The monoclonal antibody described by Lin and colleagues (anti-BR3) can both deplete B cells directly and block the B-cell survival factor BAFF. It thus achieves more profound biological effects in vivo, which may have therapeutic advantages.
In their study, Lin and colleagues have described anti-BR3 mAb, a novel monoclonal antibody therapeutic agent that combines 2 separate mechanisms of action in a single molecule (see figure). Both mechanisms target B cells but in different ways that have previously been shown to have potential synergies.1 By comparing variant constructs, the authors provide supportive evidence that the proposed double action indeed functions in vivo. The agent binds to the BR3 cell surface BAFF receptor on B cells and, thereby, depletes these cells by ADCC. It also inhibits the binding of BAFF to BR3, which should block the survival effect of BAFF, presumably on B cells that express BR3 but are not directly depleted. Compared with a “pure” B cell–depleting mAb, anti-CD20, or a “pure” BAFF blocker, BR3-Fc, anti-BR3 provides more profound depletion of certain resistant B-cell subsets, such as marginal zone cells, germinal center cells, and plasma cells, and causes a greater fall in serum immunoglobulins. Interestingly, the biological effects of anti-BR3 are more pronounced in mice than in cynomologous monkeys, which, as the authors discuss, may have important implications for the translation of their results to humans.
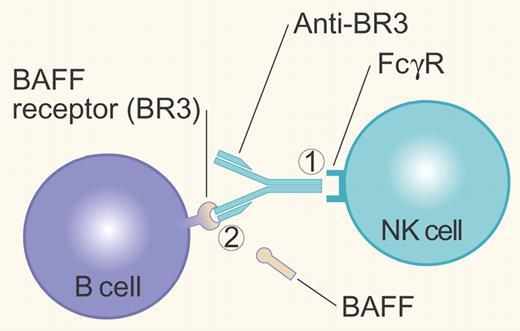
Anti-BR3 mAb can control B cells either by (1) crosslinking cell surface BR3 with the FcγR on NK cells, resulting in Antibody Dependent Cell-mediated Cytotoxicity; or (2) blocking binding of the survival factor BAFF to its BR3 receptor. Illustration by Paulette Dennis.
Anti-BR3 mAb can control B cells either by (1) crosslinking cell surface BR3 with the FcγR on NK cells, resulting in Antibody Dependent Cell-mediated Cytotoxicity; or (2) blocking binding of the survival factor BAFF to its BR3 receptor. Illustration by Paulette Dennis.
Why would we want such a dual-acting agent? First, several of the clinical situations in which we want to deplete B cells, such as lymphoma/leukemia or autoimmune diseases, can be associated with elevated serum levels of BAFF.2,3 Second, the depletion of B cells by anti-CD20 (rituximab) has been accompanied by further increases in serum BAFF, which may be a compensatory mechanism to attempt to maintain homeostasis.4 Third, the efficiency of B-cell depletion by existing modalities may not be adequate in certain malignancies, such as CLL, or in some autoimmune diseases, such as SLE.
On the other hand, we could raise several theoretical concerns about the proposed new approach. At least in nonmalignant conditions, the depth of B-cell depletion may not be critical to therapeutic effect. Furthermore, profound depletion, with concomitant fall in serum immunoglobulins, is worrisome for infectious complications. Finally, the elegance of the dual-action molecule is counterbalanced by the major drawback of all combination medicines: the relative doses of the contained activities are fixed and may not be optimal for a given patient. It is quite feasible to deplete B cells with one agent (eg, rituximab), block BAFF with another (eg, belimumab or anti-BAFF), and get the same synergistic effect but have the option of titrating each agent separately. In addition, the complexity of the BAFF/APRIL system and their 3 receptors, and their variability in disease, might suggest the need for more flexibility not only in dose but also in the specific method of targeting in each patient.5
In any case, the current paper illustrates the in vivo validity of a combined approach. We should expect additional sophisticated biologics with manifold-specific targets. It is daunting to consider the complexity of evaluating their use in patient populations.
Conflict-of-interest disclosure: The author has consulted for Genentech and has been supported by Genentech in the past for clinical and laboratory studies, all related to B-cell depletion. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal