Abstract
MCL-1 is a Bcl-2 family member that has been described as antiapoptotic in various myeloid neoplasms. Therefore, MCL-1 has been suggested as a potential new therapeutic target. Systemic mastocytosis (SM) is a myeloid neoplasm involving mast cells (MCs) and their progenitors. In the present study, we examined the expression and functional role of MCL-1 in neoplastic MCs and sought to determine whether MCL-1 could serve as a target in SM. As assessed by RT-PCR and immunohistochemical examination, primary neoplastic MCs expressed MCL-1 mRNA and the MCL-1 protein in all SM patients examined. Moreover, MCL-1 was detectable in both subclones of the MC line HMC-1—HMC-1.1 cells, which lack the SM-related KIT mutation D816V, and HMC-1.2 cells, which carry KIT D816V. Exposure of HMC-1.1 cells or HMC-1.2 cells to MCL-1–specific antisense oligonucleotides (ASOs) or MCL-1–specific siRNA resulted in reduced survival and increased apoptosis compared with untreated cells. Moreover, MCL-1 ASOs were found to cooperate with various tyrosine kinase inhibitors in producing growth inhibition in neoplastic MCs, with synergistic effects observed with PKC412, AMN107, and imatinib in HMC-1.1 cells and with PKC412 in HMC-1.2 cells. Together, these data show that MCL-1 is a novel survival factor and an attractive target in neoplastic MCs.
Introduction
Mastocytosis is a term collectively used for a group of disorders characterized by abnormal accumulation of mast cells (MCs) in one or more organ systems.1-7 Cutaneous and systemic variants of the disease have been described.1-7 Cutaneous mastocytosis (CM) typically develops in early childhood and follows a benign course with frequent spontaneous regression.7 Systemic mastocytosis (SM) can develop at any age and is characterized by involvement of one or more visceral organs with or without skin involvement.1-6 In contrast to CM, SM is a persistent clonal disorder of MCs.1-6 In a high proportion of patients, the transforming KIT mutation D816V is detectable.8-12 In patients with advanced SM, the mutation may also be detectable in other myeloid lineages or even in B lymphocytes.13,14 Thus, SM is a disease of multilineage hematopoietic progenitors. The concept that SM arises from a multilineage progenitor is also supported by the notion that associated hematologic non–mast cell lineage diseases (AHNMDs) can develop in these patients.1-6,8,15-17
The WHO consensus classification defines 4 categories of SM: indolent systemic mastocytosis (ISM), mastocytosis with AHNMD (SM-AHNMD), aggressive SM (ASM), and mast cell leukemia (MCL).18,19 SM variants differ from each other in their clinical behavior and prognosis, and they usually require different therapies.2-6,18-27 Notably, in contrast to ISM, patients with ASM or MCL are candidates for treatment with cytoreductive or targeted drugs.18-27
Thus far, only a few drugs, such as interferon-alpha (IFN-α) and cladribine (2CdA), have been described to suppress the growth of neoplastic MCs in ASM and MCL, and only a few patients show major and durable responses.21-27 Some of the novel tyrosine kinase (TK) inhibitors, such as midostaurin (PKC412)28 or nilotinib (AMN107),29 may also counteract the growth of neoplastic MCs.30,31 Again, however, long-lasting responses have not yet been described. Other studies suggest that imatinib (STI571) inhibits the growth of neoplastic MCs in patients with SM.32-35 However, the effect of imatinib is only seen in patients in whom neoplastic MCs harbor wild-type KIT32 or the KIT mutation F522C33 or in patients who have coexistent eosinophilia associated with the FIP1L1/PDGFRA fusion gene.34,35 By contrast, imatinib showed few, if any, effects on neoplastic MCs exhibiting KIT D816V.32,36,37 In fact, this KIT mutation, which is detectable in most patients with SM, confers resistance to imatinib.
A number of attempts have recently been made to identify new targets in neoplastic MCs and to develop more effective therapeutic approaches and more effective drug combinations.38-40 One promising approach may be to investigate survival-related molecules that are expressed in MCs in high-grade MC neoplasms.
MCL-1 is a well-characterized member of the Bcl-2 family that is considered to act in an antiapoptotic manner in various neoplastic cells.41-46 Originally, MCL-1 was described as a survival-enhancing molecule, expressed during TPA-induced differentiation of ML-1 cells.41 In consecutive studies, we and others have shown that MCL-1 is constitutively expressed in neoplastic cells in chronic myeloid leukemia (CML).43-46 However, little is known about the expression and role of MCL-1 in other myeloid neoplasms.
In the present study, we examined the expression and functional role of MCL-1 in neoplastic human MCs. Results of our study showed that primary neoplastic MCs in all variants of SM, including ASM and MCL and the MCL cell line HMC-1, express MCL-1 in a constitutive manner. In addition, we showed that targeting MCL-1 in these cells is associated with reduced growth and induction of apoptosis and with increased sensitivity to TK inhibitors, including PKC412, AMN107, and imatinib.
Patients, materials, and methods
Reagents
Imatinib (STI571), midostaurin (PKC412),28 and nilotinib (AMN107)29 were kindly provided by Dr P. W. Manley, Dr E. Buchdunger, and Dr D. Fabbro (Novartis Pharma, Basel, Switzerland). Stock solutions of PKC412 and AMN107 were prepared by dissolving in dimethyl sulfoxide (DMSO; Merck, Darmstadt, Germany). Interleukin-4 (IL-4) was purchased from PeproTech (Rocky Hill, NJ), RPMI 1640 medium and fetal calf serum (FCS) from PAA Laboratories (Pasching, Austria), l-glutamine and Iscove modified Dulbecco medium (IMDM) from Gibco Life Technologies (Gaithersburg, MD), 3 H-thymidine from Amersham (Aylesbury, United Kingdom), and cladribine (2CdA) from Sigma (St Louis, MO). Antisense oligonucleotides (ASOs; designed according to published sequences46 ) were obtained from VBC Genomics (Vienna, Austria) or ISIS Pharmaceuticals (Carlsbad, CA), and siRNA was obtained from Dharmacon (Lafayette, CO).
