Abstract
To identify novel autoantibodies in acquired aplastic anemia (AA), we screened the sera of patients with AA possessing small populations of paroxysmal nocturnal hemoglobinuria (PNH)–type cells for the presence of antibodies (Abs) which recognize proteins derived from a leukemia cell line, UT-7. Immunoblotting using proteins derived from lysates or culture supernatants of UT-7 cells revealed the presence of IgG Abs specific to an 80-kDa protein. Peptide mass fingerprinting identified this 80-kDa protein as moesin. Enzyme-linked immunosorbent assay (ELISA) using recombinant moesin showed high titers of antimoesin Abs in 25 (37%) of 67 patients with AA. Moesin was secreted from several myeloid leukemia cell lines other than UT-7, such as OUN-1 and K562, as an exosomal protein. The presence of antimoesin Abs was significantly correlated with the presence of PNH-type cells and antidiazepam-binding inhibitor-related protein-1 (DRS-1) Abs. Patients with AA that did not show any of these 3 markers tended to respond poorly to immunosuppressive therapy. These findings suggest that a B-cell response to moesin, possibly derived from hematopoietic cells, frequently occurs in patients with AA and that detection of antimoesin Abs in combination with other markers may be useful in diagnosing immune pathophysiology in patients with AA.
Introduction
Acquired aplastic anemia (AA) is a syndrome characterized by pancytopenia and bone marrow hypoplasia. Immune-mediated suppression of hematopoiesis is considered to be the most important mechanism responsible for the development of this syndrome,1–3 but this mechanism does not underlie all patients with AA. Approximately 30% of patients with AA do not respond to immunosuppressive therapy (IST) with antithymocyte globulin (ATG) plus cyclosporine (CsA),4,5 and some of the patients treated with IST undergo progression to myelodysplastic syndrome (MDS) or acute myelogenous leukemia (AML). For patients with nonimmune mediated AA, IST might even be harmful because of an increased risk of opportunistic infections. Despite the understanding that not all patients with AA have an immune pathophysiology, patients with AA have been unconditionally placed on IST because there is no good marker to differentiate nonimmune-mediated AA from immune-mediated AA
We recently reported that the presence of a small population of CD55−CD59− blood cells serves as a marker for immune pathophysiology of AA.6–8 However, the highly sensitive flow cytometric assay which determines whether there is an increase in the proportion of such paroxysmal nocturnal hemoglobinuria (PNH)–type cells requires fresh blood. The assay to detect PNH-type granulocytes is difficult to use on patients with AA whose neutrophil counts are less than 0.1 × 109/L. Moreover, even in the absence of an increased proportion of PNH-type cells, greater than 40% of patients with AA respond to IST.8 Accordingly, laboratory markers other than small populations of PNH-type cells are necessary to diagnose immune pathophysiology prior to IST in patients with AA.
In autoimmune diseases in which T cells play a primary role in the pathogenesis, antibodies (Abs) specific to antigens derived from T-cell target proteins, such as myelin basic protein in multiple sclerosis (MS) and glutamate decarboxylase in insulin-dependent diabetes mellitus (IDDM), are often detected, and they have served as a marker of immune pathophysiology of the diseases.9–12 If Abs specific to the antigens abundantly expressed by hematopoietic progenitor cells were detectable in the serum of patients with AA, they might reflect the immune pathophysiology of their patients. We previously identified Abs specific to diazepam-biding inhibitor-related protein-1 (DRS-1) in the serum of 38% of patients with AA showing small populations of PNH-type cells (patients with PNH+ AA).13 The serum of patients with PNH+ AA may also contain other autoantibodies (auto-Abs) specific to self-antigens because of a breakdown of tolerance that is thought to occur in organ-specific autoimmune diseases such as MS,14 IDDM,15 and Basedow disease.16 The detection of such Abs may complement the diagnosis of immune pathophysiology in patients with AA.
To identify novel auto-Abs in AA, we screened sera from patients with PNH+ AA for the presence of Abs recognizing antigens derived from a megakaryocytic leukemia cell line, UT-7, a cDNA library of which was used for the identification of anti–DRS-1 Abs.13 A new method taking advantage of culture supernatant of the cells for Western blotting followed by mass spectrometry identified an Ab specific to a membrane-cytoskeleton linking protein, moesin.
Patients, materials, and methods
Study subjects
Sera or plasma were obtained from 67 patients with AA (23 with severe AA and 44 with moderate AA), 21 patients with refractory anemia (RA) of MDS, 49 patients with rheumatoid arthritis, and 48 healthy individuals. The severity of AA was classified according to the criteria proposed by Camitta et al.17 Samples were cryopreserved at −80°C until use. All patients and healthy volunteers were provided informed consent in accordance with the Declaration of Helsinki, before collecting samples. This study was approved by the human research committee of Kanazawa University Graduate School of Medical Science. AA was diagnosed in patients at Kanazawa University Hospital and other hospitals taking part in the bone marrow failure study led by the Ministry of Health, Labor, and Welfare of Japan.
