In this issue of Blood, Li and colleagues describe a critical role for B cells in xenoreactivity and provide evidence for marginal zone B cell–NK cell interactions in the regulation of T-independent xenoantibody production.
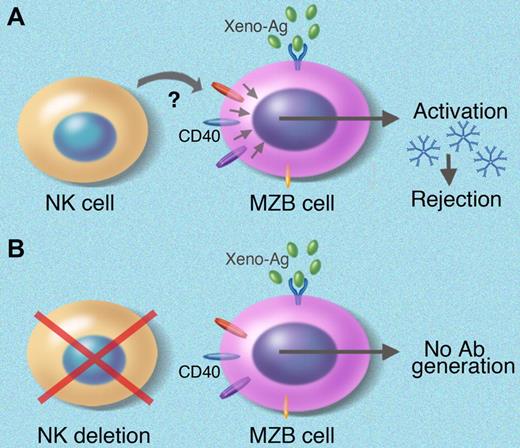
Solid organ transplantation is the preferred treatment for end-organ failure. Many patients die before a precious organ becomes available due to the donor organ shortage, prompting a strong research focus on xenotransplantation. However, xenotransplantation has remained an elusive goal in solving the organ shortage. Most research to date has focused on preexisting natural antibody and the role of T cells and natural killer (NK) cells in xenoreactivity.1,2 The work of Li and colleagues in this issue of Blood identifies a novel collaboration between marginal zone B (MZB) cells (CD19+CD21highCD23low) and NK cells in the rapid induction of T-cell–independent, non–Gal-xenoantibody production and xenorejection (as shown in the figure).
The authors have demonstrated a novel interaction between marginal-zone B cells and NK cells in xenotransplantation. They have shown that (A) NK-cell help is required for T-independent xenoantibody production using C57BL/6 nude mice as recipients, and (B) depletion of NK cells abrogates xenoantibody production.
The authors have demonstrated a novel interaction between marginal-zone B cells and NK cells in xenotransplantation. They have shown that (A) NK-cell help is required for T-independent xenoantibody production using C57BL/6 nude mice as recipients, and (B) depletion of NK cells abrogates xenoantibody production.
CD154:CD40 is a specific costimulatory pathway in B-cell activation and antibody production in T-cell–dependent allogeneic3 and xenogeneic4 antigens. In their report, Li and colleagues note that the CD154:CD40 pathway seems to be involved in the inducible T-cell–independent xenoantibody response, as it could be partially suppressed by CD154 blockade, providing indirect evidence that this pathway may be involved in the interaction between MZB and NK cells. The interaction between MZB cells and NK cells for xenoantibody production is independent of IFN-γ production or cytolytic activity by NK cells. These studies suggest that NK cells can regulate MZB-cell function and possibly could be used for cell-based immunotherapy to prevent xenograft rejection. However, caution is needed, given the complexity of non-Gal, T-independent antigens in xenotransplantation. For example, the anti–TNP-Ficoll IgM response is not completely abrogated in MZB-cell–deficient, Pyk-2–deficient mice. Nevertheless, this study provides new insight into the collaborative crosstalk of cell-cell interactions in the regulation of T-independent xenoantibody production. This report also affirms the critical role of B cells not only in allotransplantation,5 but also in xenotransplantation. These findings may provide a mechanistically rational approach for using costimulatory blockade and/or targeting these specific effector cells to promote xenograft acceptance. Perhaps most importantly, Li and colleauges can further test their findings in a normal animal model, which would provide further evidence for their clinical applicability in xenotrans-plantation.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■