T cells highly inhibited alloproliferation (n = 10, mean percentage inhibition of 88%, P < .001, Student t test) compared with T cells primed with HLA-G–negative APCs (Figure 1A). This inhibition was associated with an increased IL-10 secretion (n = 3, P < .001, Student t test), while IL-2 levels remained stable (Figure 1B). Similar results were obtained with HLA-G5–primed T cells that inhibited alloproliferation by 70% when used as third-party cells (n = 3, P < .001, Student t test), concomitantly to a significant increase of IL-10 secretion (n = 4, P < .001, Student t test) (Figure 1C,D). These results were strengthened by (1) the correlation between the ratio of third-party T cells primed with APC-HLA-G1s or HLA-G5+ SNs (suppressors) and the inhibition level of alloresponses (Figure 1G), and (2) transwell chamber experiments showing that HLA-G–induced T cells do not require cell contact to exert their suppressive function but act through soluble factor(s), including IL-10 (Figure 1E,F).
IL-10 is required for the suppressive function of HLA-G–induced T cells
We then analyzed the direct contribution of IL-10 to the suppressive function of HLA-G–induced T cells. Results showed that neutralizing IL-10 with Ab reversed the suppressive activity of HLA-G–induced T cells (Figure 1H). We next investigated whether a particular cytokinic microenvironment could be related to their generation. Results showed that increased levels of IL-10 were observed at day 6 in MLR supernatants when APC-HLA-G1s (Figure 1I) or HLA-G5 (Figure 1J) was used to induce suppressor T cells. By contrast, IL-4, IFN-γ, and IL-2 levels did not vary in the same experiments (data not shown and Figure 1I,J). Taken together, these results show that IL-10 is required for the suppressive function of HLA-G–primed T cells whose emergence occurs from IL-10 microenvironment.
APC-HLA-G1s and soluble HLA-G5 induce CD3+ T-cell subpopulations expressing lowered levels of CD4 and CD8
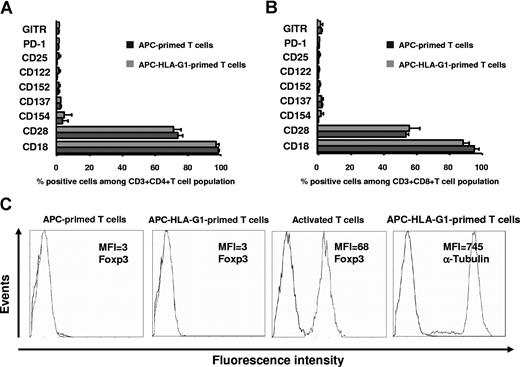
To identify the phenotype of suppressor T cells induced by HLA-G, we then analyzed the expression of T-cell markers associated with suppressive function. Flow cytometric analysis showed that the percentage of positive cells as well as mean fluorescence intensity for PD-1 (programmed cell death-1 protein), CD25 (IL-2 receptor α chain), CD122 (IL-2 receptor β chain), CD152 (CTLA-4), CD137 (4–1BB), CD154 (CD40L), CD28, CD18 (integrin β2 chain), and GITR (glucocorticoid-induced tumor necrosis factor receptor family related) did not vary among CD3+CD4+ (Figure 2A) and CD3+CD8+ (Figure 2B) T cells that had been primed for 6 days with APCs expressing or not HLA-G1. In addition, T cells primed with APCs or APC-HLA-G1s did not express intracellular Foxp3 protein, while activated T cells did as expected (Figure 2C). Similar results were obtained at day 3 (data not shown).
HLA-G–induced CD4+ and CD8+ T cells are Foxp3− and their expression of CD18, CD25, CD28, CD122, CD137, CD152, CD154, GITR, and PD1 is not modulated. (A,B) T cells were primed with either APCs or APC-HLA-G1s and were analyzed by 4-color flow cytometry at day 6 of MLR for expression of PD1, GITR, CD25, CD122, CD152, CD137, CD154, CD28, and CD18 among the CD3+CD4+ (A) and CD3+CD8+ (B) T cells. Results are shown as histograms representing the percentage of positive cells. One representative experiment of 5 is shown. (C) Intracellular Foxp3 protein expression was analyzed by flow cytometry in T cells primed with either APCs or APC-HLA-G1s at day 6 of MLR. Analysis was done after cell permeabilization on CD3+-gated cells. Positive control of Foxp3 staining was provided by anti-CD3/anti-CD28 activated T cells. Permeabilization was assessed by α-tubulin staining. One representative experiment of 3 is shown.
HLA-G–induced CD4+ and CD8+ T cells are Foxp3− and their expression of CD18, CD25, CD28, CD122, CD137, CD152, CD154, GITR, and PD1 is not modulated. (A,B) T cells were primed with either APCs or APC-HLA-G1s and were analyzed by 4-color flow cytometry at day 6 of MLR for expression of PD1, GITR, CD25, CD122, CD152, CD137, CD154, CD28, and CD18 among the CD3+CD4+ (A) and CD3+CD8+ (B) T cells. Results are shown as histograms representing the percentage of positive cells. One representative experiment of 5 is shown. (C) Intracellular Foxp3 protein expression was analyzed by flow cytometry in T cells primed with either APCs or APC-HLA-G1s at day 6 of MLR. Analysis was done after cell permeabilization on CD3+-gated cells. Positive control of Foxp3 staining was provided by anti-CD3/anti-CD28 activated T cells. Permeabilization was assessed by α-tubulin staining. One representative experiment of 3 is shown.