Detection of PNH-type cells
Proportions of CD55−CD59− cells in CD11b+ granulocytes and in glycophorin A+ red blood cells were determined using 2-color flow cytometry as described previously.8
Cell lines
The following leukemia cell lines and synovial cells were kindly provided by each respective researcher. A megakaryoblastic leukemia cell line UT-7 by Dr N. Komatsu of Jichi Medical School; myeloid leukemia cell lines KH88, OUN-1, SAS413, NB4, and KG-1 by Dr M. Yasukawa of Ehime University; a myelodysplastic syndrome cell line TF-1 by Dr S. Ogawa of the University of Tokyo; synovial cells of a patient with rheumatoid arthritis by Dr M. Kawano of Kanazawa University. K562 was purchased from the Health Science Research Resources Bank (Osaka, Japan).
Isolation of CD34+ cells, CD3+ T cells, and CD19+ B cells
Peripheral blood buffy coat cells were collected from a healthy volunteer donor after G-CSF administration. CD34+ cells were isolated from the buffy coat cells using a CD34 progenitor cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. CD3+ T cells and CD19+ B cells were isolated from the same cells using CD3 and CD19 microbeads (Miltenyi Biotec), respectively. The purity of isolated CD34+, CD3+, and CD19+ cells was greater than 95% as demonstrated by flow cytometry.
Immunofluorescence analysis of UT-7 cells
UT-7 cells were fixed with 4% formaldehyde for 5 minutes and treated with 0.1% Tween 20 for 10 minutes for permeabilization. The cells were incubated in PBS containing 0.5% sera from a patient with AA for 30 minutes. After washing, the cells were incubated in PBS containing 0.1% FITC-labeled goat anti–human IgG and viewed with an immunofluorescent microscope (Axioplan2 imaging; Carl Zeiss, Jena, Germany) equipped with a 100×/0.4 NA oil objective lens and M1 digital camera (Carl Zeiss); Isis software version 5.0 (Carl Zeiss) was used to acquire digital images.
Purification of bacterially expressed moesin proteins
Full-length human moesin cDNA and the cDNA fragments of human moesin were synthesized by the reverse transcription–polymerase chain reaction (RT-PCR) amplification method. Briefly, cDNA was reverse transcribed from the mRNA of UT-7 cells by using SuperScript First-Strand Synthesis (Invitrogen, Carlsbad, CA). Thirty cycles of PCR were performed using each set of primers; for the full-length moesin termed M0 (S0, 5′-CGGAATTCGCCTTTGCCGCCACCATGCCC-3′; A0, 5′-CGGTCGACTCCCTAGAGGCTGGGTGCCCA-3′), for the carboxyl (C)–terminal portion of moesin (moesin 399-500) termed M1 (S1, 5′-CGCGGATCCGCCAAGGAGGCCTTGCTGCAG-3′; A1, 5′-CGGAATTCGGCCATAGCATCAGCCCGTAG-3′), and for the C-terminal portion of moesin (moesin 494-577) termed M2 (S2, 5′-CGCGGATCCCTACGGGCTGATGCTATGGCC-3′; A2, 5′-CGGAATTCTCCCTAGAGGCTGGGTGCCCA3-′). Each cycle consisted of denaturation at 94°C for 30 seconds, annealing at 60°C for 1 minute, and extension at 72°C for 1 minute. PCR products purified from an agarose gel were inserted into the pGEX-6P-1 vector (GE Healthcare, Fairfield, CT) between the EcoRI and SalI sites for expression of a GST-tag fusion protein M0, and between the EcoRI and BamHI sites for the GST-tag fusion proteins M1 and M2 using BL21 competent cells (Novagen, Madison, WI). Synthesized proteins were purified using glutathione Sepharose 4B (Amersham Biosciences, Piscataway, NJ). Native moesin protein and C-terminal moesin protein fragments were released from GST-tag moesin proteins using PreScission Protease (Amersham Biosciences) according to the manufacturer's instructions. The proper size of the recombinant proteins was confirmed by Western blotting with mouse antimoesin mAb (Clone 38; BD Biosciences, San Jose, CA). To detect a specific Ab in serum, blotted membranes were incubated in 3% BSA-PBS containing serum diluted 1:200.
Western blotting
UT-7 cells were suspended in Laemmli sample buffer and sonicated for the preparation of cell lysates. Approximately 10 μg UT-7 lysate, 5 μg Grp78, and 5 μg recombinant native moesin protein per lane were electrophoresed in a 12% polyacrylamide gel and transferred to a PVDF membrane. The membrane was incubated in 3% BSA-PBS containing serum diluted 1:200 from patients with AA or healthy persons.
