Abstract
Despite considerable success in treating newly diagnosed childhood acute lymphoblastic leukemia (ALL), relapsed disease remains a significant clinical challenge. Using a NOD/SCID mouse xenograft model, we report that immunostimulatory DNA oligonucleotides containing CpG motifs (CpG ODNs) stimulate significant immune activity against primary human ALL cells in vivo. The administration of CpG ODNs induced a significant reduction in systemic leukemia burden, mediated continued disease control, and significantly improved survival of mice with established human ALL. The death of leukemia cells in vivo was independent of the ability of ALL cells to respond directly to CpG ODNs and correlated with the production of IL-12p70, IFN-α, and IFN-γ by the host. In addition, depletion of natural killer cells by anti–asialo-GM1 treatment significantly reduced the in vivo antileukemic activity of CpG ODN. This antileukemia effect was not limited to the xenograft model because natural killer cell–dependent killing of ALL by human peripheral blood mononuclear cells (PBMCs) was also increased by CpG ODN stimulation. These results suggest that CpG ODNs have potential as therapeutic agents for the treatment of ALL.
Introduction
Despite the fact that more than 95% of children with newly diagnosed acute lymphoblastic leukemia (ALL) achieve a first complete remission, relapsed ALL is the fifth most common pediatric malignancy. For the 20% to 25% of pediatric ALL patients in remission who subsequently have relapses, therapy options are limited and the prognosis is poor.1 In adults, ALL is less common but has proven even more difficult to treat, with initial therapy generally achieving only short remissions.2,3 For patients with relapsed ALL, hematopoietic stem cell transplantation (HSCT) is the best treatment option, achieving significant disease-free survival in 30% to 50% of cases.4–7 Relapse is the primary cause of treatment failure in transplant recipients, and there is an urgent need for novel therapies for this disease.
Comparison of HSCT outcomes using different donor sources reveals that immune activity, the graft-versus-leukemia (GVL) effect, is capable of significantly reducing the incidence of ALL relapse.8–12 The poorer outcome associated with T-cell depletion of the graft13 and the strong correlation between development of chronic graft-versus-host disease and reduced relapse rates,14,15 implicate donor-derived T-cell activity in protection against leukemia progression. A role for natural killer (NK) cells in control of pediatric ALL is suggested by significant remissions obtained in patients undergoing haploidentical HSCT selected for killer cell immunoglobulin-like receptor (KIR) mismatches in the graft-versus-host direction.16 These observations suggest that intervention strategies that optimize antileukemia immune activities may contribute significantly to therapy for ALL.
Synthetic DNA oligonucleotides containing unmethylated CpG motifs (CpG ODNs) have been shown to provoke strong immune responses. CpG ODNs are thought to mimic the activity of bacterial DNA and serve as a “danger” signal for the immune system, triggering rapid responses from innate immune cells, including plasmacytoid dendritic cells and NK cells.17 In addition, CpG ODN stimulation leads to the generation of Th1-type adaptive immune responses. These properties have led to considerable interest in the clinical use of CpG ODNs in vaccines and cancer and autoimmune disease therapy.18 Although the effects of CpG ODN stimulation of human leukemia cells in vitro have been described, there have been no reports of the in vivo consequences of CpG ODN treatment on primary human leukemia. In this study, we investigated the ability of CpG ODNs to stimulate antileukemia immune responses in vivo using the NOD/SCID mouse model of human ALL.19–23
Materials and methods
Cells and tissue culture
The human precursor B ALL cell lines 697 and 380 were generously provided by Dr Dario Campana (St Jude Children's Research Hospital, Memphis, TN). These cell lines have previously been shown to be directly responsive (380) and nonresponsive (697) to CpG ODN stimulation.24 The cell lines were cultured in RPMI medium with 10% FBS and Normocin (InVivogen, San Diego, CA). The primary pediatric ALL sample A199 has been extensively described previously.24 This is a t(11;19), CD19+, CD10− early pre-B ALL obtained from a patient at British Columbia (BC's) Children's Hospital (Vancouver, BC, Canada), with approval from the University of British Columbia and the BC's Children's Hospital institutional review boards. Informed consent was provided in accordance with the Declaration of Helsinki. A199 leukemic cells were expanded in NOD/SCID mice to generate sufficient cell numbers for the described experiments. A199 cells obtained from mice at several time points during sequential in vivo passaging were confirmed to be derived from the original clone by cytogenetic detection of the t(11;19) translocation and immunophenotyping.