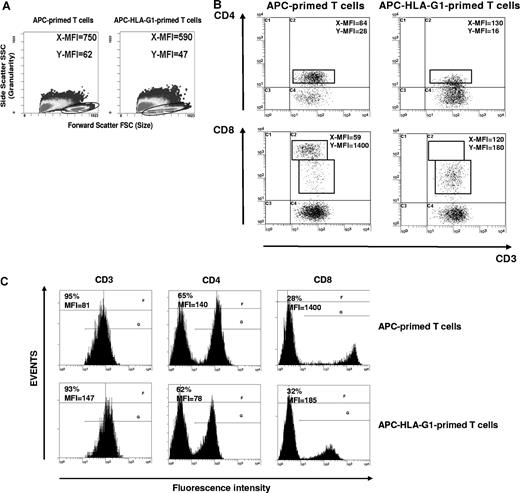
By contrast, there were striking differences in T-cell morphology between APC- and APC-HLA-G1–primed T cells. APC-primed T cells were enlarged, while APC-HLA-G1–primed T cells were smaller with fewer granularities (Figure 3A). We showed that allostimulation by APC-HLA-G1s induced T-cell subsets with lower cell surface expression of CD4 or CD8 coreceptor compared with APC-primed T cells (Figure 3B,C). Indeed, flow cytometric analysis showed a 2-fold and 8-fold decrease of CD4 and CD8 surface expression, respectively, on CD3+ T cells primed with APC-HLA-G1s compared with APC-primed T cells (Figure 3C). Interestingly, these CD3+CD4low and CD3+CD8low T cells showed higher surface expression levels of CD3 (Figure 3B,C). Such CD3+CD4low and CD3+CD8low T cells were found after 6 days of allostimulation with APC-HLA-G1s. Similar results were observed with T cells primed in presence of HLA-G5 (data not shown). Since HLA-G–induced T cells emerge from IL-10 microenvironment (Figure 1I,J), we investigated whether this cytokine was directly involved in their differentiation into CD3+CD4low and CD3+CD8low T cells. In our experimental conditions, addition of neutralizing IL-10 mAb did not affect this differentiation. Indeed, CD4 MFI was 58 (± 4) versus 55 (± 1) and CD8 MFI was 1182 (± 202) versus 944 (± 105) with and without anti–IL-10 mAb, respectively (n = 3, P > .05, Student t test). In these experiments, CD4 and CD8 MFI was 84 (± 3) and 2192 (± 104), respectively, after T-cell priming in the absence of HLA-G.
CD3+CD4low and CD3+CD8low T cells are induced after APC-HLA-G1 priming. (A) Morphology and granularity of T cells primed with either APCs or APC-HLA-G1s are provided on a forward scatter (size) and side scatter (granularity) graph. APC-primed T cells are enlarged (mean fluorescence intensity = MFI, X-MFI = 750) and granulated (Y-MFI = 62), while APC-HLA-G1–primed T cells are smaller (X-MFI = 590) with fewer granularities (Y-MFI = 47). (B,C) T cells primed with either APCs (left panel) or APC-HLA-G1s (right panel) were analyzed for surface expression of CD3, CD4, and CD8 at day 6 of MLR using 4-color flow cytometry. Results are expressed as (B) dot plots gated on CD3+ T cells or (C) histograms. (B) X-MFI corresponds to CD3-MFI, and Y-MFI indicates CD4-MFI or CD8-MFI. (C) Numbers on the left of each graph represent the percentage of positive cells (top) and the MFI calculated on the positive cells (bottom). Two representative experiments of 6 are shown.
CD3+CD4low and CD3+CD8low T cells are induced after APC-HLA-G1 priming. (A) Morphology and granularity of T cells primed with either APCs or APC-HLA-G1s are provided on a forward scatter (size) and side scatter (granularity) graph. APC-primed T cells are enlarged (mean fluorescence intensity = MFI, X-MFI = 750) and granulated (Y-MFI = 62), while APC-HLA-G1–primed T cells are smaller (X-MFI = 590) with fewer granularities (Y-MFI = 47). (B,C) T cells primed with either APCs (left panel) or APC-HLA-G1s (right panel) were analyzed for surface expression of CD3, CD4, and CD8 at day 6 of MLR using 4-color flow cytometry. Results are expressed as (B) dot plots gated on CD3+ T cells or (C) histograms. (B) X-MFI corresponds to CD3-MFI, and Y-MFI indicates CD4-MFI or CD8-MFI. (C) Numbers on the left of each graph represent the percentage of positive cells (top) and the MFI calculated on the positive cells (bottom). Two representative experiments of 6 are shown.