Peptide mass fingerprinting
UT-7 cells (1× 107) were suspended in 1 mL RPMI 1640 medium containing 10% fetal calf serum (FCS) and incubated for 1 hour at 37°C in the CO2 incubator. Culture supernatants were collected by centrifugation at 500g for 5 minutes. Mass spectrometric identification of an 80-kDa protein derived from the UT-7 cell supernatants was performed as previously described.18 Briefly, proteins fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis were visualized by Coomassie Brilliant Blue (CBB) staining, and the 80-kDa band was excised from gels, followed by in-gel digestions with trypsin (Promega, Madison, WI) in a buffer containing 50 mM ammonium bicarbonate (pH 8.0) and 2% acetonitrile overnight at 37°C. Molecular mass analyses of the triptic peptides were performed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF/MS) using an ultraflex TOF/TOF (Bruker Daltonics, Billerica, MA). Proteins were identified by comparison between the molecular weights determined by MALDI-TOF/MS and the theoretical peptide masses of proteins registered in NCBInr.
Enzyme-linked immunosorbent assay (ELISA)
Each well of a 96-well Nunc-Immuno plate (Nalge-Nunc, Roskilde, Denmark) was filled with 50 μL coating buffer (50 mM carbonate/bicarbonate buffer, pH 9.6) containing 5 μg/mL purified recombinant moesin protein and was incubated overnight at 4°C. The plates were washed and filled with PBS containing 10% FCS for 2 hours at 37°C to block nonspecific binding of proteins to moesin. Sera from the patients were added to the wells at a final dilution of 1:200, and the plates were incubated at 37°C for 1 hour. After washing, peroxidase-conjugated goat anti–human IgG antibodies (1:80 000; Jackson ImmunoResearch, Baltimore, PA) were added to the wells, and the plates were incubated at 37°C for 1 hour. Finally, the plates were washed and incubated with 3,3,5,5-tetramethylbenzidine substrate (Pierce, Rockford, IL) at room temperature for 30 minutes, and the optical density (OD) absorbance at 450 nm was determined using a SLTEAR 340 ATELISA reader (SLT-Labinstruments, Grödig, Austria). A positive reaction was defined as an absorbance value exceeding the mean +2 SDs of the OD absorbance values from the sera of the 20 or 48 controls.
Enrichment of exosomes from culture supernatants of leukemia cell lines and Western blotting
Leukemia cell lines (K562, OUN-1, TF-1, and UT-7) cells, CD34+ cells, CD3+ T cells, CD19+ B cells, and synovial cells were cultured at 2 × 109/L for 48 hours, and the supernatants were collected. The culture supernatants of leukemia cell lines and synovial cells were subjected to 3 successive centrifugations to remove cells and cell debris at 300g for 10 minutes, 2000g for 20 minutes, and finally at 10 000g for 30 minutes, all at 4°C. Exosomes were then pelleted at 64 000g for 100 minutes. Pellets were resuspended and washed in PBS and centrifuged at 100 000g for 1 hour using a SW28 rotor (Beckman Coulter Instruments, Fullerton, CA).19 The exosomes were fixed with 2% paraformaldehyde in 0.1 M phosphate buffer of pH 7.4 for 1 hour and then were postfixed with 0.5% OsO4 for 20 minutes at 4°C. Next, they were stained with 0.5% uranyl acetate for 20 minutes, dehydrated with a graded ethanol series, and were embedded in an epoxy resin (Selva Feinbiochemica GmbH, Heidelberg, Germany). Ultrathin sections were prepared and examined using an electron microscope (JEM-1210; JEOL, Tokyo, Japan) after brief staining with uranyl acetate and lead citrate. Exosomes were resuspended in PBS, divided into aliquots, and stored at −80°C. Approximately 1 μg exosomal protein per lane was electrophoresed in a 12% polyacrylamide gel and transferred to a PVDF membrane. The membrane was incubated in PBS containing a mouse antimoesin mAb (Clone 38/87; NeoMarkers, Fremont, CA) at 0.2 μg/mL or a rabbit antimoesin polyclonal Ab (TK89) which was kindly provided by Dr S. Tsukita of Kyoto University.
Immunosuppressive therapy and response criteria
ATG (Lymphoglobuline; Aventis Behring, King of Prussia, PA; 15 mg/‘kg/day’, 5 days) plus cyclosporine (CsA; Novartis, Basel, Switzerland; 3-6 mg/‘kg/day’), or CsA alone was administered following the standard protocol. The dose of CsA was adjusted to maintain trough levels between 150 and 250 ng/mL, and the appropriate dose was administered for at least 6 months. G-CSF (filgrastim, 300 μg/m2 or lenograstim, 5 μg/kg) was administered to some patients. Response to IST was evaluated according to the response criteria described by Camitta.20
Statistics
Differences in the prevalence of antimoesin Ab titers in serum among different patient groups were examined using Fisher exact test.
Results
Detection of Abs specific to proteins derived from UT-7 cells in the sera of patients with AA
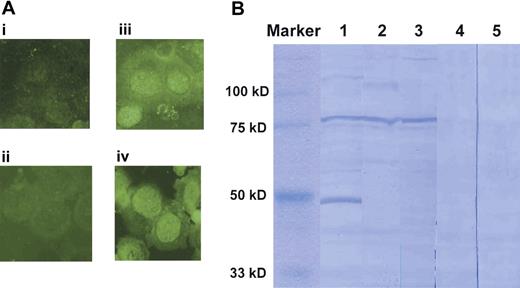
We first screened sera of patients with AA showing increased PNH-type cells for the presence of Abs reactive to UT-7 cell proteins using an immunofluorescence analysis. Among the sera from 9 patients with AA, 6 patients' sera stained the cytoplasm of UT-7 cells as shown in Figure 1A, indicating the presence of IgG antibodies specific to UT-7 proteins. To identify these proteins, UT-7 lysate was subjected to Western blotting using sera from patients with AA. Sera from several patients with PNH+ AA produced a clear band with a size of 80 kDa (Figure 1B). The Ab specific to the 80-kDa protein was detected in the sera of 9 (43%) of 21 patients with PNH+ AA, whereas it was undetectable in any of the 7 patients not showing increased PNH-type cells (PNH− patients) and 11 healthy persons.
Detection of antibodies (Abs) specific to proteins derived from UT-7 cells in the sera of patients with AA. (A) Immunofluorescence analysis of UT-7 cells using 1:200 diluted sera and FITC-labeled anti–human IgG. (i-ii) Healthy persons; (iii-iv) patients with AA. (B) Western blotting for UT-7 lysates with sera from 3 patients with PNH+ AA (lanes 1-3) and 2 healthy persons (lanes 4-5). Images were obtained using 1000× magnification.
Detection of antibodies (Abs) specific to proteins derived from UT-7 cells in the sera of patients with AA. (A) Immunofluorescence analysis of UT-7 cells using 1:200 diluted sera and FITC-labeled anti–human IgG. (i-ii) Healthy persons; (iii-iv) patients with AA. (B) Western blotting for UT-7 lysates with sera from 3 patients with PNH+ AA (lanes 1-3) and 2 healthy persons (lanes 4-5). Images were obtained using 1000× magnification.
Identification of the 80-kDa protein
To identify the 80-kDa protein, we cut out the clear band from a CBB-stained gel and tried to determine the amino acid sequence of the protein using peptide mass fingerprinting. However, this attempt failed to identify a single protein because of the presence of various other proteins in the total amount eluted from the 80-kDa band. We then examined culture supernatants of UT-7 cells for the presence of the 80-kDa protein because some intracellular proteins are reportedly secreted from cell lines into culture medium.19,21,22 Figure 2 shows the results of Western blotting. Incubation of the membrane blotted with the culture supernatant proteins in PBS containing sera from patients with AA revealed a similar but more distinct 80-kDa band. When the corresponding band was cut out from the original CBB-stained gel (Figure 2, lane 5) and subjected to peptide mass fingerprinting, this protein was identified as either moesin or Grp 78. Serum of patients with AA failed to reveal an 80-kDa band when a Grp 78 protein blotted membrane was used. The approximately 70-kDa bands in lanes 3 and 6 of Figure 2 were thought to be nonspecific bands because of a low purity of the recombinant Grp 78 protein.
Identification of the 80-kDa protein recognized by IgG Abs of patients with AA. UT-7 lysates (lanes 1, 4), culture supernatants of UT-7 cells (lanes 2, 5, 7), and recombinant Grp 78 protein (lanes 3, 6) were subjected to Western blotting using sera from a healthy person (lanes 1-3), a patients with PNH+ AA (lanes 4-6), and antimoesin monoclonal Ab (lane 7). The arrow indicates 80-kDa protein bands.
Identification of the 80-kDa protein recognized by IgG Abs of patients with AA. UT-7 lysates (lanes 1, 4), culture supernatants of UT-7 cells (lanes 2, 5, 7), and recombinant Grp 78 protein (lanes 3, 6) were subjected to Western blotting using sera from a healthy person (lanes 1-3), a patients with PNH+ AA (lanes 4-6), and antimoesin monoclonal Ab (lane 7). The arrow indicates 80-kDa protein bands.