Mice
NOD/SCID (NOD/LtSz-scid/scid) mice were maintained as breeding colonies at BC's Children's Hospital and the Children's Hospital of Philadelphia, housed under specific pathogen-free conditions in micro-isolators. All experiments were performed with approval from the University of British Columbia and the Children's Hospital of Philadelphia institutional review boards. ALL cells (10-15 × 106) were injected intravenously, via lateral tail vein, into NOD/SCID mice given no prior conditioning regimen. Leukemia cell burden was measured every 5 to 10 days by phenotyping peripheral blood for human CD45+ cells. Leukemia burden was defined as the percentage of the total cell population that was human leukemia cells. Mice were killed when peripheral blood contained more than 70% leukemic blasts or at overt sign of morbidity. Preliminary studies revealed that ALL cell lines produced different disease kinetics than the primary A199 ALL cells, with morbidity occurring more rapidly and at lower peripheral blood leukemia burden (data not shown). At indicated times, mice were injected intravenously with active B-type CpG ODN (10103) or control ODN (2137), both in 100 μL PBS, or with 100 μL PBS. Both ODNs were obtained from the Coley Pharmaceutical Group (Wellesley, MA). In all experiments, except the titration described in Figure 1, mice were given 300 μg CpG ODNs per injection. For NK cell depletion studies, 50 μL anti–asialo GM1 (aGM1) antibody (Cedarlane, Hornby, ON, Canada) in 100 μL total volume with PBS was injected intravenously on day −2, day 0 (with or without CpG ODNs) and every fourth day subsequently for 24 days. PBS control mice followed the same injection schedule. After the mice were killed, spleen and liver were removed and single-cell suspensions generated by homogenization and passage through a cell strainer. Bone marrow cells were obtained by flushing femurs with PBS.
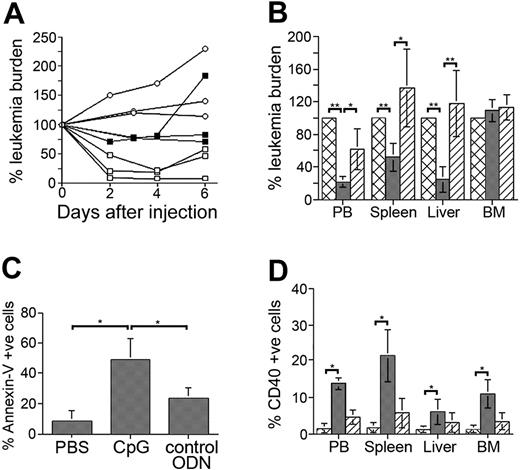
CpG ODNs stimulate ALL cells and induce systemic ALL cell death. (A) A199-bearing mice were treated with 300 μg CpG ODN (□), 100 μg CpG ODN (▪), or PBS (○) and monitored for leukemia cells in peripheral blood. Results presented are leukemia burden as percentage of burden for each animal immediately prior to CpG ODN or PBS administration. (B) A199-bearing mice with significant peripheral leukemia burden were treated with PBS (cross-hatched bars), CpG ODN (dark gray bars), or control ODN (diagonally striped bars) and killed 72 to 96 hours later. Organs were harvested and analyzed for the presence of leukemia cells. Results are presented as leukemia burden in treated mice as a percentage of burden in PBS-treated mice. PBS-treated mice are thus 100%. (C) The percentage of annexin V+ ALL cells in spleens from CpG ODN-treated and control-treated (PBS and control ODN) mice. (D) NOD/SCID mice bearing A199 leukemia cells were treated with PBS (cross-hatched bars), CpG ODN (dark gray bars), or control ODN (diagonally striped bars) and then killed 72 hours later. CD40 expression by ALL cells was then determined by flow cytometry. All results (except panel A) are presented as mean ± SD for at least 4 mice/group. **P < .005, *P < .05.
