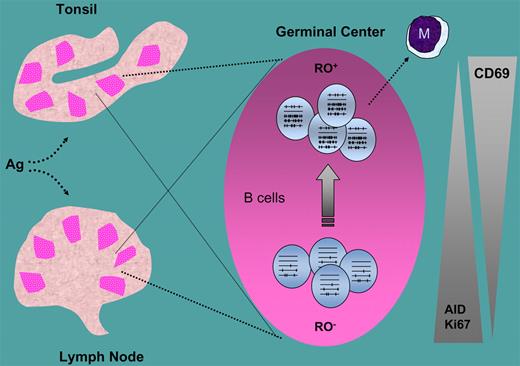
During adaptive immune responses, lymphocyte activation takes place in the germinal centers (GCs) of secondary lymphoid organs. Dissecting the populations that participate in the GC process is, by definition, difficult. In this issue of Blood, Jackson and colleagues report that a small percentage (10%) of human tonsil B cells expresses the CD45RO (RO+) surface phenotype and contains the highest mutation rate in the V-region genes. These cells are activated centrocytes that still contain transcripts for activation-induced cytidine deaminase (AID), albeit at reduced levels compared with RO− GC B cells.
Antibodies that are secreted by B lymphocytes upon activation by antigen via the B-cell receptor (BCR) mediate the individual's defense against extracellular pathogens. In T-cell–dependent responses, this process requires help from CD4 T cells whose cytokines determine the quality of the antibody response. Simultaneously, antibodies improve their affinity for antigen, a process known as affinity maturation, due to somatic hypermutation (SHM) in the antibody variable (V) region.1 Affinity maturation occurs late in the primary immune response and is the hallmark of memory B-cell responses. Both affinity maturation and class switch are controlled by AID.2
Surface expression of CD45 isoforms, a protein tyrosine phophatase present on all lymphocyte lineages, is commonly used to characterize T cells that can be subdivided into different subsets and stages of differentiation. Little information exists for B cells. Jackson and colleagues have taken a closer look at this issue by using a clever strategy in which the gate for CD45RA and CD45RO analysis of B lymphocytes in human tonsils was set on T cells from the same tissue source. Then they divided the total GC B-cell pool into separate subpopulations with distinctly different mutation averages, including pools that are enriched for either germ line or highly mutated V-region sequences. They found, as expected, great developmental heterogeneity among the GC B-cell pool, extending beyond the standard centroblast and centrocyte populations. Interestingly, RO+ fractions had both a greater percentage of cells with more mutations and a decreased number of unmutated germ-line sequences (see figure). Yet, unlike in RO-subdivided centrocytes, mutation averages did not differ between centroblast RO fractions. Thus, their work provides new insights into the somatic mutation process, both in terms of temporal and developmental regulation.
RO− and RO+ B cells in germinal centers and their relationship with somatic hypermutation, and activity, cell proliferation (Ki67), and activation (CD69). M indicates memory B cells.
RO− and RO+ B cells in germinal centers and their relationship with somatic hypermutation, and activity, cell proliferation (Ki67), and activation (CD69). M indicates memory B cells.
What is the link between CD45RO on GC B cells and SHM? The new data underscore an important correlation, but no cause-effect relation was established. A recent report in mice with CD45 deficiency limited to B cells shows that GC maintenance, but not initiation, requires CD45.3 This implies that CD45 is not necessary for class-switch recombination, or in all likelihood, for the initiation of SHM. These studies argue, however, that CD45 is important for survival of GC B cells, hence for maintaining T-cell–dependent antibody responses. Thus, although a direct extrapolation from mouse to human dictates caution, the data gathered by Jackson and colleagues fit a scenario in which RO+ GC B cells are transitional cells with SHM. RO isoform expression may then underscore the stabilization of class-switch recombination and SHM, for which BCR signaling in concert with AID activation seem to be necessary.
An easy phenotypic grip on GC B cells with SHM, such as the RO+ phenotype, may provide us with a valuable new tool to better understand the seemingly contradictory nature of SHM. The consequences of altering (quasirandomly) the germ-line immunoglobulin V-region DNA sequence disclose at least 2 juxtaposed outcomes: (1) improved antibody affinity for cognate foreign antigen (presumably beneficial), and (2) the generation of new specificities that target “self” (presumably detrimental). Ineffective counterselection against self-recognizing B cells could ultimately lead to autoimmune disease and, in some cases, leukemic transformation.
The new phenotypic way to identify GC B cells transitioning through different stages of SHM could lead to practical advances. On the one hand, a controlled induction of SHM could be used to undo and silence specific disease-promoting replacement mutations that were incorporated and retained during the GC response. On the flip side, SHM could be temporarily inhibited during a strong antiviral response in individuals who are genetically predisposed to develop familial blood-related diseases such as systemic lupus erythematosus. Collectively, it appears as if we get closer to the day when disease control can be achieved by manipulating the GC process.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■