Prevalence of antimoesin Abs in patients with AA or MDS
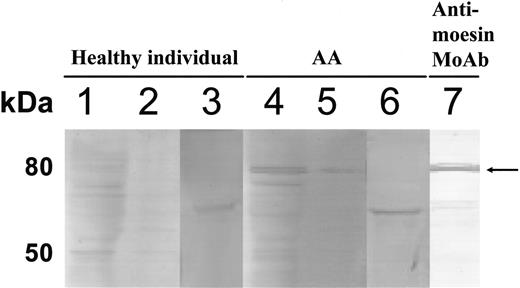
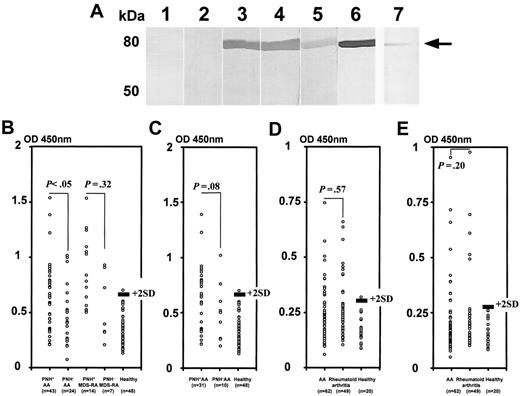
To confirm the presence of Abs specific to moesin in the sera of patients with AA, a recombinant human native moesin protein was prepared. Figure 3A shows the results of Western blotting using the native moesin. Clear bands corresponding to the moesin were produced by sera of 3 patients with PNH+ AA but not by sera from 2 healthy persons. When we used the patient serum at different dilutions of more than 1:200, including 1:400, 1:800, 1:1600, 1:6400, and 1:25 600, the serum diluted up to 1:6400 could detect moesin by Western blotting. To measure the titers of moesin Abs in the serum, we established an ELISA using recombinant moesin protein. Higher antimoesin Ab titers than the cutoff value were detected in 25 (37%) of 67 patients. The prevalence was 44% in 41 patients whose duration of illness was shorter than 1 year. Figure 3B shows titers of antimoesin Ab in the sera of 67 patients with AA consisting of 43 patients with PNH+ and 24 patients with PNH−, 21 patients with MDS-RA consisting of 14 patients with PNH+ and 7 patients with PNH−, and 48 healthy persons. Twenty (47%) of the patients with PNH+ AA showed Ab titers greater than the cutoff value, whereas 5 (21%) of the patients with PNH− AA showed increased titers. There was a significant difference in the prevalence of higher antimoesin Ab titers between patients with PNH+ AA and patients with PNH− AA (P = .03). Nine (64%) of the patients with PNH+ MDS-RA showed Ab titers greater than the cutoff value, whereas 3 (43%) of the patients with PNH− MDS-RA showed increased titers. No significant difference was observed in the prevalence of higher antimoesin Ab titers between patients with PNH+ MDS-RA and patients with PNH− MDS-RA (P = .32). Among patients with recently diagnosed AA examined before therapy, the prevalence of patients showing higher antimoesin Ab titers than the cutoff value in 31 patients with PNH+ (52%) was also higher than that in 10 patients with PNH− (20%), although the difference in the prevalence between the 2 groups was not statistically significant (P = .08; Figure 3C). None of the sera from 6 patients undergoing chemotherapy (3 with acute myelogenous leukemia and 3 with non-Hodgkin lymphoma) were positive for antimoesin Abs.
Detection of antimoesin Abs in the sera of patients with AA, MDS-RA, and rheumatoid arthritis. (A) Detection of specific Abs to recombinant moesin in the sera of patients with AA. Purified recombinant native moesin protein was used to detect Abs specific to moesin in sera from 2 healthy persons (lanes 1-2) and 3 patients with PNH+ AA (lanes 3-5). Antimoesin monoclonal Ab was used as a positive control (lane 6). The serum of a patient with PNH+ AA diluted to 1:6400 was also used (lane 7). The arrow indicates recombinant moesin bands. (B-C) Titration of moesin Abs in sera of patients with AA and MDS-RA using ELISA. Antimoesin Ab titers were determined for the sera of 43 patients with PNH+ AA, 24 patients with PNH− AA, 14 patients with PNH+ MDS-RA, 7 patients with PNH− MDS-RA, and 48 healthy persons. (B) All patients. (C) Patients with recently diagnosed AA examined before therapy. (D-E) Titration of Abs specific to C-terminal moesin fragment in the sera of patients with AA and rheumatoid arthritis. Titers of anti–C-terminal moesin fragment Abs were determined in the sera of 62 patients with AA, 49 patients with rheumatoid arthritis, and 20 healthy persons. (D) Antimoesin C-terminal fragment (399-500) M1 Abs. (E) Antimoesin C-terminal fragment (494-577) M2 Abs. The solid line denotes a cutoff value defined as the mean + 2 SDs of the absorbance in healthy persons. P values indicate the differences in the prevalence of patients showing higher antimoesin or moesin-fragment Ab titers between 2 different groups.
Detection of antimoesin Abs in the sera of patients with AA, MDS-RA, and rheumatoid arthritis. (A) Detection of specific Abs to recombinant moesin in the sera of patients with AA. Purified recombinant native moesin protein was used to detect Abs specific to moesin in sera from 2 healthy persons (lanes 1-2) and 3 patients with PNH+ AA (lanes 3-5). Antimoesin monoclonal Ab was used as a positive control (lane 6). The serum of a patient with PNH+ AA diluted to 1:6400 was also used (lane 7). The arrow indicates recombinant moesin bands. (B-C) Titration of moesin Abs in sera of patients with AA and MDS-RA using ELISA. Antimoesin Ab titers were determined for the sera of 43 patients with PNH+ AA, 24 patients with PNH− AA, 14 patients with PNH+ MDS-RA, 7 patients with PNH− MDS-RA, and 48 healthy persons. (B) All patients. (C) Patients with recently diagnosed AA examined before therapy. (D-E) Titration of Abs specific to C-terminal moesin fragment in the sera of patients with AA and rheumatoid arthritis. Titers of anti–C-terminal moesin fragment Abs were determined in the sera of 62 patients with AA, 49 patients with rheumatoid arthritis, and 20 healthy persons. (D) Antimoesin C-terminal fragment (399-500) M1 Abs. (E) Antimoesin C-terminal fragment (494-577) M2 Abs. The solid line denotes a cutoff value defined as the mean + 2 SDs of the absorbance in healthy persons. P values indicate the differences in the prevalence of patients showing higher antimoesin or moesin-fragment Ab titers between 2 different groups.