CpG ODNs stimulate ALL cells and induce systemic ALL cell death. (A) A199-bearing mice were treated with 300 μg CpG ODN (□), 100 μg CpG ODN (▪), or PBS (○) and monitored for leukemia cells in peripheral blood. Results presented are leukemia burden as percentage of burden for each animal immediately prior to CpG ODN or PBS administration. (B) A199-bearing mice with significant peripheral leukemia burden were treated with PBS (cross-hatched bars), CpG ODN (dark gray bars), or control ODN (diagonally striped bars) and killed 72 to 96 hours later. Organs were harvested and analyzed for the presence of leukemia cells. Results are presented as leukemia burden in treated mice as a percentage of burden in PBS-treated mice. PBS-treated mice are thus 100%. (C) The percentage of annexin V+ ALL cells in spleens from CpG ODN-treated and control-treated (PBS and control ODN) mice. (D) NOD/SCID mice bearing A199 leukemia cells were treated with PBS (cross-hatched bars), CpG ODN (dark gray bars), or control ODN (diagonally striped bars) and then killed 72 hours later. CD40 expression by ALL cells was then determined by flow cytometry. All results (except panel A) are presented as mean ± SD for at least 4 mice/group. **P < .005, *P < .05.
Flow cytometry
Four-color flow cytometry was performed on a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Human and mouse CD45 antibodies were used to permit specific gating on the leukemic blast population. For analysis of cell-surface molecules, all samples were labeled with directly conjugated fluorescent antibodies at 4°C for 20 minutes. The samples were then washed and resuspended in 1.5% paraformaldehyde and analyzed within 24 hours. All antibodies were obtained from BD PharMingen (San Diego, CA). The relevant labeled isotype control antibodies were included in all experiments. Leukemia cell apoptosis was detected using annexin V and 7-amino actinomycin D (7-AAD) staining, following the manufacturer's recommended protocol (BD PharMingen). The data presented are the numbers of all annexin V+ cells, including 7-AAD double-positive cells, and thus represent early and late apoptotic cells as well as possibly necrotic cells. Analysis was performed using CellQuest software (Becton Dickinson) after gating on the specific population according to forward and side scatter and CD45 expression. The effectiveness of the NK cell depletion protocol was confirmed by staining splenocytes with anti-CD49b antibody (DX5; BD PharMingen).
Cytokine analysis
Serum was collected from NOD/SCID mice at 6 and 24 hours after injection of PBS or CpG ODN, and stored at −80°C until analyzed. The IL-12p70 and IFN-γ content of the serum samples collected at 24 hours was determined using a mouse inflammation cytometric bead array (CBA) kit (BD PharMingen) following the manufacturer's recommended protocol. IFN-α levels in serum samples collected at 6 hours were measured by enzyme-linked immunosorbent assay (ELISA; PBL-Biomedical Laboratories, Piscataway, NJ).
In vitro cytotoxicity assays
Peripheral blood mononuclear cells (PBMCs) obtained from healthy donors by centrifugation over Ficoll-Paque Plus (Amersham Pharmacia Biotech, Uppsala, Sweden) were used as effector cells. 697 ALL target cells were labeled with 51 Cr for 2 hours and washed extensively prior to the assays. Effectors and target cells were then mixed in 96-well round-bottom plates at ratios starting at 100:1 in the presence or absence of 6 μg/mL CpG ODN 10103. Where indicated, PBMCs were depleted of NK cells using EasySep CD56+ cell separation reagent (StemCell Technologies, Vancouver, BC, Canada) prior to mixing with target cells. Assay plates were incubated for 6 hours at 37°C, and supernatants were harvested and analyzed for 51 Cr content.
Statistics
The significance of differences between survival rates for experimental and control groups was determined using the log-rank test. Nonparametric Mann-Whitney U tests were used to analyze the significance of all other experimental data, except Figure 1B. Figure 1B was analyzed using a paired t test. The α (P) value was set at .05, making all values of P < .05 statistically significant. All results are presented as mean ± standard deviation (SD).
Results
CpG ODNs reduce systemic ALL burden
We have previously shown that the primary B-cell precursor ALL sample, A199, responds to CpG ODN stimulation with increased CD40 expression and cytokine production.24 To investigate the influence of CpG ODNs on ALL in vivo, we gave A199 leukemia-bearing NOD/SCID mice injections with 100 μg or 300 μg active CpG ODNs or PBS and monitored for changes in peripheral leukemia burden. For mice with an initial leukemia burden of 20% to 30%, there was a detectable decline in the percentage of leukemia cells in the peripheral blood of the mice receiving 300 μg CpG ODNs within 48 hours (Figure 1A). The reduction in peripheral leukemia burden was most pronounced 4 days after injection, with a gradual increase observed at later time points. A smaller reduction in peripheral leukemia burden was detected in mice receiving 100 μg CpG ODNs.
To determine if the reduction in the percentage of leukemia cells in peripheral blood was indicative of a systemic decline or simply the result of altered leukemia trafficking, we examined the leukemia content of organs from CpG ODN-, control ODN-, and PBS-treated mice. Mice with similar peripheral blood leukemia burdens (∼20%) were grouped in these experiments to control for the effect of leukemia burden on outcome. CpG ODN administration significantly reduced the percentage of leukemia cells in peripheral blood (P < .001), spleen (P < .004), and liver (P < .004), but not bone marrow (P = .254) compared to PBS-treated mice (Figure 1B). Because a reduced percentage of leukemia cells could be the result of an increase in number of murine cells, we compared the total number of cells, human and mouse, in the spleens of the differently treated mice. Spleens from CpG ODN-treated mice contained fewer cells (26 ± 3 million) compared to control mice (PBS = 85 ± 13 million; control ODN = 81 ± 12 million), indicating that murine cell expansion does not account for the reduced leukemia percentage. In addition to their reduced number, the leukemia cell populations present in the spleens of CpG ODN-treated mice contained a significantly higher percentage of annexin V–binding ALL cells (P = .008 for PBS versus CpG; P = .016 for CpG versus control ODN), indicating significantly more leukemia cell death in these mice (Figure 1C). In contrast, we did not observe the presence of annexin V–binding leukemia cells in the bone marrows of CpG ODN-treated mice (data not shown). To determine if CpG ODNs were acting directly on ALL cells in vivo, we measured CD40 expression on leukemia cells present in the organs of the differently treated mice. A significant increase in CD40 expression by ALL cells was observed in peripheral blood, spleen, liver, and bone marrow of mice receiving active CpG ODNs (P = .028 for each organ; Figure 1D). The smaller increase in CD40+ ALL cells in the organs of control ODN-treated mice is consistent with our previous report of reduced but detectable activity of ODN 2137 in ALL cells.24
CpG ODNs inhibit ALL progression in mice
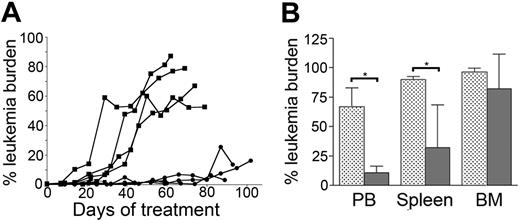
To investigate if the reduction in leukemia burden observed following CpG ODN administration was sufficient to mediate continued control of ALL in vivo, we examined the influence of CpG ODN treatment on ALL progression in mice. A199-bearing mice were first treated with CpG ODNs or PBS at an early detectable peripheral blood leukemia burden (< 0.5%), and subsequently received CpG ODN or PBS injections every 7 days. Peripheral leukemia burden was monitored 3 days after each injection. Control of leukemia was maintained in CpG ODN-treated mice for several months (Figure 2A). All PBS mice and 2 CpG ODN mice died of disease, whereas 2 CpG ODN-treated mice were killed prior to any overt signs of morbidity. The leukemia burden in organs of all mice was measured at time of death and the treatment groups compared (Figure 2B). Significantly lower leukemia burden was detected in peripheral blood and spleen (P = .028), but not bone marrow, of CpG ODN-treated mice compared to PBS mice.
CpG ODN treatment improves survival of mice bearing established ALL. (A) NOD/SCID mice bearing A199 primary ALL cells were injected weekly with CpG ODN (•) or PBS (▪), starting 1 day after human leukemia cells were first detected in peripheral blood (day 0). Peripheral leukemia burden was then measured each week until the animals were killed. (B) The leukemia burden in organs of CpG ODN- (dark gray bars) or PBS-treated (light gray bars) mice (from panel A) was measured at death (4 mice/group). *P < .05.