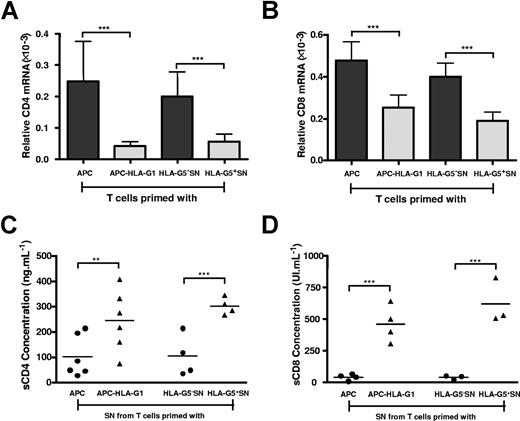
To define the level at which HLA-G downmodulates CD4 and CD8 expression, we first analyzed CD4 and CD8 gene transcription. Results showed that both CD4 (Figure 4A) and CD8 (Figure 4B) transcriptional levels were significantly decreased in, respectively, HLA-G–induced CD3+CD4low and CD3+CD8low T cells compared with T cells primed in absence of HLA-G (P < .001, Student t test) (Figure 4A,B). Second, we investigated whether HLA-G–mediated CD4/CD8 downmodulation may also result from a release of both molecules from T-cell surface. Results showed that the amount of soluble CD4 (Figure 4C) and CD8 (Figure 4D) molecules was significantly higher in supernatants from HLA-G–induced T cells than in those of T cells primed in absence of HLA-G (P < .001, Student t test).
HLA-G affects both CD4 and CD8 gene transcription and expression. T cells were primed either with APCs or APC-HLA-G1s or with HLA-G5− or HLA-G5+ SNs for 6 days. T cells and supernatants were then collected for further analysis. (A,B) Total RNA was isolated from T-cell populations and used for real-time RT-PCR analysis using specific CD4 (A) or CD8 (B) primer and probe. Relative quantities of CD4 (A) and CD8 (B) transcripts in each T-cell population are shown in comparison with those of control healthy donor's PBMCs (assigned a value of 1). Results are expressed as mean plus or minus SEM from 4 independent experiments. (C,D) Levels of soluble CD4 (C) and CD8 (D) molecules were measured by specific ELISA in supernatants from T cells primed either with APCs or APC-HLA-G1s or with HLA-G5− or HLA-G5+ SNs. Concentrations are given as mean plus or minus SEM.
HLA-G affects both CD4 and CD8 gene transcription and expression. T cells were primed either with APCs or APC-HLA-G1s or with HLA-G5− or HLA-G5+ SNs for 6 days. T cells and supernatants were then collected for further analysis. (A,B) Total RNA was isolated from T-cell populations and used for real-time RT-PCR analysis using specific CD4 (A) or CD8 (B) primer and probe. Relative quantities of CD4 (A) and CD8 (B) transcripts in each T-cell population are shown in comparison with those of control healthy donor's PBMCs (assigned a value of 1). Results are expressed as mean plus or minus SEM from 4 independent experiments. (C,D) Levels of soluble CD4 (C) and CD8 (D) molecules were measured by specific ELISA in supernatants from T cells primed either with APCs or APC-HLA-G1s or with HLA-G5− or HLA-G5+ SNs. Concentrations are given as mean plus or minus SEM.
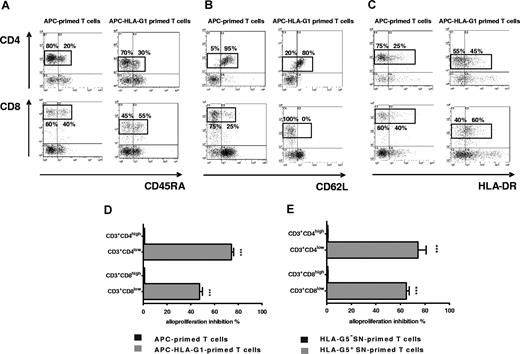
Extensive phenotypic analysis showed that the percentage of cells expressing CD45RA, HLA-DR, and CD62L differed among CD3+CD4low and CD3+CD8low T cells compared with CD3+CD4high and CD3+CD8high T cells primed without HLA-G (Figure 5). Indeed, CD4highCD45RA+ T cells represented 20% of CD3+CD4high T cells primed with APCs, whereas CD4lowCD45RA+ T cells represented 30% of CD3+CD4low T cells (Figure 5A). CD45RA+ cells reached 55% among CD3+CD8low T cells, while they represented 40% of the CD3+CD8high T cells primed with APCs (Figure 5A). These differences were significant with a mean of CD4highCD45RA+ versus CD4lowCD45RA+ T cells: 20 (± 1) versus 33 (± 2), and a mean of CD8highCD45RA+ versus CD8lowCD45RA+ T cells: 37 (± 1) versus 53 (± 4); (n = 3, P > .05, Student t test). Interestingly, the percentage of HLA-DR+ cells was increased in both CD3+CD4low and CD3+CD8low T cells by more than 1.5-fold (Figure 5C). This enhancement was significant with a mean of CD4highHLA-DR+ versus CD4lowHLA-DR+ T cells: 28 (± 4) versus 48 (± 4), and a mean of CD8highHLA-DR+ versus CD8lowHLA-DR+ T cells: 39 (± 4) versus 56 (± 3; n = 3, P < .05, Student t test). Concomitantly, CD62L+ T-cell subsets decreased among CD3+CD4low and CD3+CD8low T cells (Figure 5B). This diminution was significant with a mean of CD4highCD62L+ versus CD4lowCD62L+ T cells: 94 (± 3) versus 76 (± 3), and a mean of CD8highCD62L+ versus CD8lowCD62L+ T cells: 21 (± 2) versus 5 (± 2; n = 3, P < .05, Student t test). All these results were similarly observed with T cells primed in presence of HLA-G5 (data not shown).
CD3+CD4low and CD3+CD8low cells are suppressor T-cell subsets whose CD45RA, CD62L, and HLA-DR expression is modulated. (A-C) T cells primed with either APCs (left panel) or APC-HLA-G1s (right panel) were analyzed by 4-color flow cytometry at day 6 of MLR for expression of (A) CD45RA, (B) CD62L, and (C) HLA-DR among CD4+ and CD8+ T cells. Results are shown as dot plots. Numbers represent the percentage of cells within the corresponding region. One representative experiment of 3 is shown. (D-E) T cells were primed either with (D) APCs or APC-HLA-G1s or with (E) HLA-G5− or HLA-G5+ SNs for 6 days. Then, CD4+ and CD8+ T cells were sorted. These purified cells were used in an immunosuppressive assay as γ-irradiated third-party cells in MLR with HLA-mismatched PBMCs at a responder–stimulator–third-party cell ratio of 1:1:1. Results are expressed as percentage of alloproliferation inhibition (%) ± SEM in triplicate wells. Alloproliferation inhibition percentage observed with CD3+CD4low or CD3+CD8low T cells was calculated, based on the alloproliferation observed with CD3+CD4high and CD3+CD8high T cells, respectively.
CD3+CD4low and CD3+CD8low cells are suppressor T-cell subsets whose CD45RA, CD62L, and HLA-DR expression is modulated. (A-C) T cells primed with either APCs (left panel) or APC-HLA-G1s (right panel) were analyzed by 4-color flow cytometry at day 6 of MLR for expression of (A) CD45RA, (B) CD62L, and (C) HLA-DR among CD4+ and CD8+ T cells. Results are shown as dot plots. Numbers represent the percentage of cells within the corresponding region. One representative experiment of 3 is shown. (D-E) T cells were primed either with (D) APCs or APC-HLA-G1s or with (E) HLA-G5− or HLA-G5+ SNs for 6 days. Then, CD4+ and CD8+ T cells were sorted. These purified cells were used in an immunosuppressive assay as γ-irradiated third-party cells in MLR with HLA-mismatched PBMCs at a responder–stimulator–third-party cell ratio of 1:1:1. Results are expressed as percentage of alloproliferation inhibition (%) ± SEM in triplicate wells. Alloproliferation inhibition percentage observed with CD3+CD4low or CD3+CD8low T cells was calculated, based on the alloproliferation observed with CD3+CD4high and CD3+CD8high T cells, respectively.
Both CD3+CD4low and CD3+CD8low T-cell subsets induced by HLA-G are suppressor cells
We next investigated whether the HLA-G–induced suppressive function was associated with the CD3+CD4low/CD3+CD8low phenotype. We sorted the CD3+CD4low and CD3+CD8low T cells after priming with APC-HLA-G1s or HLA-G5, and the CD3+CD4high and CD3+CD8high T cells after priming in the absence of HLA-G after 6 days of MLR. These cells were then used as third-party cells in suppression assays. CD3+CD4low and CD3+CD8low T cells induced either by APC-HLA-G1s (Figure 5D) or HLA-G5 (Figure 5E) significantly inhibited alloproliferation compared with CD3+CD4high and CD3+CD8high. Indeed, CD3+CD4low and CD3+CD8low T cells induced by APC-HLA-G1s inhibited alloproliferation by 74% (± 2%) and 47% (± 2%; n = 3, mean ± SEM), respectively, compared with CD3+CD4high and CD3+CD8high T cells (P < .001, Student t test) (Figure 5D). Similar results were found using CD3+CD4low and CD3+CD8low T cells induced by HLA-G5 leading to alloproliferation inhibition of 74% (± 7%) and 65% (± 2%; n = 3, mean ± SEM, P < .001, Student t test), respectively (Figure 5E).