Patient characteristics and purification of neoplastic mast cells
Thirty patients with mastocytosis (ISM, n = 20; ASM, n = 3; MCL, n = 3; smoldering SM [SSM], n = 3; mast cell sarcoma [MCS], n = 1), 17 with CML (chronic phase [CP], n = 8; accelerated phase [AP], n = 5; blast phase [BP], n = 4), 9 with myelodysplastic syndromes (MDS; refractory anemia [RA], n = 2; refractory anemia with ringed sideroblasts [RARS], n = 2; refractory cytopenia with multilineage dysplasia [RCMD], n = 2; RA with excess of blasts [RAEB], n = 2; chronic myelomonocytic leukemia [CMML], n = 1), 8 with myeloproliferative disorders (MPD; essential thrombocythemia [ET], n = 4; idiopathic myelofibrosis [IMF], n = 2; polycythemia vera [PV], n = 2), one with SCF-induced MC hyperplasia, and 9 with normal bone marrow (BM; lymphoma staging) were examined. Patient characteristics are shown in Table 1
BM cells were obtained from the posterior iliac crest. Informed consent was obtained from each patient before BM biopsy and aspiration. In 2 patients with MCL and one with MCS, MCs were purified to homogeneity (purity greater than 98%) using PE-labeled mAb YB5.B8 (PharMingen, San Diego, CA) and FACS Vantage (Becton Dickinson, San Jose, CA), as described.47 All experiments were conducted after approval was received from the institutional review board of the Medical University of Vienna and in accordance with the Declaration of Helsinki.
Culture of HMC-1 cells exhibiting or lacking KIT D816V
The MCL cell line HMC-148 was kindly provided by Dr J. H. Butterfield (Mayo Clinic, Rochester, MN). Two HMC-1 subclones were used, namely HMC-1.1 harboring KIT G560V, but not KIT D816V, and HMC-1.2 cells exhibiting both KIT mutations.32 HMC-1 was grown in IMDM with 10% FCS, l-glutamine, and antibiotics at 37°C. HMC-1 cells were rethawed from an original stock every 4 to 8 weeks and were passaged weekly. As control of phenotypic stability, HMC-1 cells were periodically checked for expression of KIT and the downmodulating effect of IL-4.49
Treatment with inhibitors
HMC-1 cells were incubated with various concentrations of PKC412 (100 pM-10 μM), AMN107 (1 nM-100 μM), imatinib (3 nM-300 μM), 2CdA (0.1-10 000 ng/mL), or control medium at 37°C for up to 48 hours. In a separate set of experiments, HMC-1 cells were transfected with MCL-1–specific ASOs before they were exposed to drugs. In other experiments, cells were transfected with various concentrations of MCL-1–specific ASO (50-250 nM) for up to 12 hours or with MCL-1–specific siRNA (200 nM) for 12 hours without further exposure to inhibitors. After exposure to drugs or ASO, cells were subjected to 3H-thymidine uptake experiments, analysis of cell viability and apoptosis, or Western blotting.
Northern blot analysis, RT-PCR, and Western blot analysis
Total RNA was isolated from HMC-1 cells using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. RNA preparation and Northern blot analysis were performed essentially as described.46,50 Hybridization was performed in rapid-hyb buffer (Amersham) using 32P-labeled cDNA specific for MCL-1 or β-actin. The MCL-1 probe was generated by excising the full-length MCL1 cDNA from pBSK-MCL-151,52 (a kind gift from S. J. Korsmeyer, Dana Farber Cancer Institute, Boston, MA) with EcoRI and XbaI (New England Biolabs, Beverly, MA), as described.46 Bound radioactivity was visualized by exposure to Biomax MS film (Kodak, Rochester, NY) at −80°C using intensifying screens (Kodak). mRNA expression levels were quantified by densitometry using E.A.S.Y. Win32 software (Herolab, Wiesloch, Germany).
RT-PCR reactions were performed with the Protoscript First Strand cDNA Synthesis kit (New England Biolabs, Beverly, MA) using 1 μg RNA in a 50-μL reaction volume. PCR conditions were as follows: initial denaturation at 94°C for 60 seconds, annealing at 55°C for 60 seconds, polymerization at 72°C for 60 seconds (35 cycles), and terminal extension at 72°C for 10 minutes. The following primer pairs were used: MCL-1, 5′-TGC TGG AGT TGG TCG GGG AA-3′ (forward) and 5′-TCG TAA GGT CTC CAG CGC CT-3′ (reverse); β-actin, 5′-ATG GAT GAT GAT ATC GCC GCG-3′ (forward) and 5′-CTA GAA GCA TTT GCG GTG GAC GAT GGA GGG GCC-3′ (reverse). PCR products were resolved in 1% agarose gels containing 0.5 μg/mL ethidium bromide.
Western blot analysis was performed as described46 using lysates of HMC-1 cells and a polyclonal rabbit anti–human MCL-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). To confirm equal loading, membranes were reprobed with a rabbit anti–human β-actin antibody (Sigma). Chemiluminescence was detected by exposure to a Biomax MS film (Kodak).
Immunohistochemistry and immunocytochemistry
Immunohistochemistry was performed on 2-μm sections prepared from paraffin-embedded, formalin-fixed BM specimens in all patients (Table 1) using the indirect immunoperoxidase staining technique as described.53,54 Endogenous peroxidase was blocked by CH3OH/H2O2. After pretreatment in a microwave oven, BM sections were incubated with a polyclonal anti–MCL-1 antibody (Santa Cruz Biotechnology) (work dilution, 1:50) overnight. To examine MCL-1 expression in MCs, serial BM sections were stained with antitryptase antibody G3 (1:5000; Chemicon, Temecula, CA), as described.47 Antibodies were diluted in 0.05 M Tris-buffered saline (TBS, pH 7.5) and 1% bovine serum albumin (BSA; Sigma). After washing, slides were incubated with biotinylated goat anti–rabbit or horse anti–mouse IgG for 30 minutes, washed, and exposed to streptavidin-biotin-peroxidase complex (30 minutes); 3-amino-9-ethyl-carbazole (AEC) was used as chromogen. Slides were counterstained in Mayer hemalaun.
Immunocytochemistry was performed on cytospin preparations of HMC-1 cells, as described,46 using anti–MCL-1 antibody (work dilution, 1:200) and biotinylated goat anti–rabbit IgG (Biocarta, San Diego, CA). In select experiments, an MCL-1–blocking peptide (Santa Cruz Biotechnology) was applied (work dilution, 1:40). As chromogen, alkaline phosphatase complex (Biocarta) was used. Antibody reactivity was made visible using Neofuchsin (Nichirei, Tokyo, Japan). Images were visualized using an Olympus DP11 camera connected to an Olympus BX50F4 microscope equipped with a 40×/0.85 numerical aperture (NA) (Figure 1A) or a 100×/1.35 NA (Figure 1B) UPlan-Apo objective lens (Olympus, Hamburg, Germany). Images were acquired using Adobe Photoshop CS2 software version 9.0 (Adobe Systems, San Jose, CA), and were processed using PowerPoint software (Microsoft, Redmond, WA).
Transfection with MCL-1 antisense oligonucleotides and siRNA
HMC-1 cells were transfected with MCL-1–specific 2′-O-methoxyethyl/2′-deoxynucleotide chimeric phosphorothioate ASO (5′-TTG GCT TTG TGT CCT TGG CG-3′) or with scramble control oligonucleotide pool, as described.46,55 In select experiments, an annealed, desalted, double-stranded MCL-1 siRNA (AAG AAA CGC GGU AAU CGG ACU)56 and a control siRNA against luciferase (CUU ACG CUG AGU ACU UCG A), both obtained from Dharmacon, were applied. For transfection, 800 000 cells were seeded in 75-cm2 culture plates at 37°C for 24 hours, and MCL-1 ASO and MCL-1 siRNA were complexed with Lipofectin (Invitrogen), as described.46 HMC-1 cells were then incubated with MCL-1 ASO (50-250 nM) or 200 nM MCL-1–specific siRNA at 37°C for 4 hours. Thereafter, cells were washed and cultured in the presence or absence of various concentrations of TK inhibitors for 12 hours before they were analyzed. siRNA-treated cells were kept in control medium for 12 hours (without TK inhibitors) before they were examined.
3H-thymidine incorporation assay and determination of cell viability
To investigate antiproliferative drug effects, 3 H-thymidine incorporation experiments were conducted as described.31,46 TK inhibitors and 2CdA were applied on untreated cells or cells transfected with MCL-1–specific ASO or scramble control. In particular, 4 hours after transfection with MCL-1 ASO or control oligonucleotides, cells were cultured in 96-well microtiter plates (5 × 104 cells/well) in the absence or presence of various concentrations of PKC412 (100 pM-10 μM), AMN107 (1 nM-100 μM), imatinib (3 nM-300 μM), or cladribine (2CdA; HMC-1.1, 0.1-10 000 ng/mL; HMC-1.2, 0.5- 500 ng/mL) for 24 hours. All experiments were performed in triplicate. Cell viability was determined by the trypan blue exclusion test. The percentage of apoptotic cells was determined on Wright-Giemsa–stained cytospin slides. Apoptosis was defined by generally accepted cytomorphologic criteria, including cell shrinkage, nuclear condensation, and chromatin clumping.57
Statistical analysis
To determine the level of significance in differences found in data evaluation, the paired Student t test was applied. Results were considered significantly different at P < .05. To determine synergistic effects of MCL-1 ASO and drugs, combination index values were calculated according to published guidelines58 using commercially available software (CalcuSyn; Biosoft, Ferguson, MO). Fractional effects (x-axis) refer to the number (percentage) of apoptotic cells. Combination index (CI) values (y-axis) define additive as a CI of 1.0 (dots hitting the interface line), synergism as a CI less than 1.0, and antagonism as a CI greater than 1.0.
Results
Detection of the MCL-1 protein in neoplastic mast cells
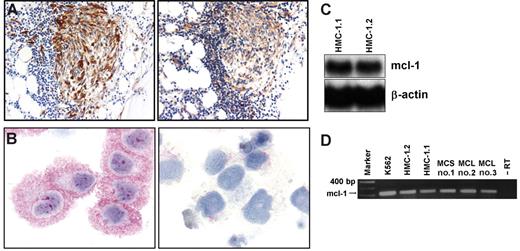
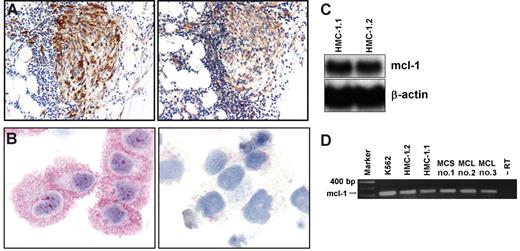
As assessed by immunohistochemistry and serial section staining, neoplastic MCs were found to react with an antibody against MCL-1 in all examined patients with mastocytosis (n = 30) (Table 1). Figure 1A shows the coexpression of tryptase (left panel) and MCL-1 (right panel) in spindle-shaped neoplastic BM MCs in a patient with ISM. Virtually all neoplastic MCs in these infiltrates were found to stain positively for MCL-1. No differences were observed in MCL-1 expression (with regard to percentage of stained MCs or intensity of staining) in MCs on immunohistochemical analysis when comparing patients in various categories of SM. In particular, MCL-1 was found to be expressed in neoplastic MCs in patients with ISM and SSM and in high-grade MC malignancies (ASM, MCL) (Table 1). Moreover, BM MCs in other myeloid neoplasms (MPD, CML, MDS) and MCs in normal/reactive BM were found to display MCL-1 (Table 1). Apart from MCs, megakaryocytes and myeloid (non–MC-lineage) progenitors were found to react with the anti–MCL-1 antibody in these BM sections (Table 1). The human MCL-derived leukemia cell line HMC-1 (HMC-1.1 and HMC-1.2) was also found to express the MCL-1 protein by immunocytochemistry. Figure 1B shows the reactivity of the anti–MCL-1 antibody with HMC-1.1 cells after preincubation with control medium (left panel) or an MCL-1–specific blocking peptide (right panel). The same staining result was obtained with HMC-1.2 cells (not shown).
Expression of MCL-1 in neoplastic human mast cells. (A) Immunohistochemical detection of tryptase (left) and MCL-1 (right) in neoplastic MCs in a patient with indolent SM (ISM). Adjacent bone marrow sections were incubated with antibodies against tryptase or MCL-1. Immunohistochemistry was performed as described. (B) Immunocytochemical detection of MCL-1 in HMC-1.2 cells exhibiting the KIT mutation D816V (left). Immunocytochemistry was performed using a polyclonal anti–MCL-1 antibody. Preincubation of the antibody with a specific blocking peptide resulted in a negative stain (right). Similar staining results were obtained with HMC-1.1 cells lacking KIT D816V (not shown). (C) Northern blot analysis of HMC-1.1 cells and HMC-1.2 cells using an MCL-1–specific cDNA probe. β-Actin loading control is also shown. (D) RT-PCR analysis of MCL-1 mRNA expression in K562 cells, HMC-1.1 cells, HMC-1.2 cells, and purified (purity greater than 98%) neoplastic human mast cells obtained from one patient with mast cell sarcoma (MCS) (patient 1) and 2 patients (patients 2 and 3) with mast cell leukemia (MCL). The PCR reaction was controlled by omitting the RT step (−RT).
Expression of MCL-1 in neoplastic human mast cells. (A) Immunohistochemical detection of tryptase (left) and MCL-1 (right) in neoplastic MCs in a patient with indolent SM (ISM). Adjacent bone marrow sections were incubated with antibodies against tryptase or MCL-1. Immunohistochemistry was performed as described. (B) Immunocytochemical detection of MCL-1 in HMC-1.2 cells exhibiting the KIT mutation D816V (left). Immunocytochemistry was performed using a polyclonal anti–MCL-1 antibody. Preincubation of the antibody with a specific blocking peptide resulted in a negative stain (right). Similar staining results were obtained with HMC-1.1 cells lacking KIT D816V (not shown). (C) Northern blot analysis of HMC-1.1 cells and HMC-1.2 cells using an MCL-1–specific cDNA probe. β-Actin loading control is also shown. (D) RT-PCR analysis of MCL-1 mRNA expression in K562 cells, HMC-1.1 cells, HMC-1.2 cells, and purified (purity greater than 98%) neoplastic human mast cells obtained from one patient with mast cell sarcoma (MCS) (patient 1) and 2 patients (patients 2 and 3) with mast cell leukemia (MCL). The PCR reaction was controlled by omitting the RT step (−RT).
Detection of MCL-1 mRNA in neoplastic mast cells
As assessed by Northern blot analysis, HMC-1.1 and HMC-1.2 cells expressed MCL-1 mRNA (Figure 1C). Expression of MCL-1 mRNA in HMC-1 cells was confirmed by RT-PCR analysis (Figure 1D). Moreover, through RT-PCR analysis, we were able todemonstrate MCL-1 mRNA expression in highly purified primary neoplastic MCs in patients with MCL or MCS (Figure 1D).
Down-regulation of MCL-1 expression counteracts viability of neoplastic MCs
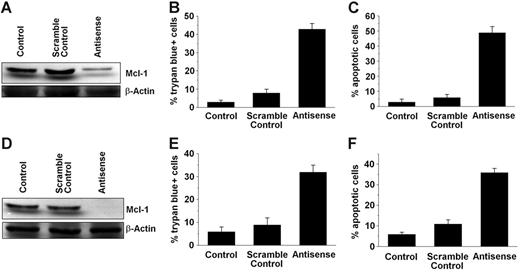
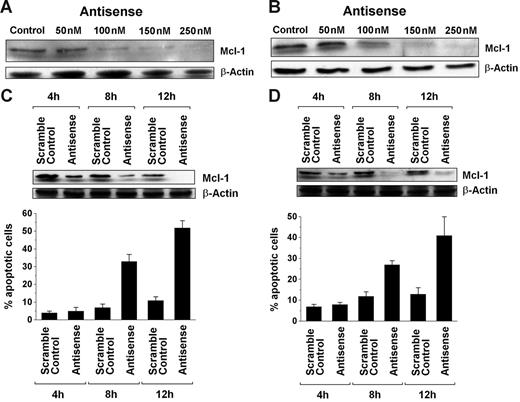
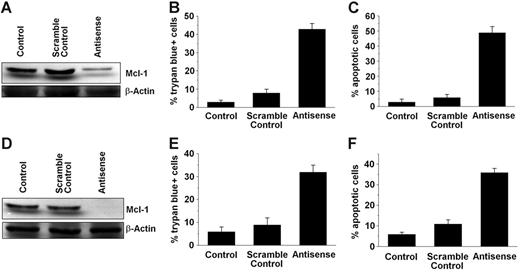
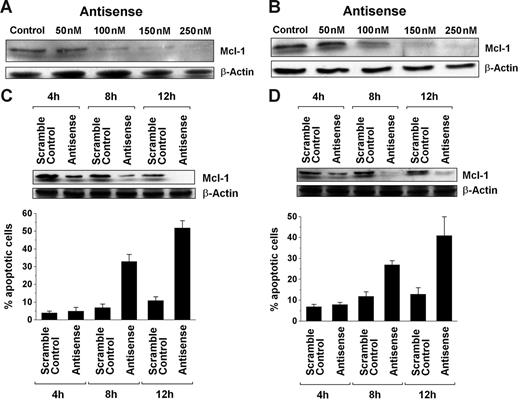
To investigate the role of MCL-1 as a survival molecule and a potential target in SM, MCL-1 was knocked down in HMC-1.1 and HMC-1.2 cells by an ASO approach. As determined by Western blot analysis, transfection of both cell lines with MCL-1 ASO (250 nM) virtually abolished expression of the MCL-1 protein compared with scramble control or untransfected cells (Figure 2). As expected, transfection with MCL-1 ASOs also led to a substantial increase in trypan blue–positive cells and to a substantial increase in the percentage of apoptotic cells in both HMC-1 subclones, namely HMC-1.1 (Figure 2A–2C) and HMC-1.2 (Figure 2D–2F). The effects of MCL-1 ASO on the growth and survival of HMC-1 cells were dose and time dependent (Figure 3). These data show that targeting of MCL-1 in neoplastic MCs is associated with the loss of cell viability and the induction of apoptosis.
Effects of MCL-1 antisense oligonucleotides on MCL-1 protein expression and cell viability in HMC-1.1 and HMC-1.2 cells. HMC-1.1 cells (A-C) and HMC-1.2 cells (D-F) harboring KIT D816V were transfected with an MCL-1 antisense oligonucleotide at 250 nM (Antisense), a scramble control, or were left untransfected (Control) for 12 hours before analysis. (A, D) Western blot analysis of MCL-1 expression was performed using an anti–MCL-1 antibody. β-Actin served as loading control. One representative blot is shown for each cell line. (B, E) Evaluation of nonviable (trypan blue-positive) expressed as a percentage of all nucleated cells. (C, F) Numbers (%) of apoptotic cells. Results represent the mean ± SD from 3 independent experiments.
Effects of MCL-1 antisense oligonucleotides on MCL-1 protein expression and cell viability in HMC-1.1 and HMC-1.2 cells. HMC-1.1 cells (A-C) and HMC-1.2 cells (D-F) harboring KIT D816V were transfected with an MCL-1 antisense oligonucleotide at 250 nM (Antisense), a scramble control, or were left untransfected (Control) for 12 hours before analysis. (A, D) Western blot analysis of MCL-1 expression was performed using an anti–MCL-1 antibody. β-Actin served as loading control. One representative blot is shown for each cell line. (B, E) Evaluation of nonviable (trypan blue-positive) expressed as a percentage of all nucleated cells. (C, F) Numbers (%) of apoptotic cells. Results represent the mean ± SD from 3 independent experiments.
Dose- and time-dependent effects of MCL-1 antisense oligonucleotides on neoplastic mast cells. (A-B) Western blot analysis of MCL-1 expression in HMC-1.1 cells (A) and HMC-1.2 cells (B) after exposure to various concentrations of MCL-1 antisense oligonucleotides (Antisense) (50-250 nM) or control medium (Control) for 12 hours. β-Actin served as loading control. (C-D) Time-dependent effects of MCL-1 antisense oligonucleotides (Antisense) (250 nM) and a scramble Control (250 nM) on expression of the MCL-1 protein in HMC-1.1 cells (C) and HMC-1.2 cells (D). MCL-1 expression was determined by Western blotting, with β-actin serving as a loading control. (C-D, lower) Time-dependent effects of the MCL-1 antisense oligonucleotides (250 nM) and of the scramble Control (250 nM) on cell viability (ie, percentage) of apoptotic HMC-1.1 cells (C) and HMC-1.2 cells (D). Results represent the mean ± SD from 3 independent experiments.
Dose- and time-dependent effects of MCL-1 antisense oligonucleotides on neoplastic mast cells. (A-B) Western blot analysis of MCL-1 expression in HMC-1.1 cells (A) and HMC-1.2 cells (B) after exposure to various concentrations of MCL-1 antisense oligonucleotides (Antisense) (50-250 nM) or control medium (Control) for 12 hours. β-Actin served as loading control. (C-D) Time-dependent effects of MCL-1 antisense oligonucleotides (Antisense) (250 nM) and a scramble Control (250 nM) on expression of the MCL-1 protein in HMC-1.1 cells (C) and HMC-1.2 cells (D). MCL-1 expression was determined by Western blotting, with β-actin serving as a loading control. (C-D, lower) Time-dependent effects of the MCL-1 antisense oligonucleotides (250 nM) and of the scramble Control (250 nM) on cell viability (ie, percentage) of apoptotic HMC-1.1 cells (C) and HMC-1.2 cells (D). Results represent the mean ± SD from 3 independent experiments.
Effects of MCL-1 siRNA on neoplastic mast cells
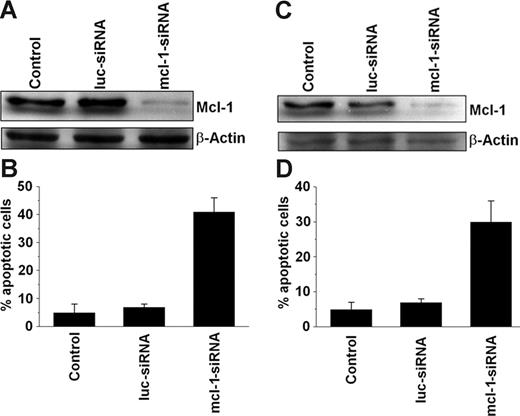
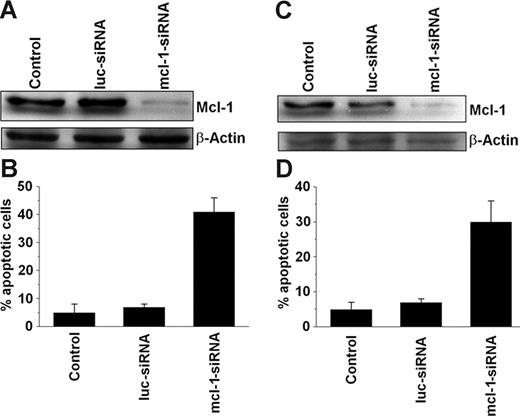
We applied MCL-1–specific siRNA to further demonstrate the role of MCL-1 as a survival molecule in neoplastic MCs. In these experiments, the MCL-1 siRNA was found to down-regulate expression of the MCL-1 protein in HMC-1.1 cells and HMC-1.2 cells compared with a luciferase control siRNA or untransfected cells (Figure 4). The siRNA-induced down-regulation of MCL-1 was found to be associated with an increase in apoptotic HMC-1.1 cells (Figure 4B) and HMC-1.2 cells (Figure 4D). These data provide further evidence that MCL-1 is a survival factor in neoplastic MCs.
Effects of MCL-1–specific siRNA on neoplastic mast cells. Effects of MCL-1-siRNA on MCL-1 protein expression (A, C) and cell viability (B, D) in HMC-1.1 cells (A-B) and HMC-1.2 cells (C-D). Cells were left untransfected (Control) or were transfected with an MCL-1–specific siRNA (MCL-1-siRNA; 200 nM) or a luciferase-specific (control) siRNA (luc-siRNA; 200 nM). MCL-1 protein expression (A, C) was determined after 12 hours by Western blotting using a polyclonal anti–MCL-1 antibody. Equal loading was confirmed by probing for β-actin. Cell viability was analyzed by recording the percentage of apoptotic cells. Results represent the mean ± SD from 3 independent experiments in each set of experiments (C-D).
Effects of MCL-1–specific siRNA on neoplastic mast cells. Effects of MCL-1-siRNA on MCL-1 protein expression (A, C) and cell viability (B, D) in HMC-1.1 cells (A-B) and HMC-1.2 cells (C-D). Cells were left untransfected (Control) or were transfected with an MCL-1–specific siRNA (MCL-1-siRNA; 200 nM) or a luciferase-specific (control) siRNA (luc-siRNA; 200 nM). MCL-1 protein expression (A, C) was determined after 12 hours by Western blotting using a polyclonal anti–MCL-1 antibody. Equal loading was confirmed by probing for β-actin. Cell viability was analyzed by recording the percentage of apoptotic cells. Results represent the mean ± SD from 3 independent experiments in each set of experiments (C-D).
Effects of TK inhibitors on expression of MCL-1 in neoplastic mast cells
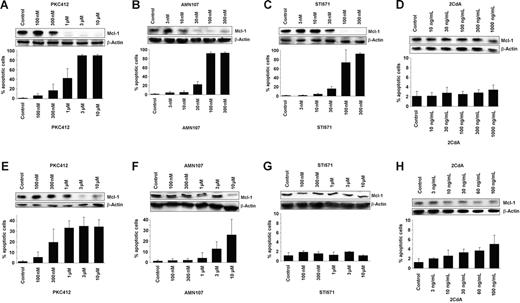
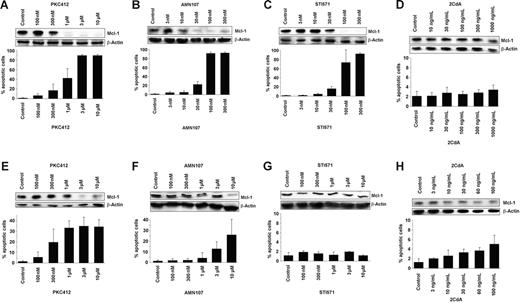
We then explored the effects of various TK inhibitors on the expression of MCL-1 in HMC-1 cells. In these experiments, we found that PKC412 (1-10 μM), AMN107 (10-300 nM), and imatinib (STI571; 30-300 nM), but not 2CdA (up to 1000 ng/mL), down-regulates the expression of MCL-1 in HMC-1.1 cells lacking KIT D816V (Figure 5A–D). When applying the same drugs on HMC-1.2 cells exhibiting KIT D816V, we found that PKC412 and 2CdA decreased the expression of MCL-1 at drug concentrations that may be achieved in vivo, whereas no significant effect was seen with imatinib. Similarly, AMN107 decreased MCL-1 expression only when applied at 10 μM, but did not decrease MCL-1 expression at lower drug concentrations (Figure 5E–H). In both cell lines, the drug-induced decrease in MCL-1 expression was accompanied by an increase in apoptotic cells (Figure 5).
Effects of PKC412, imatinib, AMN107, and 2CdA on MCL-1 protein expression and cell viability in neoplastic mast cells. Effects of PKC412 (A, E), AMN107 (B, F), imatinib (STI571) (C, G), or 2CdA (D, H) on MCL-1 protein expression (upper panel) and on cell viability (lower panel) in HMC-1.1 cells lacking KIT D816V (A-D) and in HMC-1.2 cells exhibiting KIT D816V (E-H). MCL-1 protein expression was determined by Western blotting using a polyclonal anti–MCL-1 antibody. Equal loading was confirmed by probing for β-actin. Cell viability was analyzed by recording the percentage of apoptotic cells. Results documenting drug effects on cell viability represent the mean ± SD from 3 independent experiments.
Effects of PKC412, imatinib, AMN107, and 2CdA on MCL-1 protein expression and cell viability in neoplastic mast cells. Effects of PKC412 (A, E), AMN107 (B, F), imatinib (STI571) (C, G), or 2CdA (D, H) on MCL-1 protein expression (upper panel) and on cell viability (lower panel) in HMC-1.1 cells lacking KIT D816V (A-D) and in HMC-1.2 cells exhibiting KIT D816V (E-H). MCL-1 protein expression was determined by Western blotting using a polyclonal anti–MCL-1 antibody. Equal loading was confirmed by probing for β-actin. Cell viability was analyzed by recording the percentage of apoptotic cells. Results documenting drug effects on cell viability represent the mean ± SD from 3 independent experiments.
MCL-1 ASOs cooperate with PKC412, AMN107, and imatinib in producing growth inhibition in neoplastic MCs
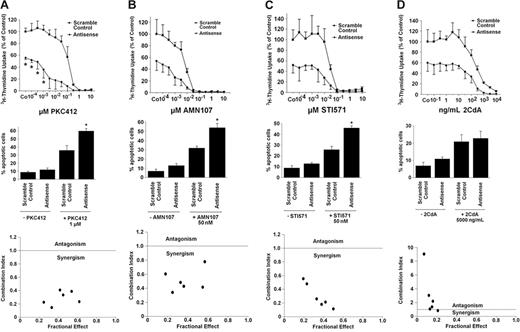
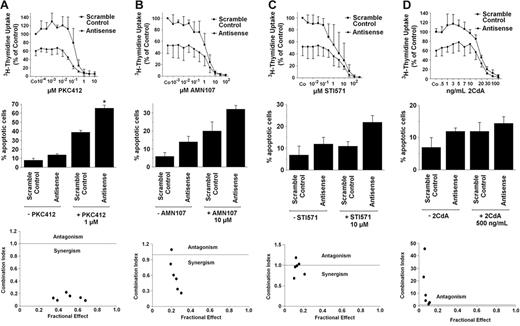
Based on the striking effects of the TK inhibitors and of the MCL-1–specific ASOs, we were interested in learning whether combinations of drugs and ASOs would result in synergistic antiproliferative (or apoptosis-inducing) effects on HMC-1.1 cells and HMC-1.2 cells. In a first step, IC50 values were determined for each single drug in 3H-thymidine uptake experiments. In addition, the numbers of apoptotic cells after exposure to drugs (EC50 values) were determined. Respective results are summarized in Table 2 The concentrations of inhibitors required to block proliferation usually were lower than concentrations required to induce apoptosis. In line with our previous data,31 PKC412, 2CdA, and, to a lesser degree, AMN107 (but not imatinib) counteracted cell growth in HMC-1.2 cells at pharmacologic concentrations. By contrast, in HMC-1.1 cells, PKC412, AMN107, and imatinib reduced cell growth and induced apoptosis, whereas no significant effect was seen with 2CdA. The MCL-1 ASO inhibited proliferation and induced apoptosis in HMC-1.1 cells and HMC-1.2 cells, with slightly higher IC50 values seen in HMC-1.2 cells.
When comparing drug-induced responses of MCL-1–ASO-transfected HMC-1 cells with those of HMC-1 cells transfected with a scramble control oligonucleotide, substantial differences were found. In fact, in most cases, transfection with MCL-1 ASO was found to sensitize HMC-1 cells against TK inhibitors and against 2CdA. Interestingly, in HMC-1.1 cells, most combinations were found to be synergistic in nature (Figure 6). By contrast, in HMC-1.2 cells, synergistic effects were seen with combinations of ASO and PKC412 but not with ASO and AMN107 or ASO and imatinib (Figure 7), indicating that MCL-1 can be used as a new drug target in neoplastic MCs and that MCL-1–specific ASO is capable of sensitizing HMC-1 cells against 2CdA and novel TK inhibitors.
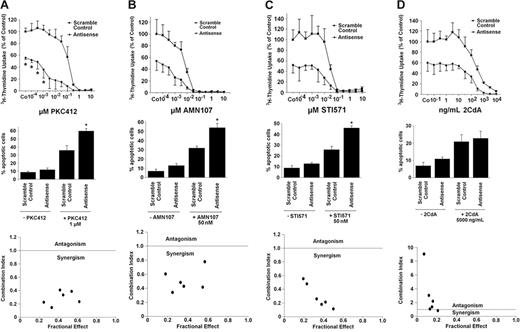
Effects of MCL-1 antisense oligonucleotides and TK inhibitors on growth and viability of neoplastic mast cells lacking KIT D816V. (Top row) Effects of various concentrations of PKC412 (A), AMN107 (B), STI571 (imatinib) (C), or 2CdA (D) on 3H-thymidine uptake by HMC-1.1 cells transfected with a scramble control (▪) or with MCL-1 antisense oligonucleotides (Antisense) (each 50 nM) (•). Results are expressed as percentage of control (scramble control) without drug and represent the mean ± SD of 3 independent experiments (*P < .05). (Middle row) Effects of MCL-1 antisense oligonucleotides (Antisense) or scramble control (each 100 nM) applied with PKC412 (A), AMN107 (B), STI571 (imatinib) (C), or 2CdA (D) or without the respective drug on cell viability (ie, percentage of apoptotic cells). Results represent the mean ± SD of 3 independent experiments (*P < .05). (Bottom row) Using CalcuSyn software, analyses of dose-effect relationships of PKC412 (A), AMN107 (B), STI571 (imatinib) (C), or 2CdA (D) and MCL-1 antisense-induced apoptosis in HMC-1.1 cells were calculated according to the median effect method of Chou and Talalay.57 CI less than 1 indicated synergism.