CpG ODN treatment improves survival of mice bearing established ALL. (A) NOD/SCID mice bearing A199 primary ALL cells were injected weekly with CpG ODN (•) or PBS (▪), starting 1 day after human leukemia cells were first detected in peripheral blood (day 0). Peripheral leukemia burden was then measured each week until the animals were killed. (B) The leukemia burden in organs of CpG ODN- (dark gray bars) or PBS-treated (light gray bars) mice (from panel A) was measured at death (4 mice/group). *P < .05.
CpG ODN-induced ALL death requires a host response
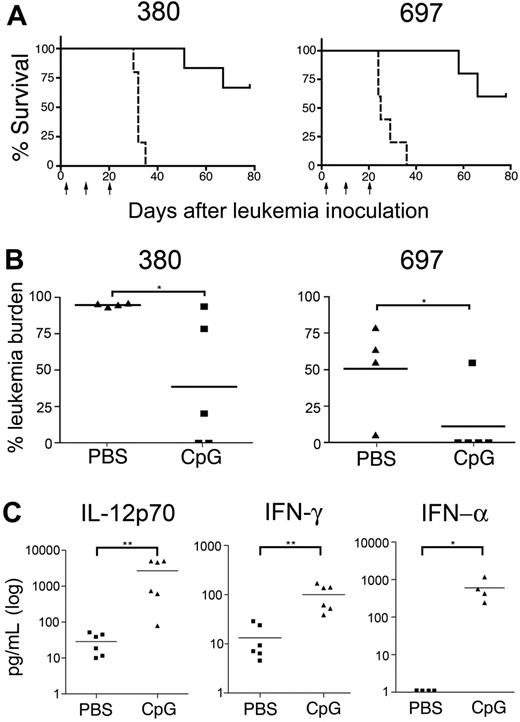
The increase in CD40 expression by A199 cells indicated that ALL cells respond to CpG ODNs in vivo (Figure 1D). To determine if direct stimulation of leukemia cells was required for the observed reduction in leukemia burden, we compared the influence of CpG ODNs on the in vivo growth of 2 B-cell precursor ALL cell lines. We have previously shown that the 380 cell line is directly responsive to CpG ODN stimulation in vitro, whereas the 697 cell is unresponsive.24 Mice were injected with 15 × 106 380 or 697 cells and were then given 3 injections of 300 μg CpG ODN or PBS at days 2, 10, and 20 after ALL injection and monitored for morbidity and survival. Significant and comparable improvements in survival were obtained by CpG ODN treatment of mice containing 380 or 697 cells (P < .002 and P = .002 respectively; Figure 3A). In these experiments, where CpG ODN was administered at an early stage of leukemia development, a significant reduction of leukemia burden in the bone marrow of both 380- and 697-bearing mice was achieved (P = .032; Figure 3B), with 4 of 5 mice bearing 697 cells and 2 of 5 mice bearing 380 cells containing less than 0.05% leukemia burden in bone marrow at time of death. The control of ALL progression achieved with mice bearing 697 cells indicates that a CpG ODN response by leukemia cells is not necessary to induce leukemia cell death. This implies that the response of the host is central to the in vivo control of ALL progression. Consistent with this hypothesis, we observed significantly increased amounts of mouse IL-12p70, IFN-α, and IFN-γ in the serum of leukemia-free mice treated with CpG ODN compared to PBS mice (Figure 3C).
The effect of CpG ODNs does not require direct ALL stimulation. Mice bearing 380 (directly responsive to CpG ODNs) or 697 (unresponsive to CpG ODNs) ALL cell lines were injected with CpG ODNs or PBS 2, 10, and 20 days after inoculation with leukemia cells (indicated by arrows) and monitored for morbidity and survival. (A) Survival curves of cell line–bearing mice. PBS-treated mice are indicated with a dashed line and CpG ODN-treated mice are indicated with a solid line. (P < .002 for 380 and P = .002 for 697; n = 5 mice/group). (B) Leukemia burden in the bone marrow of mice from panel A, measured at time of death. (C) Mouse cytokines IL-12p70 and IFN-γ (n = 6), and IFN-α (n = 4) present in the serum of PBS- and CpG-treated leukemia-free NOD/SCID mice. Bars indicate mean values for each group. **P < .005, *P < .05.
The effect of CpG ODNs does not require direct ALL stimulation. Mice bearing 380 (directly responsive to CpG ODNs) or 697 (unresponsive to CpG ODNs) ALL cell lines were injected with CpG ODNs or PBS 2, 10, and 20 days after inoculation with leukemia cells (indicated by arrows) and monitored for morbidity and survival. (A) Survival curves of cell line–bearing mice. PBS-treated mice are indicated with a dashed line and CpG ODN-treated mice are indicated with a solid line. (P < .002 for 380 and P = .002 for 697; n = 5 mice/group). (B) Leukemia burden in the bone marrow of mice from panel A, measured at time of death. (C) Mouse cytokines IL-12p70 and IFN-γ (n = 6), and IFN-α (n = 4) present in the serum of PBS- and CpG-treated leukemia-free NOD/SCID mice. Bars indicate mean values for each group. **P < .005, *P < .05.
NK cells mediate significant CpG ODN-induced killing of ALL
Although the innate immune system of NOD/SCID mice is deficient, it is not absent. To further investigate the mechanism responsible for CpG ODN-induced killing of ALL blasts, we depleted NK cells, using the asialo-GM1 antibody, prior to administration of CpG ODNs (Figure 4A). This treatment reduced the percentage of spleen cells expressing the pan-NK marker CD49b from more than 15% to less than 2% (data not shown). The prior depletion of NK cells significantly reduced the effectiveness of CpG ODN treatment at prolonging the survival of mice bearing the 697 cell line (aGM1+CpG versus CpG, P < .005). This reduced effectiveness was not complete, however, because mice receiving both asialo-GM1 and CpG ODNs demonstrated a significant enhancement in survival compared to control mice (aGM1+CpG versus PBS or aGM1, P < .005). Consistent with this finding, IL-12p70 and IFN-γ levels after CpG ODN stimulation were not significantly reduced in NK cell–depleted mice (data not shown).
CpG ODNs stimulate killing of ALL by murine and human NK cells. (A) In vivo depletion of NK cells by asialo-GM1 antibody prior to CpG ODN stimulation significantly reduces antileukemia activity. Mice were injected with 697 cells and aGM1 antibody (or PBS) on day −2 and with aGM1 antibody (or PBS) every fourth day for 24 days. CpG ODN or PBS treatment was given on days 0, 12, and 20. P < .005 for CpG versus aGM1+CpG, and for aGM1+CpG versus PBS or aGM1. There were 5 mice per group. (B) Depletion of NK cells (NK dep) from human PBMCs prior to addition of CpG ODN abrogated the increase in ALL cell killing achieved using PBMCs from 3 healthy donors. Labeled 697 cells were incubated with unmanipulated PBMCs or NK-depleted PBMCs (NK dep) in the presence or absence of CpG ODN for 6 hours. The killing achieved at an effector-target ratio of 100:1 is shown. *P < .05. Results presented as mean ± SD.
CpG ODNs stimulate killing of ALL by murine and human NK cells. (A) In vivo depletion of NK cells by asialo-GM1 antibody prior to CpG ODN stimulation significantly reduces antileukemia activity. Mice were injected with 697 cells and aGM1 antibody (or PBS) on day −2 and with aGM1 antibody (or PBS) every fourth day for 24 days. CpG ODN or PBS treatment was given on days 0, 12, and 20. P < .005 for CpG versus aGM1+CpG, and for aGM1+CpG versus PBS or aGM1. There were 5 mice per group. (B) Depletion of NK cells (NK dep) from human PBMCs prior to addition of CpG ODN abrogated the increase in ALL cell killing achieved using PBMCs from 3 healthy donors. Labeled 697 cells were incubated with unmanipulated PBMCs or NK-depleted PBMCs (NK dep) in the presence or absence of CpG ODN for 6 hours. The killing achieved at an effector-target ratio of 100:1 is shown. *P < .05. Results presented as mean ± SD.
The demonstration of significant antileukemia activity following CpG ODN treatment of mice with established disease prompted us to investigate whether such activity could be achieved with human immune effector cells. To address this question, we determined the ability of CpG ODN-stimulated human allogeneic PBMCs to kill ALL cells. Using fresh PBMCs obtained from 3 healthy donors, we detected significantly increased killing of 697 ALL cells in vitro when incubated in the presence of CpG ODN (P = .005; Figure 4B). Depletion of CD56+ NK cells from responder PBMCs, prior to the incubation with 697 cells, completely abolished the CpG ODN-induced increase in ALL cell killing (P = .016).