Epitope analysis of antimoesin Abs in patients with AA and those with rheumatoid arthritis
A previous study demonstrated that antimoesin Abs were detectable in 14% of patients with rheumatoid arthritis.23 Our ELISA revealed high titers of antimoesin Abs in 34% of patients with rheumatoid arthritis who did not show any cytopenias (data not shown). To determine whether a difference exists in the specificity of antimoesin Abs between patients with AA and rheumatoid arthritis, 2 different C-terminal fragments of moesin (M1 and M2) were prepared and used for the ELISA. When we screened the sera from 3 patients showing high titers of antimoesin Abs against the full-length moesin for the presence of Abs specific to N-terminal proteins (amino acid residues 1-398) using Western blotting, none of the sera from these patients revealed a band of the N-terminal protein (data not shown). We therefore focused on the C-terminal protein to detect specific Abs with ELISA. As shown in Figure 3D-E, 12 (19%) patients with AA and 10 patients (20%) with rheumatoid arthritis showed anti-M1 Ab titers, whereas 11 (18%) patients with AA and 5 patients (10%) with rheumatoid arthritis showed anti-M2 Ab titers greater than the cutoff value. Therefore, the 2 groups of patients showed a similar pattern of Ab titers to each fragment.
Secretion of moesin from leukemia cell lines, CD34+ cells, CD3+ T cells, CD19+ B cells, and synovial cells
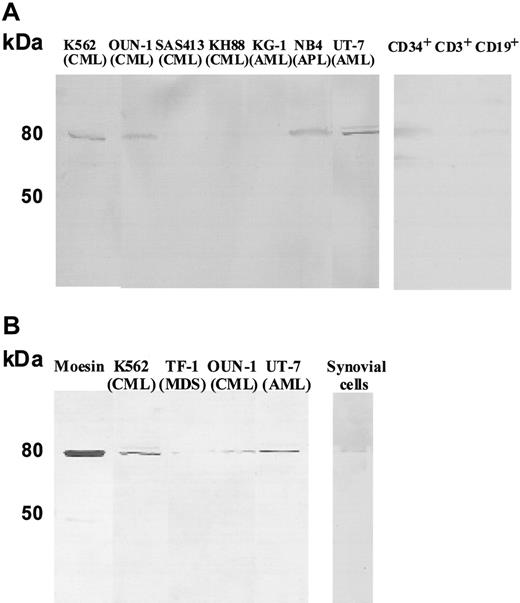
The secretion of moesin may be common to immature myeloid leukemia cells. We therefore examined culture supernatants of myeloid leukemia cell lines other than UT-7 cells as well as of CD34+ cells, CD3+ T cells, and CD19+ B cells from a healthy volunteer donor. Moesin was detectable in culture supernatant of 4 (K562, OUN-1, NB4 and UT-7) of 7 myeloid leukemia cell lines, CD34+ cells, and CD19+ B cells (Figure 4A). Because B lymphocytes reportedly secrete moesin as a form of exosome,24 we examined the exosomal fraction of culture supernatant derived from leukemia cell lines and synovial cells of a patient with rheumatoid arthritis for the presence of moesin. As shown in Figure 4B, moesin was detectable in the exosome fraction of culture supernatants from all 4 leukemia cell lines and synovial cells. When the purified exosome fraction was examined by electron microscopy, the presence of small particles which were compatible in size with that of the exosomes was observed (data not shown).
Detection of moesin in the culture supernatant of myeloid leukemia cell lines, CD34+ cells, CD3+ T cells, CD19+ B cells, and synovial cells. (A) Culture supernatants of various myeloid leukemia cell lines, CD34+ cells, CD3+ T cells, and CD19+ B cells were subjected to Western blotting using antimoesin Abs. (B) Exosomal fractions prepared from the culture supernatants of the 4 positive cell lines and synovial cells were also examined by Western blotting.
Detection of moesin in the culture supernatant of myeloid leukemia cell lines, CD34+ cells, CD3+ T cells, CD19+ B cells, and synovial cells. (A) Culture supernatants of various myeloid leukemia cell lines, CD34+ cells, CD3+ T cells, and CD19+ B cells were subjected to Western blotting using antimoesin Abs. (B) Exosomal fractions prepared from the culture supernatants of the 4 positive cell lines and synovial cells were also examined by Western blotting.
Relationship of antimoesin Abs with either other markers or the response to IST
We previously reported that Abs specific to DRS-1 are detectable in 38% of patients with PNH+ AA.13 We then studied the relationship between the presence of antimoesin Abs with the presence of anti–DRS-1 Abs in 45 patients with AA. Antimoesin Abs were detectable in 68% of patients with anti–DRS-1 Ab+, whereas they were detectable in only 27% of patients with anti–DRS-1 Ab− (P = .007). Twenty-eight patients recently diagnosed underwent ATG + CsA or CsA therapy after examination of their blood for the presence of antimoesin Abs, anti–DRS-1 Abs, and small populations of PNH-type cells (Table 1). There were no significant differences in the rate of response to ATG + CsA or CsA between patients showing antimoesin Abs and those not showing antimoesin Abs (75% versus 67% for ATG + CsA; 100% versus 75% for CsA). When all 3 markers were assessed, 13 (76%) of 17 and 9 (100%) of 9 patients showing at least 1 of the 3 makers improved with ATG + CsA and CsA alone, respectively, whereas none of 2 patients not showing any of these markers responded.
Discussion
The present study revealed that Abs specific to moesin are detectable in the serum of approximately 37% of patients with AA. Moesin is an intracellular protein that links the cell membrane and cytoskeleton and mediates the formation of microtubules and cell-adhesion sites as well as ruffling of the cell membrane.25 This membrane-linking protein is expressed by various blood cells, including megakaryocytes and granulocytes, but its expression is localized inside the cell membrane and not on the cell surface. It is therefore unlikely for antimoesin Abs to affect the function and viability of hematopoietic cells. It is also unlikely that T cells specific for moesin play a role in the inhibition of hematopoietic stem cells in patients with AA because moesin is expressed by various kinds of cells other than blood cells.26,27 Nevertheless, the presence of antimoesin Abs appears to reflect the immune pathophysiology of bone marrow failure because it correlates with the presence of small population of PNH-type cells which are well associated with the immune pathophysiology of AA.8
A previous study demonstrated the presence of antimoesin Abs in 14% of patients with rheumatoid arthritis.23 None of the patients with antimoesin Abs+ with rheumatoid arthritis in the previous report and of the 11 patients in our study showed cytopenia. In contrast, none of our patients with AA with antimoesin Abs showed symptoms characteristic of rheumatoid arthritis or laboratory findings such as positive rheumatoid factors. There was no difference in the specificity pattern of antimoesin Abs in patients with AA and rheumatoid arthritis. However, a case-control study on AA conducted by IAAS revealed that a past history of rheumatoid arthritis is significantly associated with the later development of AA,28 and moesin was detectable in the exosomes derived from leukemia cell lines as well as from synovial cells of a patients with rheumatoid arthritis. It is therefore possible that AA and rheumatoid arthritis may share pathogenetic mechanisms leading to a breakdown of immunologic tolerance to moesin.
In T-cell diseases such as AA, the presence of auto-Abs has not attracted much attention from hematologists for a long time. Recently, 3 different auto-Abs specific to kinectin,29 DRS-1,13 and anti-postmeiotic segregation increased 130 were detected in the serum of patients with AA. All of these Abs were identified through the immunoscreening of serum with a cDNA library derived from fetal liver cells or a leukemia cell line. The Abs to moesin are unique in that they were identified through their direct binding to protein in the denatured lysate of UT-7 cells. Moreover, moesin is the first autoantigen that was successfully identified in autoimmune diseases by using auto-Abs in patient's serum and peptide mass fingerprinting.
Although the serologic identification of antigens by recombinant expression cloning (SEREX) is a useful assay for identifying novel antigens recognized by a small amount of auto-Abs in serum,13,29 insignificant antigens are often identified because of the low specificity of the assay. Antigens detected by immunoblotting with serum may be significant when the assay revealed distinct bands. However, it is often difficult to identify the amino acid sequence of target antigens in cell lysates with peptide mass fingerprinting because of the presence of many proteins other than the target antigens which are eluted from the cut band. Moesin was successfully identified using the culture supernatant of UT-7 cells as a template because it was secreted from the cell line as a protein in the exosome and formed a single band in the polyacrylamide gel. Immunoblotting of exosomal fractions from culture supernatants followed by peptide mass fingerprinting thus appears to be a powerful method for identifying novel antigens which potentially elicit antibody production.
Our study showed that moesin was secreted into culture medium from various leukemia cell lines and synovial cells of a rheumatoid arthritis patient as an exosomal protein. Previous studies have shown that exosomes secreted from B lymphocytes and mesothelioma cells contain various intracellular proteins, including moesin,19,24 and that some leukemia/lymphoma cell lines such as K56221 and Daudi22 secrete exosome-containing cytosolic proteins. However, moesin has not yet been detected in the exosomal fractions derived from cells from leukemia cell lines and synovial cells. Immunoblotting in the present study has shown the exosome fraction derived from the culture supernatants of the leukemia cell lines to contain moesin. Exosomes are known to be capable of presenting antigen by itself directly or indirectly through being ingested by antigen-presenting cells to helper T cells and eliciting specific Ab production.31,32 It is conceivable that immature hematopoietic cells and B lymphocytes of patients with AA may also secrete a low amount of moesin as an exosome, leading to the induction of antimoesin Abs. The presence of moesin in the culture supernatant of CD34+ cells and B cells from a healthy person supports this hypothesis.
The prevalence of high antimoesin Abs was significantly higher in patients with PNH+ than in patients with PNH−. A similar association was observed between anti–DRS-1 Ab and PNH-type cells in our previous study. In immune-mediated bone marrow failure such as PNH+ AA, the immune system attack, particularly by CD4+ T cells, is thought to damage hematopoietic stem cells. Various cytokines produced by CD4+ T cells in the process of the immune attack may stimulate hematopoietic cells and B cells to secrete moesin, leading to a breakdown of immune tolerance toward moesin. Although the presence of antimoesin Abs was not associated with a good response to IST in our small series of patients, the detection of antimoesin Abs in combination with anti–DRS-1 Abs and PNH-type cells may help predict a good response to IST. Indeed, the rate of response to IST in 28 patients with AA showing at least 1 of the 3 makers was 85%, whereas none of the 2 patients with AA not showing any of the markers responded. The presence of such auto-Abs may particularly serve as a good marker for immune pathophysiology when sufficient numbers of blood cells for flow cytometry to detect PNH-type cells are not available because of severe bone marrow failure. The predictive value of these autoantibodies needs to be tested in a prospective clinical study.
Authorship
Contribution: H.T., S.N., and T.C. designed the research, analyzed data, and wrote the paper; M.Y. and S.I. performed electron microscopic examination; X.F. and X.L. performed research; K.O. performed peptide mass fingerprinting; C.S. analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shinji Nakao, Cellular Transplantation Biology, Division of Cancer Medicine, Kanazawa University Graduate School of Medical Science, 13-1 Takaramachi, Kanazawa, Ishikawa 920-8641, Japan; e-mail: snakao@med3.m.kanazawa-u.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms M. Yoshii and Ms A. Hamano of Cellular Transplantation Biology of Kanazawa University for their technical assistance. We also thank the following doctors for providing us with patient samples and clinical information: Y. Haseyama of Tonan Hospital; N. Uchida and T. Azuma of Ehime University Hospital; R. Imamura of Kurume University Hospital; S. Kikuchi, K. Kuribayashi, and A. Ueno of Sapporo Medical University; A. Matsuda, Y. Sato, and M. Misumi of Saitama Medical University; H. Yokoyama, I. Koni, T. Wada, Y. Goto, S. Shimadoi, and J. Ozaki of Kanazawa University Hospital; T. Kurokawa and K. Ishiyama of Toyama Prefectural Central Hospital; S. Okamoto of Keio University Hospital; T. Mori of Yaizu City Hospital; T. Azuma and M. Teramura of Tokyo Women's Medical College Hospital; N. Yonetani of Wakayama Red Cross Hospital; K. Naito of Hamamatsu Medical University Hospital; Y. Terasaki of Toyama City Hospital; T. Saga, A. Sawazaki, and T. Kotani of NTT Kanazawa Hospital; T. Komeno of Mito Medical Center; M. Sugiyama of Kinki University Hospital; S. Senda of Toyama Red Cross Hospital; J. Tadokoro of Dokkyo Medical University; K. Shindo and M. Tashima of Nagahama City Hospital; A. Wakita of Nagoya City Higashi Hospital; N. Takahashi of Chichibu Daiichi Hospital; T. Tamaki of Izumisano City Hospital; F. Nakahara and S. Iki of NTT Kanto Hospital; K. Sugiura of Kyoritu Sogo Hospital; M. Iida, Y. Nakamura, and M. Yamaguchi of Ishikawa Prefectural Central Hospital; K. Koike of Asahikawa Red Cross Hospital; M. Morioka of Aiiku Hospital; K. Kanaya of Tokyo Medical University Hachioji Medical Center; T. Fukushima of Kanazawa Medical University Hospital; Y. Tamai of Hirosaki University Hospital; T. Oonaka of University of Occupational and Environmental Health; N. Hyakunan of Ryukyu University Hospital; K. Kyoda of Kouseiren Takaoka Hospital; K. Kumano of Hokkaido University Hospital; Y. Maesako of Tenri Hospital; K. Suzuki of Matsusaka Chuo General Hospital; H. Ishida and N. Uoshima of Matsushita Memorial Hospital; E. Ishii of Saga Medical University Hospital; N. Ichikawa of Nagano Red Cross Hospital; S. Yagima of Hamamatsu Medical Center; Y. Asano of Chihaya Hospital; H. Gondo of Hamanomachi Hospital.
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Technology, Sports, and Culture of Japan (KAKENHI 15390298) and grants from the Research Committee for Idiopathic Hematopoietic Disorders, the Ministry of Health, Labor, and Welfare, Japan.