These data show that HLA-G1 and HLA-G5 affect T-cell activation during in vitro allogeneic challenge inducing CD3+CD4low and CD3+CD8low suppressor T cells. To assess the clinical relevance of these HLA-G–induced suppressor T-cell subsets, we studied patients who received a combined liver-kidney transplant (LKT). Previous studies showed that LKT patients have high HLA-G plasma levels and peripheral blood T cells with suppressive function.21 They better accept their kidney graft compared with patients who received a single kidney transplant (KT), and this improved allograft acceptance could be associated with HLA-G expression.18,19
CD3+CD4low and CD3+CD8low suppressor T-cell subsets are overrepresented in the peripheral blood of patients who underwent transplantation, with high HLA-G plasma levels and better graft acceptance
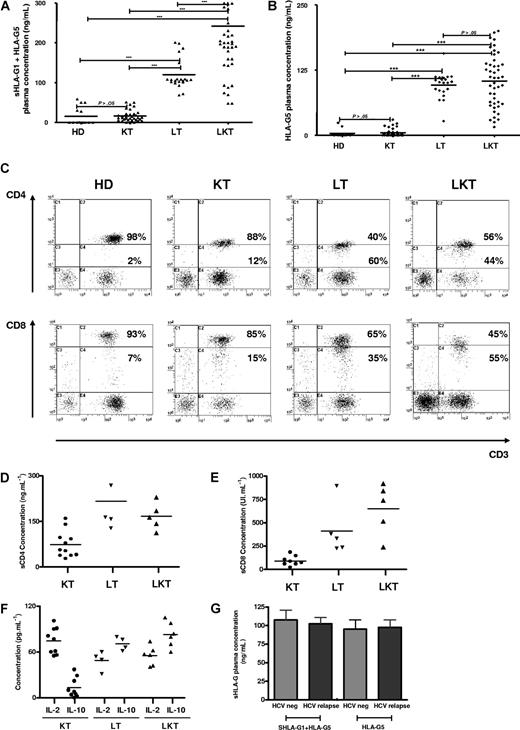
Here, 44 newly recruited LKT patients were studied. We first checked HLA-G plasma levels in this novel panel of LKT patients in comparison with healthy donors (HDs, n = 14). For this purpose, 2 different ELISAs were performed that detect either shed HLA-G1 plus HLA-G5 (sHLA-G1 + HLA-G5) (Figure 6A) or only HLA-G5 (Figure 6B). Since HLA-G can be trapped within the clot when serum is collected,29 we worked with plasma that better reflects the in vivo concentration of circulating HLA-G. In agreement with our previous results,21 HLA-G plasma levels were significantly higher in LKT patients compared with HDs (Figure 6A,B). The respective roles played by the liver and the kidney graft were examined by measuring HLA-G in plasma from patients who received a single liver transplant (LT, n = 21) and patients who received a single kidney transplant (KT, n = 34). Considering the concentrations from HDs as basal levels of HLA-G in plasma (normal values), all LT and LKT patients had high HLA-G plasma levels (P < .001 comparing LT or LKT to HD, Student t test) (Figure 6A,B). By contrast, KT patients had HLA-G plasma concentrations close to normal values (P > .05 comparing KT to HD, Student t test) (Figure 6A,B). Significant differences were observed when comparing LT or LKT to KT (P < .001, Student t test). These results showed that high HLA-G plasma levels are always detected in patients who underwent a liver transplantation, strongly suggesting a specific role of the liver in up-regulating HLA-G expression. By delineating patients according to follow-up period posttransplantation, we found that HLA-G plasma levels were stable over time in the LKT and LT populations, while increased HLA-G levels were observed long term in KT patients (P < .05, Student t test) (Tables 1,Table 2–3). Moreover, we did not find any difference between occurrence of posttransplantation viral infections and HLA-G plasma levels in LT patients (Figure 6G).
CD3+CD4low and CD3+CD8low suppressor T cells are overrepresented in patients who underwent transplantation with high plasma levels of HLA-G, IL-10, and sCD4/sCD8 molecules. (A-B) Soluble HLA-G levels were measured in plasma from healthy donors (HDs, n = 14), and patients who received a kidney transplant (KT, n = 34), liver transplant (LT, n = 21), and combined liver-kidney transplant (LKT, n = 44). Concentrations were measured by ELISA using as capture antibody either (A) MEM-G/9 specific for both shed HLA-G1 and HLA-G5 or (B) 5A6G7 reacting only with HLA-G5 and as detection antibody either (A) anti-β2m or (B) W6/32. (C) PBMCs from HD, KT, LT, and LKT were analyzed by 3-color flow cytometry for surface expression of CD3, CD4, and CD8. Results are shown as dot plots in which the low and high coreceptor-expressing cells were defined according to the basal expression level observed in healthy donors. Numbers represent the percentage of cells within the corresponding region. Six individuals from each group were analyzed showing a similar pattern of CD4 and CD8 coreceptor expression. One representative pattern is shown. (D-E) Levels of soluble CD4 and CD8 molecules were measured by specific ELISA in plasma from KT, LT, and LKT patients. (F) IL-10 and IL-2 concentrations were measured by ELISA in plasma from KT, LT, and LKT patients. (G) Comparison of reactivation posttransplantation of viral infections due to hepatitis C virus and sHLA-G plasma levels is shown for LT patients dividing according to the occurrence of HCV relapse or not (HCV neg).
CD3+CD4low and CD3+CD8low suppressor T cells are overrepresented in patients who underwent transplantation with high plasma levels of HLA-G, IL-10, and sCD4/sCD8 molecules. (A-B) Soluble HLA-G levels were measured in plasma from healthy donors (HDs, n = 14), and patients who received a kidney transplant (KT, n = 34), liver transplant (LT, n = 21), and combined liver-kidney transplant (LKT, n = 44). Concentrations were measured by ELISA using as capture antibody either (A) MEM-G/9 specific for both shed HLA-G1 and HLA-G5 or (B) 5A6G7 reacting only with HLA-G5 and as detection antibody either (A) anti-β2m or (B) W6/32. (C) PBMCs from HD, KT, LT, and LKT were analyzed by 3-color flow cytometry for surface expression of CD3, CD4, and CD8. Results are shown as dot plots in which the low and high coreceptor-expressing cells were defined according to the basal expression level observed in healthy donors. Numbers represent the percentage of cells within the corresponding region. Six individuals from each group were analyzed showing a similar pattern of CD4 and CD8 coreceptor expression. One representative pattern is shown. (D-E) Levels of soluble CD4 and CD8 molecules were measured by specific ELISA in plasma from KT, LT, and LKT patients. (F) IL-10 and IL-2 concentrations were measured by ELISA in plasma from KT, LT, and LKT patients. (G) Comparison of reactivation posttransplantation of viral infections due to hepatitis C virus and sHLA-G plasma levels is shown for LT patients dividing according to the occurrence of HCV relapse or not (HCV neg).
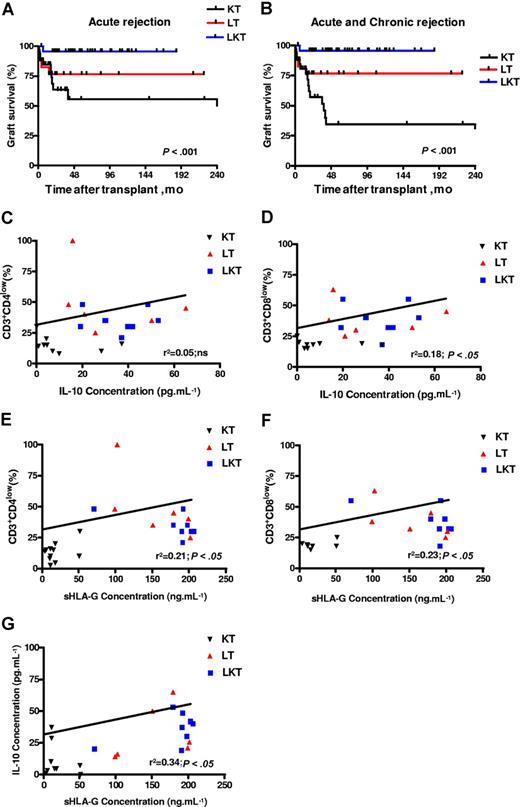
Next, the peripheral blood CD3+CD4+ and CD3+CD8+ T-cell populations from HDs were compared with those of KT, LT, and LKT patients. We found an increase of CD3+CD4low and CD3+CD8low cells among circulating T cells from both LT and LKT patients compared with peripheral CD3+CD4+ and CD3+CD8+ T cells from HD and KT patients. One representative individual for each group is shown in Figure 6C. The low and high CD4- and CD8-expressing T cells were defined according to the basal expression level observed in healthy donors; all samples (ie, PBMCs from HD, KT, LT, and LKT) were processed together. Thus, our in vitro and in vivo data indicate that the presence of increased CD3+CD4low and CD3+CD8low T-cell subsets in both LT and LKT patients can be attributed to HLA-G. Supporting this conclusion and in agreement with in vitro results described in Figure 1I,J, we found (1) significantly higher amounts of IL-10 (Figure 6F) and of both soluble CD4 and CD8 molecules (Figure 6D,E) in plasma from HLA-G+ LT and LKT compared with those from HLA-G− KT patients (P < .001, Student t test), and (2) a significant correlation between sHLA-G plasma levels and the presently described HLA-G–related immunologic parameters (ie, IL-10 and suppressor CD3+CD4low and CD3+CD8low subpopulations) (Table 4; Figure 7C–G
Correlation between HLA-G and HLA-G–related immunologic parameters
| . | KT, n = 9 . | LT, n = 6 . | LKT, n = 8 . | Correlation with HLA-G . | Correlation with IL-10 . |
|---|---|---|---|---|---|
| sHLA-G, ng/mL | 19.4 ± 18.1 | 154.8 ± 45.9 | 178.6 ± 44.7 | — | — |
| IL-10, pg/mL | 10.7 ± 13.1 | 32 ± 20.9 | 36.3 ± 12.4 | r2 = 0.34; P < .05 | — |
| CD3+CD4low, % | 15.3 ± 6.6 | 48.8 ± 26.3 | 34.6 ± 9.3 | r2 = 0.21; P < .05 | r2 = 0.05; ns |
| CD3+CD8low, % | 18.6 ± 3.0 | 38.8 ± 13.7 | 38 ± 12.5 | r2 = 0.23; P < .05 | r2 = 0.18; P < .05 |
| . | KT, n = 9 . | LT, n = 6 . | LKT, n = 8 . | Correlation with HLA-G . | Correlation with IL-10 . |
|---|---|---|---|---|---|
| sHLA-G, ng/mL | 19.4 ± 18.1 | 154.8 ± 45.9 | 178.6 ± 44.7 | — | — |
| IL-10, pg/mL | 10.7 ± 13.1 | 32 ± 20.9 | 36.3 ± 12.4 | r2 = 0.34; P < .05 | — |
| CD3+CD4low, % | 15.3 ± 6.6 | 48.8 ± 26.3 | 34.6 ± 9.3 | r2 = 0.21; P < .05 | r2 = 0.05; ns |
| CD3+CD8low, % | 18.6 ± 3.0 | 38.8 ± 13.7 | 38 ± 12.5 | r2 = 0.23; P < .05 | r2 = 0.18; P < .05 |
Data are means plus or minus SD. sHLA-G corresponds to sHLA-G1 + HLA-G5 levels. Correlation of HLA-G–related immunologic parameters with HLA-G or IL-10 was determined using ANOVA test and linear regression analyses. KT, LT, and LKT patients underwent their transplantation for more than 1 year.