Discussion
The results of this study demonstrate that the in vivo administration of CpG ODN induces significant immune activity against primary human ALL blasts. The systemic death of leukemia cells was independent of their ability to respond to CpG ODN stimulation, arguing against a direct cytotoxic effect of the CpG ODNs. Consistent with this finding, we have not observed any significant death of ALL cells following CpG ODN stimulation in vitro24 (and data not shown). The control of leukemia growth and improved survival achieved by repeated administration of CpG ODNs into mice with established disease demonstrate that CpG ODNs stimulate effective anti-ALL activity in vivo and suggest that these agents may have considerable therapeutic potential for the treatment of ALL. Support for further testing of this application is provided by the demonstration that the CpG ODN effect was not limited to killing ALL xenografts but was also observed in a human allogeneic setting.
It is generally believed that immune therapy for malignant disease will be most effective when applied to the eradication of residual disease, after primary therapy has reduced the tumor burden. In the case of leukemia, the ineffectiveness of immune therapy to eradicate leukemic blasts from packed bone marrow has been reported.25 Our results are consistent with this hypothesis, with a significant reduction in the leukemia content in bone marrow achieved only when CpG ODNs were administered early in disease development in the mice. In both pediatric and adult ALL populations, complete remissions are achieved in the majority of patients, although the duration of these remissions varies between the 2 groups. The success of this initial therapy, therefore, would provide an opportunity to administer CpG ODNs in a minimal residual disease setting that may contribute to the maintenance of remission.
NOD/SCID mice lack an adaptive immune system and have functional deficiencies in the innate immune system.26 The comparable control of disease progression achieved for primary ALL cells and the CpG ODN responsive and nonresponsive cell lines, however, clearly implicates the host response in the death of leukemia cells. The role of the residual innate immune compartment in CpG ODN-induced ALL killing is demonstrated directly by the reduced survival of mice treated with asialo-GM1 prior to CpG ODN injection compared to mice treated with only CpG ODNs. Because NK cells are the predominant cell type depleted by the asialo-GM1 antibody, this study indicates that these cells are responsible for a significant proportion of the leukemia cell death induced by CpG ODNs. A contribution by other depleted cell types, such as CD11c+ B220+ DX5+ IFN-producing killer dendritic cells,27,28 however, cannot be ruled out by this study. Although effective at reducing CpG ODN-induced survival, asialo-GM1 treatment did not eliminate all antileukemia activity in treated mice, indicating that other mechanisms may be involved. CD11c+ dendritic cells have been identified as the primary source of IFN-γ following CpG ODN stimulation of NK-depleted NOD/SCID mice, suggesting that these cells may also contribute to the overall antileukemic activity of CpG ODNs.29 Recent findings also suggest a potential role for macrophage in CpG ODN-induced antitumor activity.30
The demonstration of a prominent role for NK cells in the in vivo control of ALL in mice is consistent with our observation that NK cells are required for the killing of ALL cells by CpG ODN-stimulated human PBMCs. NK cells have been implicated in the control of ALL in patients, both during first remission and after HSCT.16,31,32 However, ALL cells are relatively insensitive to NK cell–mediated cytotoxicity,33,34 as evidenced in this study by the poor killing of 697 cells by nonstimulated human PBMCs. The ability of CpG ODN to overcome the resistance of ALL cells to NK cytotoxicity may therefore present a strategy to exert control over ALL progression at distinct stages of disease. NK-cell cytotoxicity can be triggered through a variety of activating receptors. We have examined human NK cells for CpG ODN-induced changes in expression of the immune tyrosine-based activating motif (ITAM) bearing receptors NKp30, NKp44, and NKp46. To date, however, we have not identified any expression changes consistent with the observed increase in leukemia cell killing (data not shown). In addition, no up-regulation of NKG2D ligand expression was detected on ALL cells stimulated with CpG ODN in the presence of PBMCs (data not shown).
Results achieved in different experimental models implicate IL-12, IFN-α, and TNF-α in the activation of NK cells leading to antitumor activity after CpG ODN treatment.35–42 The detection of increased amounts of murine IL-12 and IFN-γ production after CpG ODN administration, coupled with loss of ALL killing after depletion of NK cells, suggests that the in vivo activity observed in these studies involves a similar pathway of immune activation. The in vitro augmentation of leukemia cell killing by human PBMCs is most likely the result of NK activation by IFN-α. In the absence of CD40 costimulation, CpG ODNs do not induce IL-12 production by dendritic cells but do stimulate significant IFN-α and TNF-α release. These cytokines have been demonstrated to be sufficient to induce increased human NK cell–mediated cytotoxicity.41,42 A199 cells produce substantial amounts of IL-8 in response to CpG ODN stimulation,24 which could feasibly stimulate NK activity.43 The lack of detectable cytokine production by either cell line following CpG treatment (data not shown) indicates that such leukemia-derived factors are not required for anti-ALL activity. These results are consistent with a previous report in which antileukemia immune activity was elicited in a mouse model of Philadelphia chromosome–positive ALL by genetic modification of leukemic blasts to produce IL-12.44 Control of murine acute myelogenous leukemia has been achieved when CpG ODNs were administered prior to transfer of the leukemia cells, a situation in which the antileukemia activity must also be mediated by the host immune system.45 These observations, coupled with our report of significant immune activity generated against ALL, suggest that CpG ODNs may be applicable to different types of leukemia.
Although the use of the NOD/SCID model limited our study to the detection of innate immune responses, the stimulation of ALL cells by CpG ODNs in vivo, as detected by the increase in CD40 expression, indicates that the use of these agents may also influence anti-ALL adaptive immune responses. ALL blasts are inefficient stimulators of T cells.46,47 We have previously reported that CpG ODN stimulation of primary ALL blasts in vitro skews allogeneic T cells toward a Th1 response.24 The ability to stimulate ALL blasts directly in vivo, together with the enhanced production of IL-12 by host immune cells, suggests that an even greater level of Th1 immune activity will be generated against ALL in immunocompetent individuals. The ability of Th1 responses generated by CpG ODN treatment to eradicate established tumors has been demonstrated in other model systems.48–51
Current treatment protocols for relapsed ALL are unsatisfactory. Treatment of relapsed ALL with chemotherapy alone achieves event-free survival rates of 10% to 40%. The use of HSCT has been shown to achieve improved survival rates compared to chemotherapy,52 but the efficacy of this approach is hindered by the low sensitivity of ALL, compared with other types of leukemia, to immune-mediated cytotoxicity.52,53 Strategies to enhance anti-ALL immune activity in patients have included the use of IL-2, but this approach is associated with significant toxicity and achieves only limited remission rates in patients.54,55 The results presented here indicate that significant immune-mediated control of ALL can be achieved in vivo by injection of CpG ODNs, without the need for exogenous cytokines or the adoptive transfer of previously activated lymphocytes. Although detrimental effects of repeated high-dose CpG ODN stimulation in vivo have been reported,56 early results from clinical trials indicate that CpG ODN treatment protocols using 2 to 3 injections are well tolerated.57,58 Our study suggests that such protocols may provide an alternative strategy to address deficiencies in both the adaptive and innate immune responses against ALL.
Authorship
Contribution: H.F. designed and performed research and analyzed data; J.D.T. designed and performed research and analyzed data; D.T.T. performed research; J.D.F. performed research; S.A.G. analyzed data; K.R.S. analyzed data; and G.S.D.R. designed and performed research and analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gregor Reid, Arc 904G, Children's Hospital of Philadelphia, Joseph Stokes Jr Research Institute, Abramson Research Center, 34th St and Civic Center Blvd, Philadelphia, PA 19104; e-mail: reidg@email.chop.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Dario Campana for providing the ALL cell lines and Dr Jordan Orange for critically reading the manuscript and providing reagents. The excellent technical assistance of Dr Patrice Eydoux, Wendy Duey, Angela Tsang, Junior Hall, and the staff of the Child and Family Research Institute Animal Care Facility (CFRI ACF) is gratefully acknowledged.
This work was supported in part by the BC Children's Hospital Foundation (GSDR) and the American Cancer Society (grant RSG0507101) (S.A.G.).