Effects of MCL-1 antisense oligonucleotides and TK inhibitors on growth and viability of neoplastic mast cells lacking KIT D816V. (Top row) Effects of various concentrations of PKC412 (A), AMN107 (B), STI571 (imatinib) (C), or 2CdA (D) on 3H-thymidine uptake by HMC-1.1 cells transfected with a scramble control (▪) or with MCL-1 antisense oligonucleotides (Antisense) (each 50 nM) (•). Results are expressed as percentage of control (scramble control) without drug and represent the mean ± SD of 3 independent experiments (*P < .05). (Middle row) Effects of MCL-1 antisense oligonucleotides (Antisense) or scramble control (each 100 nM) applied with PKC412 (A), AMN107 (B), STI571 (imatinib) (C), or 2CdA (D) or without the respective drug on cell viability (ie, percentage of apoptotic cells). Results represent the mean ± SD of 3 independent experiments (*P < .05). (Bottom row) Using CalcuSyn software, analyses of dose-effect relationships of PKC412 (A), AMN107 (B), STI571 (imatinib) (C), or 2CdA (D) and MCL-1 antisense-induced apoptosis in HMC-1.1 cells were calculated according to the median effect method of Chou and Talalay.57 CI less than 1 indicated synergism.
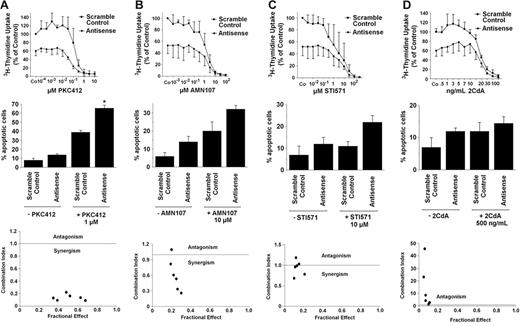
Effects of MCL-1 antisense oligonucleotides and inhibitory drugs on growth and viability of neoplastic mast cells exhibiting KIT D816V. (Top row) Effects of various concentrations of PKC412 (A), AMN107 (B), STI571 (imatinib) (C), or 2CdA (D) on 3H-thymidine uptake by HMC-1.2 cells transfected with a scramble control (▪) or MCL-1 antisense oligonucleotides (Antisense) (each 80 nM) (•). Results are expressed as percentage of control (ie, scramble control) without inhibitory drug and represent the mean ± SD of 3 independent experiments. *P < .05. (Middle row) Effects of MCL-1 antisense oligonucleotides (Antisense) or scramble control (each 100 nM) applied with PKC412 (A), AMN107 (B), STI571 (imatinib) (C), or 2CdA (D) or without the respective drug on cell viability (ie, percentage of apoptotic cells). Results represent the mean ± SD of 3 independent experiments (*P < .05). (Bottom row) Using CalcuSyn software, analyses of dose-effect relationships of PKC412 (A), AMN107 (B), STI571 (imatinib) (C), or 2CdA (D) and MCL-1 antisense–induced apoptosis in HMC-1.2 cells were calculated according to the median effect method of Chou and Talalay.57 CI less than 1 indicated synergism.
Effects of MCL-1 antisense oligonucleotides and inhibitory drugs on growth and viability of neoplastic mast cells exhibiting KIT D816V. (Top row) Effects of various concentrations of PKC412 (A), AMN107 (B), STI571 (imatinib) (C), or 2CdA (D) on 3H-thymidine uptake by HMC-1.2 cells transfected with a scramble control (▪) or MCL-1 antisense oligonucleotides (Antisense) (each 80 nM) (•). Results are expressed as percentage of control (ie, scramble control) without inhibitory drug and represent the mean ± SD of 3 independent experiments. *P < .05. (Middle row) Effects of MCL-1 antisense oligonucleotides (Antisense) or scramble control (each 100 nM) applied with PKC412 (A), AMN107 (B), STI571 (imatinib) (C), or 2CdA (D) or without the respective drug on cell viability (ie, percentage of apoptotic cells). Results represent the mean ± SD of 3 independent experiments (*P < .05). (Bottom row) Using CalcuSyn software, analyses of dose-effect relationships of PKC412 (A), AMN107 (B), STI571 (imatinib) (C), or 2CdA (D) and MCL-1 antisense–induced apoptosis in HMC-1.2 cells were calculated according to the median effect method of Chou and Talalay.57 CI less than 1 indicated synergism.
Discussion
MCL-1 is a well-characterized member of the Bcl-2 family that has recently been implicated in the pathogenesis of myeloid neoplasms.41-46 SM is a myeloid neoplasm characterized by abnormal growth and accumulation of MCs in visceral organs.1-6,13-22 We provide evidence that neoplastic MCs in SM and HMC-1 cells express MCL-1 in a constitutive manner. Moreover, our data show that MCL-1 is a critical survival molecule in neoplastic MCs and that targeting of MCL-1 is associated with decreased survival of neoplastic MCs and with increased responses to PKC412 and AMN107.
Normal and neoplastic MCs are extremely long-lived cells compared with other myeloid cells such as basophils.59 The mechanisms and factors responsible for long-term survival of MCs are largely unknown. In fact, though members of the Bcl-2 family have been implicated as essential survival factors in granulomonocytic cells, little is known about the expression and role of these molecules in MCs.38,60-64 In this study, we demonstrate MCL-1 expression in neoplastic MCs at the mRNA and protein levels and show that MCL-1 is an important survival factor for neoplastic MCs.
The clinical course in SM is variable and ranges from asymptomatic, with low proliferative capacity of MCs and normal life expectancy, to highly aggressive, with rapid proliferation of neoplastic MCs.1-6 However, no apparent differences in MCL-1 expression in MCs were detected when comparing low-grade and high-grade MC disorders. This observation is best explained by the fact that MCL-1 expression is related to the survival of MCs rather than to their proliferative capacity. In fact, even in patients with indolent SM, MCs exhibit extremely long survival.4,38,59
Little is known about the regulation of expression of MCL-1 in neoplastic cells. In CML, the disease-related oncoprotein BCR/ABL was found to promote MCL-1 expression.46 In SM, the D816V-mutated variant of KIT serves as a disease-related oncoprotein. To explore whether KIT D816V is involved in the regulation of MCL-1 expression in neoplastic MCs, we applied specific pharmacologic inhibitors. In these experiments, PKC412, AMN107, and imatinib down-regulated MCL-1 expression in HMC-1.1 cells (lacking KIT D816V but expressing KIT G560V), but in HMC-1.2 cells expressing KIT D816V, only PKC412 and, to a lesser degree, AMN107 decreased MCL-1 expression. Given that PKC412 is known as a potent inhibitor of TK activity of KIT D816V and that this mutation confers resistance against imatinib,30,31 these observations favor the hypothesis that KIT D816V contribute to abnormal MCL-1 expression in neoplastic MCs. On the other hand, our data also suggest that MCL-1 expression may be triggered by other variants of KIT. In fact, MCL-1 was also expressed in HMC-1.1 cells lacking KIT D816V, and MCL-1 expression in these cells was down-regulated by PKC412 as in HMC-1.2 cells. This observation is of particular interest because MCs in high-grade malignancies (ASM or MCL) often lack KIT D816V or have KIT D816V-negative subclones that may be selected during therapy with TK inhibitors.18,22,65 In this regard it is also noteworthy that MCs in other myeloid neoplasms, including CML, MDS, MPD, and MC in the normal/reactive BM, were found to display MCL-1. Thus, depending on the type of disease and the availability of cytokines in the tissues, MCL-1 expression in MCs (and other myeloid cells) may be controlled by (activated) wild-type (wt) KIT, mutated KIT, or other factors and disease-specific molecules such as BCR/ABL in CML.46
MCL-1 is a well-established survival molecule that counteracts apoptosis in neoplastic myeloid cells.41-46 To provide evidence for the functional significance of MCL-1 expression in neoplastic MCs, HMC-1 cells were transfected with MCL-1–specific ASO or MCL-1–specific siRNA. In both HMC-1 subclones, MCL-1 knock-down resulted in a decrease in proliferation and an increase in apoptotic cells. These data provide evidence that MCL-1 is an important survival factor in HMC-1 cells.
We next compared the growth-inhibitory and apoptosis-inducing effects of MCL-1 ASO with those of 2CdA and various TK inhibitors. An interesting observation was that proliferation was suppressed at lower concentrations of TK inhibitors compared with apoptosis. This discrepancy may have significant implications when comparing effects of various drugs and may be explained by the fact that 3H-thymidine uptake is a more sensitive parameter and may be affected before signs of apoptosis occur.
ASM and MCL are high-grade MC malignancies with grave prognoses and short survival times.13-22 It is difficult to identify antineoplastic drugs for these patients. However, during the past few years, several effective TK inhibitors have been developed.38,39 One of these drugs is PKC412, which counteracts the TK activity of wild-type KIT and KIT D816V.28,30,31,38 Correspondingly, in the present study, PKC412 counteracted the growth and expression of MCL-1 in HMC-1.2 cells expressing KIT D816V. With regard to growth inhibition, these data confirm previous observations.30,31 However, even PKC412 as a single drug may not be able to induce durable complete remission in patients with ASM or MCL.65
An attractive approach in TK-driven, drug-resistant myeloid neoplasm is to combine TK inhibitors with each other or with conventional drugs. Results of our study show that MCL-1 ASO and PKC412 and that MCL-1 ASO and AMN107 cooperate with each other in inhibiting growth in HMC-1.1 cells and HMC-1.2 cells. An interesting observation was that the cooperative drug effects between MCL-1 ASO and PKC412 were synergistic in both HMC-1 subclones, whereas the interactions between MCL-1 ASO and AMN107 were only synergistic in HMC-1.1 cells but not in HCM-1.2 cells. These differences are best explained by the less pronounced antiproliferative effect of AMN107 on HMC-1.2 cells.31 The effective concentration of AMN107 (10 μM) concerning its effect on MCL-1 expression in MCs may not be reached in vivo when using standard doses of the drug (2 × 600 mg/day).
2CdA has recently been introduced as a novel effective cytoreductive agent for the treatment of advanced SM.26,27 In the current study, we found that 2CdA inhibits the growth and expression of MCL-1 in HMC-1.2 cells more potently than in HMC-1.1 cells, which may be of great clinical importance because most patients with SM, including those with ASM and MCL, display this KIT mutation.8-12 Thus, 2CdA is the first drug described to be a more potent inhibitor in MCs expressing KIT D816V than in MCs lacking this KIT mutant. However, despite these effects, 2CdA did not produce synergistic antiproliferative effects with MCL-1 ASO in HMC-1 cells.
Recent data suggest that Bcl-2 family members are promising targets in various solid tumors and leukemias and that targeting can be achieved in these neoplasms using antisense strategies.43,44,46,66-68 Therefore, clinical phase I/II trials have attempted to use ASO-type drugs.69,70 However, despite initial encouraging results, some reservations have occurred in the past concerning the possibility of applying such drugs in cancer patients.69,70 With regard to MCL-1 and SM, further preclinical and clinical studies will be required to define whether MCL-1–targeting treatment concepts can be developed far enough to enter clinical trials in ASM and MCL.
In summary, our data show that neoplastic MCs express MCL-1 and that targeting of MCL-1 is associated with decreased survival and increased responsiveness against TK inhibitors such as PKC412. These data suggest that MCL-1 is a novel, interesting target in neoplastic MCs that may help to overcome resistance to TK inhibitors.
Authorship
Contribution: K.J.A. designed the research plan, performed key laboratory experiments, and wrote the paper; M.M. performed key laboratory experiments including RT-PCR; C.S., E.S., and V.W. provided logistical support and patients; M.-T.K., L.M., and H.A. performed immunocytochemical and immunohistochemical staining experiments; K.V.G., A.G., and W.F.P. performed laboratory experiments on cell growth, proliferation, and survival; and P.V. designed the study, provided logistics, and approved the data and the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Valent, Department of Internal Medicine I, Division of Hematology and Hemostaseology, Medical University of Vienna, AKH-Wien, Waehringer Guertel 18-20, A-1097 Vienna, Austria; e-mail: peter.valent@meduniwien.ac.at.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Fonds zur Förderung der Wissenschaftlichen Forschung in Österreich (FWF) grants P-16412 and P-17205-B14 and by the Austrian Ministry of Education, Science, and Culture (GENAU II, GZ 200.136/1-VI/1/2005). H.A. is the recipient of FWF Charlotte Bühler grant H-185. A.G. and W.F.P. were supported by grant CeMM 20030 from the Center for Molecular Medicine of the Austrian Academy of Sciences.
We thank Regina Haslinger and Hans Semper for skillful technical assistance.