— indicates not applicable.
sHLA-G plasma levels correlate with IL-10 plasma levels, percentage of CD3+CD4low and CD3+CD8low T cells, and graft survival in patients who underwent transplantation. (A,B) Kaplan-Meier estimated graft survival without acute (A) or without acute and chronic (B) rejection among the 3 populations that received a transplant: KT, LT, and LKT. Survival differences are provided with log-rank test. (C-G) Correlation and significance between IL-10 and CD3+CD4low (C), and CD3+CD8low (D); and between sHLA-G plasma levels and CD3+CD4low (E), and CD3+CD8low (F) and IL-10 (G) are shown using ANOVA test and linear regression analyses. Correlation coefficients and P values are shown for each analysis.
sHLA-G plasma levels correlate with IL-10 plasma levels, percentage of CD3+CD4low and CD3+CD8low T cells, and graft survival in patients who underwent transplantation. (A,B) Kaplan-Meier estimated graft survival without acute (A) or without acute and chronic (B) rejection among the 3 populations that received a transplant: KT, LT, and LKT. Survival differences are provided with log-rank test. (C-G) Correlation and significance between IL-10 and CD3+CD4low (C), and CD3+CD8low (D); and between sHLA-G plasma levels and CD3+CD4low (E), and CD3+CD8low (F) and IL-10 (G) are shown using ANOVA test and linear regression analyses. Correlation coefficients and P values are shown for each analysis.
Finally, regarding allograft acceptance status of these patients: one acute and no chronic graft rejection were observed in the 44 LKT patients studied (98% acceptance); 4 of 21 LT patients suffered from acute or chronic liver graft rejection (81% acceptance); and 16 of 34 KT patients suffered from acute or chronic kidney graft rejection (53% acceptance). Graft acceptance was significantly higher in HLA-G+ patients compared with HLA-G− patients. Indeed, LKT and LT patients had a significantly higher survival time without acute rejection (P < .001, log-rank test; Figure 7A) or without acute and chronic rejection (P < .001, log-rank test; Figure 7B) compared with KT patients. Thus, allograft acceptance is observed in patient groups with high HLA-G and IL-10 plasma levels and overrepresentation of peripheral blood CD3+CD4low and CD3+CD8low T-cell subsets (ie, LT and LKT groups).
Discussion
HLA-G inhibits CD4+ T-cell proliferation, induces CD4+ T-cell long-term unresponsiveness, and causes the differentiation of T cells into immunosuppressive/regulatory T cells.9,21,25 First, attention has been focused on naturally occurring CD4+CD25+ regulatory T cells. Injection of HLA-G tetramer–coated beads to recipient mice just before skin allograft induced expansion of CD4+CD25+ regulatory T cells among splenocytes and allowed skin graft tolerance.10,11,30 However, current studies in humans have failed to demonstrate that HLA-G induces expansion of such CD4+CD25+ T cells.21,22 The aim of the present work was to characterize these HLA-G–induced T cells phenotypically and functionally, and to provide evidence of their clinical relevance in humans.
Here, we report that membrane-bound HLA-G1 on APCs, as well as soluble HLA-G5, induces T cells with suppressive function. The level of suppression is directly linked to the suppressor T-cell number added in the mixed lymphocyte reaction and remains significant with 4-fold fewer suppressors than responders. These HLA-G–induced suppressive T cells express lowered levels of CD4 and CD8 coreceptors and are phenotypically defined as CD3+CD4low and CD3+CD8low T cells. We found that the downmodulation of CD4 and CD8 surface expression on HLA-G–driven T cells results from both transcriptional and posttranslational regulation. Indeed, HLA-G induces a decrease of CD4 and CD8 mRNA transcription as well as an increase of release of both coreceptors from T-cell surface. The CD8 downmodulation may be due to direct interaction between HLA-G and CD8 coreceptor. Such hypothesis is supported by previous works demonstrating that HLA-G α3 domain interacts with CD8.31 In agreement with our present data, HLA-G was found to down-regulate the expression of both CD8α mRNA and protein in IFN-γ–treated PBMCs without inducing apoptosis.32,33 Since we previously demonstrated that blocking HLA-G receptors ILT-2 and ILT-4 prevent HLA-G inhibitory effects on T cells,21,24 we postulate that induction of CD3+CD4low and CD3+CD8low T cells is due mainly to HLA-G interaction with ILT-2/ILT-4 expressed by T cells and/or APCs.
These CD3+CD4low and CD3+CD8low T cells show an increased representation of CD45RA+ cells. It has been shown that primed CD4+ T cells expressing CD45RA secreted high amounts of IL-4, IL-10, and IL-13, whereas the production of IFN-γ was very low.34 This pattern of cytokine production is akin to lymphocytes with a Th2 phenotype. HLA-DR+ cells were also overrepresented in the HLA-G–driven CD3+CD4low and CD3+CD8low T cells compared with CD4high and CD8high T cells. Similar increased percentage of CD8+HLA-DR+ peripheral T cells was observed in human immunodeficiency virus 1 (HIV-1)–infected patients.35 Notably, HIV-1–infected patients were also described to express HLA-G.36 We also show a reduced percentage of CD62L+ cells among HLA-G–driven T cells. CD62L− cells are defined as memory T cells known to be less responsive to allostimulation than CD62L+ naive T cells.37 In agreement with this, suppressor T cells induced by HLA-G are hyporesponsive to allostimulation.21
Different types of regulatory/suppressor T cells have been described based on distinct expression patterns of markers and cytokines, suppressive mechanisms, and origin. Foxp3 is a transcription factor associated with the development of naturally occurring CD4+CD25+ regulatory T cells that express this factor at high levels constitutively.38 In the present study, HLA-G–induced suppressor T cells do not express high levels of CD25 or Foxp3. This expression pattern was previously observed for other regulatory T-cell types such as the IL-10–secreting T-regulatory type 1 (Tr1) cells.39 CD4+CD25+ Foxp3+ regulatory T cells exert their suppressive function via direct cell contact.40 The activity of Tr1 and T-helper 3 regulatory T cells is not dependent on cell contact and is based mainly on cytokines such as IL-10 and TGF-β.41 Cytokines are not only essential for function but also important for the generation of suppressor T cells.42 Here, IL-10 was identified as the cytokine directly involved in the suppressive function of HLA-G–induced T cells. This function did not occur through downmodulation of IL-2 secretion but rather through an up-regulation of IL-10 production counteracting the IL-2–mediated T-cell proliferation signal. However, although increased IL-10 levels correlated with the emergence of these HLA-G–induced suppressive T cells, IL-10 seems not necessary to their generation.
Biologic relevance was provided by analyzing groups of patients who underwent transplantation who express HLA-G at high levels (ie, LT and LKT patients) in comparison with healthy controls or KT patients who have baseline levels of HLA-G. Suppressor T cells induced by HLA-G in vitro were overrepresented in patients who express HLA-G. Indeed, we consistently observed an increased representation of CD3+CD4low and CD3+CD8low within the peripheral blood from both LT and LKT patients. Notably, a correlation was clearly obtained between HLA-G, IL-10, and CD3+CD4low and CD3+CD8low T cells. Furthermore, the posttranslational mechanism by which HLA-G induces CD4 and CD8 down-regulation was supported by the increased amount of soluble CD4 and CD8 molecules in plasma from LT and LKT versus KT patients. These findings show that HLA-G but also its related immunologic parameters such as IL-10, soluble CD4/CD8, and CD3+CD4low/CD3+CD8low suppressor T cells all would contribute to induce tolerance in HLA-G+ patients. Incidentally, soluble CD4 and CD8 coreceptors are believed to be potent inhibitors of T-cell activation by competing with membrane-anchored coreceptors, thus preventing T-cell activation via coreceptor/CD3-TCR complex-peptide/MHC interaction.34
Better graft acceptance was observed in LT and LKT patient groups that all received a liver allograft. Therefore, HLA-G can be considered as a graft acceptance–associated marker whose expression may be up-regulated by liver factors. The liver has long been known to have a positive effect on the induction of peripheral tolerance.43 Previous studies showed that patients who received a combined liver-kidney transplant experienced significantly less kidney rejection compared with patients receiving a kidney transplant alone.44 Here, we detected increased levels of soluble HLA-G in plasma from all LT and LKT patients compared with those observed in HD and KT patients. Thus, we postulate that the liver allograft plays a role in HLA-G expression. The liver may be directly involved in secretion of HLA-G since its expression was detected on biliary epithelial cells, as we previously described.19 However, only some patients were displaying HLA-G+ biliary epithelial cells in the grafted liver. Another nonexclusive hypothesis is that liver cells secrete soluble factors that up-regulate HLA-G expression in an endocrine manner. Thereby, the hepatocyte growth factor (HGF) could be one candidate because its roles in vivo in graft acceptance45,46 and in vitro on monocyte differentiation into dendritic regulatory cells47 were recently reported. Besides, dendritic cells are described as efficient HLA-G secretors under adequate stimuli.48 Another candidate is the anti-inflammatory cytokine IL-10, which is produced by the liver49 and can up-regulate HLA-G expression in dendritic cells and monocytes.50 HLA-G+ dendritic cells may be preferentially localized in draining lymph nodes, from which soluble HLA-G molecules are delivered in the blood circulation.
Otherwise, CD3+CD4low and CD3+CD8low cells have been previously identified in 3 biologic contexts: in the thymus, in HIV-infected individuals, and in patients with chronic lymphocytic leukemia.51-53 Interestingly, HLA-G has also been described in thymic epithelial cells and in the course of the same pathologies,36,54,55 supporting our observation that HLA-G generates CD3+CD4low and CD3+CD8low suppressive T cells.
Such peripheral suppressive T cells may contribute to maintain an immune tolerant environment improving graft acceptance in LT and LKT patients. Here, we propose HLA-G as a biomarker of graft acceptance in the clinical follow-up of patients who underwent transplantation. HLA-G measurement in blood may be useful to predict graft outcome and provide physicians with a new approach to better manage patients who underwent transplantation. Furthermore, this study reinforces the potential use of HLA-G as an immunosuppressive agent to prevent allograft rejection. Finally, our present findings can be extended to other contexts in which HLA-G expression is up-regulated such as pregnancy,4 cancer,56 autoimmune disorders,57 and viral infections.36,58
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Commissariat à l'Energie Atomique and the Agence de la Biomedecine.
Authorship
Contribution: A.N., J.L., E.D.C., and N.R.-F. designed and analyzed all experiments; A.N. and N.R.-F. wrote the paper; A.N., S.L.R., I.K.-R., M.D., and J.C. performed experiments; A.D. and C.C. provided samples from patients who underwent transplantation; A.D. analyzed all clinical data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nathalie Rouas-Freiss, Service de Recherches en Hemato-Immunologie, CEA-DSV-DRM, Hopital Saint-Louis, Institut Universitaire d'Hematologie, 1, avenue Claude Vellefaux, 75010 Paris, France; e-mail: nathalie.rouas-freiss@cea.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal