Abstract
Leukocytes of persons coinfected with HTLV-2 and HIV-1 secrete chemokines that prevent CCR5-dependent (R5) HIV-1 infection of CD4+ T cells and macrophages, with HTLV-2–induced MIP-1α as dominant HIV-1 inhibitory molecule. Two nonallelic genes code for CCL3 and CCL3L1 isoforms of MIP-1α, and the population-specific copy number of CCL3L1 exerts a profound effect on HIV-1 susceptibility and disease progression. Here, we demonstrate that CCL3L1 is secreted spontaneously by leukocytes of HTLV-2–infected persons and superinduced when cells of HTLV-2/HIV-1 multiply exposed-uninfected seronegative (MEU) persons were stimulated with HIV-1 Env peptides. The CCL3L1 median copy number in MEU, HTLV-2/HIV-1–coinfected long-term nonprogressors (LTNPs) and HIV-1–monoinfected LTNPs were 1, 2, and 3, respectively. An increased CCL3L1/CCL3 mRNA ratio versus PHA-activated healthy leukocytes was observed in both HIV-1–monoinfected LTNPs and in HTLV-2/HIV-1MEU subjects. An additional potential correlate of HTLV-2 infection was a rapid and persistent leukocyte secretion of GM-CSF and IFN-γ, 2 cytokines endowed with CCR5 down-regulation capacity. This study confirms a crucial protective role of CCL3L1 from both HIV infection and disease progression, highlighting a previously not described functional up-regulation of this chemokine variant in both HIV-positive and -negative persons infected with HTLV-2.
Introduction
Epidemiologic evidence confirms the existence of natural factors that protect a subset of persons from HIV infection, who remain uninfected despite multiple exposures to the virus. In addition, some infected persons experience a benign, or even arrested, disease evolution. Such persons are here referred to as “multiply exposed-uninfected seronegative (MEU)” and “long-term nonprogressors (LTNPs).” One of the clearly understood determinants of disease progression involves a genetic mutation in chemokine receptor 5 (CCR5), know as CCR5Δ32. The mutation is a deletion that compromises the structure and abolishes the availability of this HIV-1 entry coreceptor in the homozygotic configuration, which is typically observed in some MEU persons. This mutation may also reduce cell-surface CCR5 expression in heterozygotes,1 as described in some LTNPs.2 The CC chemokine ligand 3-like 1 (CCL3L1), also known as macrophage-inflammatory protein 1α (MIP-1αP) or LD78β, is a natural chemokine that is highly potent in inhibiting entry of CCR5-dependent (R5) HIV-1.3 CCL3L1 is one of the 2 isoforms of MIP-1α, the other being CCL3, which is also called LD78α. A hot spot for segmental duplications of the CCL3L1 gene is found on human chromosome 17q, encompassing other CC chemokine genes.4 The population-specific gene dose of the CCL3L1 chemokine exerts a profound effect on HIV infection susceptibility when compared with other genetic elements that affect CCR5 genotype.5 In addition to genetic factors, coinfection by other viruses may also influence HIV replication.6–10 In particular, HTLV-2/HIV-1 coinfection is a condition frequently found in LTNPs and has been associated with a delayed progression of HIV-1 infection to AIDS.11 We have previously described that up-regulation of CCR5-binding chemokine expression occurs in ex vivo cultivated peripheral blood mononuclear cells (PBMCs) of HTLV-2/HIV-1–coinfected persons as compared with persons infected with only HIV-1.8 Specifically, MIP-1α secretion from CD8+ T lymphocytes was responsible for anti–HIV-1 activity in cell cultures derived from dually infected subjects. Spontaneous production of CCR5-binding chemokines was confirmed by Lewis et al,12 who linked this phenomenon to the transactivation of CCL4 and CCL5 gene promoters by HTLV-2 regulatory proteins.
Patients, materials, and methods
Patients
PBMCs were obtained from 23 persons (16 men and 7 women; aged 36-48 years) belonging to a cohort of Italian intravenous drug users (IDUs) who had been followed since 1986. All selected subjects were homozygous for wild-type CCR2 and CXCL12 polymorphism; 22 were homozygous for the wild-type CCR5 allele; 1 was CCR5Δ32 heterozygous.13 At enrollment, they all had the same seroprevalence for HSV, CMV, and hepatitis B and C viruses. MEU subjects (n = 8) had a history of penetrative sexual intercourse without condom for at least 4 years with HIV seropositive partners and exposure to a potentially high viral inoculum. Seropositivity of all MEU subjects for HTLV-2 had been documented since 1987. MEU persons were checked at regular 6-month intervals for their HIV status by serologic and/or virologic testing, including polymerase chain reaction (PCR) amplification of proviral DNA, HIV-1 isolation by PBMC cocultivation, and plasma viremia (HIV-1 RNA) by reverse transcription (RT)–PCR. The HTLV-2 subtype, identified by sequencing LTR region of the viral isolates,14 was 2b in all cases. The HTLV-2 proviral load in PBMCs, measured using quantitative real-time PCR technique, was normalized versus the albumin gene and was found to be constant since 1996, ranging from 1668 to 2745 copies/105 cells from person to person.11 MEU persons maintained CD4+ T-cell counts of at least 800 cells/μL and a normal CD4+/CD8+ T-cell ratio after enrollment. Seven HTLV-2/HIV-1–coinfected and 8 HIV-1–monoinfected subjects selected from the same IDU cohort presented the typical features of LTNPs (CD4+ T-cell counts > 700/μL stable for approximately 12 years and HIV-1 viremia ≤ 500 copies/mL in the absence of antiretroviral therapy). The number of HTLV-2 proviral DNA copies in coinfected persons was similar to that found in HTLV-2–monoinfected subjects (mean, 1200 copies/105 PBMCs), whereas the HIV-1 DNA load in monoinfected and coinfected patients was lower (mean, 25 copies/105 PBMCs). This study does not interfere with daily clinical care and treatment of the participants but only collects information by patient interview as a part of standard care and from patient records. Prior to beginning the study and the related activities we received the Local Ethics Committee of Parma's approval and the informed consent from all participants was obtained according to local and national regulations as well as the Declaration of Helsinki. The identity of each HTLV-2 MEU person, HIV-1 and HIV-1/HTLV-2–coinfected LTNP, and control subject is protected by providing arbitrary identification codes.
In vitro HIV-1 infection
Purified PBMCs were cultivated in RPMI 1640 medium (Gibco BRL, Milan, Italy), supplemented with 10% fetal bovine serum (FBS; Hyclone Europe, Cramlington, United Kingdom). PBMCs were infected with 500 TCID50/106 cells of CCR5-dependent (R5) HIV-1BAL or HIV-1ADA strains or with the CXCR4-dependent (X4) HIV-1IIIB strain. Cells were incubated for 2 hours at 37°C, washed, and cultivated in complete medium. Culture medium was replaced with fresh medium in either the presence or absence of antichemokine neutralizing monoclonal antibodies (NmAbs, 2 μg/mL). Aliquots of cell cultures and of their supernatants were harvested after 6 days of infection and analyzed for HIV proviral load by SYBR Green Real-Time PCR technique15 and for p24 Ag production by enzyme-immunoassays (EIAs; Abbott Laboratories, North Chicago, IL), respectively.
Evaluation of CCL3L1 gene copy number by real-time quantitative PCR assay
Genotyping was determined following the method of Townson et al.16 Quantitative PCR was carried out using a Chromo4 Continuous Fluorescence Detector (MJ Research, Waltham, MA). The CCL3L1 gene was amplified by using the following primer sequences: forward, 5′-tctccacagcttcctaaccaaga; reverse, 5′-ctggacccactcctcactgg; and probe, 5′-FAM-aggccggcaggtctgtgctga-BHQ-1. For the HBB gene, the primer sequences were forward, 5′-gctggcccatcactttgg; reverse, 5′-ccagccaccactttctgatagg; and probe, 5′-Texas-red-aagaattcaccccaccagtgcaggc-BHQ-3. Five serial 1:2 dilutions (25-1.56) of genomic DNA from human A431 cells, known to contain 2 copies of CCL3L1 per diploid genome (pdg) by Southern blot densitometry,16 were used on each plate to generate standard curves of CT (threshold cycle) value against the log [DNA] for HBB (also present at 2 copies pdg) and CCL3L1 genes. Each sample was tested in duplicate and retested at least once on a separate plate. The determined CT values were converted into template quantity using the standard curves. Copy number pdg is the ratio of the template quantity for CCL3L1 to that for HBB, multiplied by 2. The amount of DNA sample loaded in each test was 5 ng.
Analysis of mRNA levels by real-time PCR
Total PBMC RNA was purified using RNaqueous-4PCR kit (Ambion, Austin, TX). Reverse transcription was performed by MessageSensor RT kit (Ambion). The following primers and labeled probes were used for CCL3L1 cDNA: forward, 5′-tctctgcaaccaggtcctctc; reverse, 5′-ggaggtgtagctgaagcagca; and probe, 5′-FAM-ccacttgctgctgacacgccg-BHQ1. For CCL3 cDNA, primers were forward, 5′-tggctctctgcaaccagttct; reverse, 5′-gccgggaggtgtagctgaa; and probe, 5′-FAM-tcacttgctgctgacacgccga-BHQ1. Primers for HBB cDNA were forward, 5′-gctggcccatcactttgg; reverse primer, 5′-ccagccaccactttctgatagg; and probe, 5′-Texas-red-aagaattcaccccaccagtgcaggc-BHQ-3. The PCR conditions were 95°C for 10 minutes, followed by 45 cycles of 95°C for 15 seconds, 56°C for 30 seconds, and 72°C for 30 seconds. The runs were performed on the Chromo4 Continuous Fluorescence Detector (MJ Research). Results were analyzed by using sequence detector software, and relative fold differences were determined applying the comparative CT method (ΔΔCT). Cultures from pooled purified PBMCs of 25 healthy HIV-negative donors were either unstimulated or stimulated with PHA for 3 days and cultivated with medium enriched with IL-2 (40 U/mL) for 2 days. The RNA extracted from culture PBMCs was used as calibrator. HBB was tested as housekeeping gene.
Production, purification, and isolation of natural MIP-1α isoforms
Unstimulated PBMCs from patients were cultivated for 48 hours at the concentration of 106 cells/mL in RPMI 1640 medium, supplemented with 2% FBS. Natural MIP-1α isoforms were isolated through a multistep procedure that included adsorption to controlled pore glass, heparin-Sepharose chromatography, and cation exchange chromatography (MonoS FPLC).17 Partially purified proteins obtained from adsorption to controlled pore glass were subjected to immunoaffinity chromatography using anti–human MIP-1α mAb (R&D Systems, Minneapolis, MN). Fractions were tested for MIP-1α immunoreactivity by a specific enzyme-linked immunosorbent assay (ELISA) kit (Endogen, Boston, MA). The molecular size of purified proteins was determined by high-performance liquid chromatography (HPLC) electrospray ionization (ESI) (LC triple quadrupole; Micromass, Hertfordshire, United Kingdom) or by matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) (Reflex III; Bruker Daltonics, Leipzig, Germany) mass spectrometry (MS). The isolation procedure and MS analysis were standardized using known concentrations of a mixture of recombinant human (r) MIP-1α isoforms (PeproTech, Rocky Hill, NJ).
CCR5 internalization assay
To study the binding affinity of MIP-1α isoforms to CCR5, the internalization of this receptor was evaluated in the presence of chemokine. The isoforms were precipitated from immunoreactive RP-HPLC fractions by 20% trichloroacetic acid, dissolved in PBS, and incubated for 1 hour at 37°C at 0.09 nM with 2.5 × 104 Hos-CCR5 transfected cells. The surface detection of the receptor was performed by flow cytometry analysis on fluorescence-activated cell sorting (FACS)Calibur (Becton Dickinson, San Jose, CA). As control, the surface expression of the receptor on Hos-CCR5 cells was analyzed in the absence of the chemokine.
Cytokine and chemokine quantification
The levels of secreted IFN-γ and GM-CSF present in the PBMC culture supernatants were assayed by commercially available ELISA kits (Endogen). Chemokine (CCL5, MIP-1α, CCL4) concentrations in the culture supernatants were measured by ELISA according to the manufacturer's instructions (R&D Systems).
Statistical analysis
Kruskal-Wallis (rank sums) test and Wilcoxon test were used for nonparametric analysis of the correlation between CCL3L1 gene copy number and patient groups.
Results
MIP-1α secretion renders HTLV-2/HIV-1MEU–derived PBMCs resistant to HIV infection
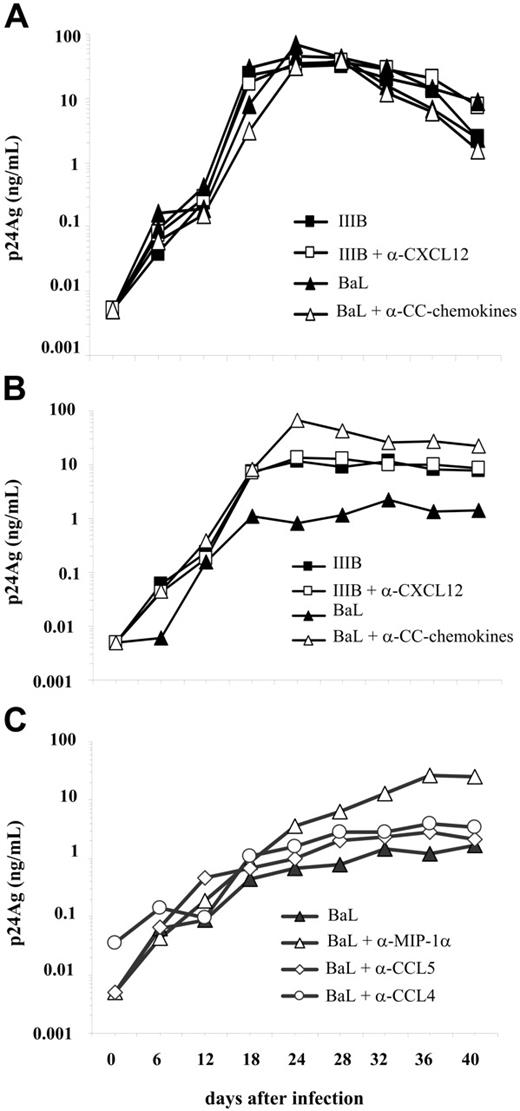
Both R5 HIV-1BaL and X4 HIV-1IIIB viruses replicated with comparable efficiency in IL-2–stimulated PBMCs of healthy seronegative control subjects either in the presence or absence of NmAb anti-CXCL12 or against CCR5-binding chemokine (Figure 1A; see also Document S1, “Resistance to HIV-1 replication in primary PBMC cultures from HTLV-2/HIV-1MEU persons” of Document S2, and Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Anti-CXCL12 NmAb did not affect the levels of X4 HIV replication in unstimulated PBMC cultures from HTLV-2/HIV-1MEU (Figure 1B). In contrast, R5 HIV-1 replication was reduced by a factor of 100 in HTLV-2/HIV-1MEU PBMCs, whereas anti–CCR5-binding chemokine NmAbs up-regulated HIV replication to levels comparable to those observed in PBMC cultures of healthy seronegative control subjects (Figure 1B). Among other CCR5-binding chemokines, the dominant role of the MIP-1α in PBMC cultures established from HTLV-2/HIV-1–coinfected persons was confirmed in this study and was correlated (Figure 1C) to the induction of CCR5 internalization (Table S1). In addition, MIP-1α secretion from PBMCs of HTLV-2/HIV-1MEU subjects was further induced by incubation with an HIV-1 Env-derived peptide (see “HIV-1 envelope-restricted MIP-1α secretion in HTLV-2/HIV-1MEU” in Document S1 and Table S2). These findings underscore a link between HTLV-2–induced MIP-1α secretion, down-regulation of CCR5, and inefficient R5 HIV-1 replication in PBMC cultures maintained in IL-2–enriched medium.
R5 HIV-1 replication in unstimulated PBMCs from HTLV-2/HIV-1MEU subjects. Dominant protective role of MIP-1α. (A) Kinetics of R5 BaL and X4 IIIB virus production in IL-2–stimulated PBMCs from a pool of 25 uninfected donors. Supernatants from PBMC cultures were harvested every 4 to 6 days and tested for HIV-1 p24 Gag Ag content. Results are representative of 5 independent experiments performed at 6-month intervals. (B) Kinetics of R5 and X4 virus production by primary cultures established from 3 HTLV-2/HIV-1MEU persons. The neutralizing activity of antichemokine NmAbs (2 μg/mL each), added either individually or as cocktails, was determined. (C) Kinetics of virus production in PBMCs infected in the presence or absence of anti-CC chemokine NmAbs added individually (2 μg/mL each). Similar results were obtained with cells infected after 6 days of cultivation (see “HIV-1 envelope-restricted MIP-1α secretion in HTLV-2/HIV-1MEU” of Document S2 and Figure S1).
R5 HIV-1 replication in unstimulated PBMCs from HTLV-2/HIV-1MEU subjects. Dominant protective role of MIP-1α. (A) Kinetics of R5 BaL and X4 IIIB virus production in IL-2–stimulated PBMCs from a pool of 25 uninfected donors. Supernatants from PBMC cultures were harvested every 4 to 6 days and tested for HIV-1 p24 Gag Ag content. Results are representative of 5 independent experiments performed at 6-month intervals. (B) Kinetics of R5 and X4 virus production by primary cultures established from 3 HTLV-2/HIV-1MEU persons. The neutralizing activity of antichemokine NmAbs (2 μg/mL each), added either individually or as cocktails, was determined. (C) Kinetics of virus production in PBMCs infected in the presence or absence of anti-CC chemokine NmAbs added individually (2 μg/mL each). Similar results were obtained with cells infected after 6 days of cultivation (see “HIV-1 envelope-restricted MIP-1α secretion in HTLV-2/HIV-1MEU” of Document S2 and Figure S1).
CCL3L1 gene dose is not the absolute parameter that confers HIV/AIDS susceptibility
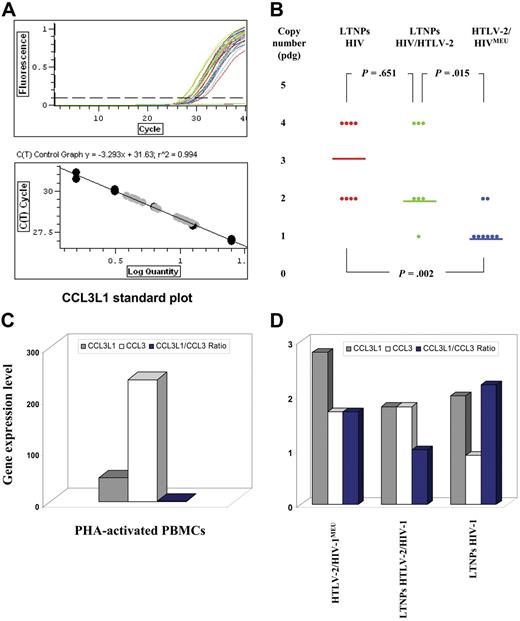
HTLV-2/HIV-1MEU subjects (n = 8), HIV-1–monoinfected LTNPs (n = 8) with more than 12 years of infection and CD4+ T-cell counts greater than 700 cells/μL, and HTLV-2/HIV-1–coinfected LTNPs with a comparable clinical profile (n = 7) were investigated in terms of CCL3L1 inhibition of HIV-1 infection and CCL3L1 gene copy number. All persons belonged to the same Italian cohort of IDUs and were matched on the basis of homogeneous criteria (see “Patients”). They were all homozygous for wild-type CCR2, CXCL12, and CCR5, except for one CCR5Δ32 heterozygote. Unexpectedly, a median CCL3L1 copy number of 1, corresponding to about half of that reported for the European population,5 was observed in HTLV-2/HIV-1MEU subjects (Figure 2B). In contrast, a median of 2 and 3 CCL3L1 gene copies were detected in HTLV-2/HIV-1–coinfected and HIV-1–monoinfected LTNPs, respectively (Figure 2B). Because a median CCL3L1 copy number lower than the population-specific value predicts a higher risk of HIV-1 infection, the MEU group should have been characterized by a higher susceptibility to HIV-1 infection. The nonparametric data distribution analysis of the differences between HTLV-2/HIV-1MEU subjects and both HIV-1–monoinfected (P = .002) and HTLV-2/HIV-1–coinfected LTNPs (P = .015) were significant.
Genotype and expression of human CCL3L1. (A) The top plot shows the standard curves with the slope and the square of the Pearson correlation coefficient (R2). The bottom plot shows the amplification curves obtained using 6 (1:2) serial dilutions in triplicate, ranging from 25 to 1.56 ng human genomic DNA extracted from the cell line A431 that contains 2 copies of CCL3L1 per diploid genome. The plots were obtained using Chromo4 Continuous Fluorescence Detector System. (B) Distribution of median CCL3L1 copy numbers in HIV-1 LTNPs, HTLV-2/HIV-1 LTNPs, and HTLV-2/HIV-1MEU subjects; P values indicate significance by the Wilcoxon test. (C) Differential CCL3L1 gene expression levels (expressed as mean of fold changes) in cellular extracts of PHA-activated PBMCs of control seronegative persons and of unstimulated PBMCs from infected persons.
Genotype and expression of human CCL3L1. (A) The top plot shows the standard curves with the slope and the square of the Pearson correlation coefficient (R2). The bottom plot shows the amplification curves obtained using 6 (1:2) serial dilutions in triplicate, ranging from 25 to 1.56 ng human genomic DNA extracted from the cell line A431 that contains 2 copies of CCL3L1 per diploid genome. The plots were obtained using Chromo4 Continuous Fluorescence Detector System. (B) Distribution of median CCL3L1 copy numbers in HIV-1 LTNPs, HTLV-2/HIV-1 LTNPs, and HTLV-2/HIV-1MEU subjects; P values indicate significance by the Wilcoxon test. (C) Differential CCL3L1 gene expression levels (expressed as mean of fold changes) in cellular extracts of PHA-activated PBMCs of control seronegative persons and of unstimulated PBMCs from infected persons.
A mechanistic link exists between CCL3L1 expression and HTLV-2 infection. PBMCs from LTNPs, but not from infected persons with progressive diseases, show an increased capacity of secreting CCR5-binding chemokines12 (C.C., unpublished data, September 7, 2005). Because the CCL3 gene exists as a single copy per haploid genome,3 the variable copy number of CCL3L1 observed in LTNPs might influence the chemokine mRNA and protein levels of expression.16 Therefore, all studied populations were tested to determine whether the CCL3L1 genotype was linked to different levels of CCL3L1/CCL3 mRNA expression ratio. Poly A+ RNA was purified from PBMCs, and the MIP-1α isoform mRNA levels were examined by real-time PCR. Each mRNA expression level was standardized using the RNA extracted from a pool of PBMCs obtained from 25 healthy persons, with a CCL3L1 mean gene copy number of 2, to which an arbitrary value of 1 was assigned. PHA-activated PBMCs showed a substantial increase in CCL3 mRNA (average of 237-fold) and a lower increase in CCL3L1 (average of 46-fold), with a CCL3L1/CCL3 mRNA ratio of 0.19 (Figure 2C). CCL3 mRNA expression was up-regulated in unstimulated PBMCs of HTLV-2–monoinfected subjects (1.7-fold) and HTLV-2/HIV-1–coinfected LTNPs (1.8-fold) but not in cells of HIV-1–monoinfected LTNPs (0.8-fold) (Figure 2D). CCL3L1 mRNA expression was up-regulated in ex vivo PBMCs from all groups (2.8-, 2-, 1.8-fold; Figure 2D), resulting in a CCL3L1/CCL3 mRNA ratio that was always greater than 1.
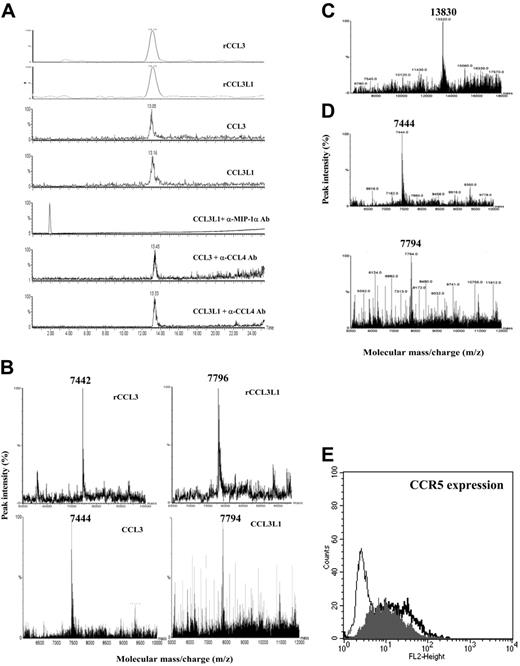
PBMCs from HTLV-2–infected persons secrete both intact and truncated CCL3/CCL3L1 isoforms with high affinity for human CCR5. Chemokine secretion in the culture supernatants of unstimulated PBMCs isolated from the different groups of persons peaked 48 hours later than the initiation of the cell culture (see “Effects of CC chemokines on CCR5-cell surface expression in PBMC cultures from HTLV-2/HIV-1MEU” of Document S2 and Figure S2). Of interest, the group of both HTLV-2–positive and –negative LTNPs, as well as the group of HTLV-2/HIV-1MEU subjects, showed a spontaneous secretion of CCR5-binding chemokines (MIP-1α, 5 ± 0.7 and 10 ± 1.2 ng/mL; CCL4, 5 ± 0.4 and 7 ± 0.6 ng/mL; CCL5, 3 ± 0.4 and 2 ± 1.1 ng/mL, respectively). To determine whether either CCL3 or CCL3L1 could account for the observed MIP-1α activity, the culture supernatant of PBMCs isolated from HTLV-2–positive persons was subjected to multistep chromatography. The relative mobility (Mr) of purified proteins was evaluated by RP-HPLC ESI-MS and confirmed by MALDI-TOF-MS analysis. Two coexisting protein peaks were detected using pH = 4 conditions for the MS chromatograms. The first peak, with an average Mr of 7444 ± 6 Da, corresponded to a mixture of truncated CCL3 and CCL3L1 isoforms. The second peak, with an average Mr of 7794 ± 6 Da, corresponded to the intact CCL3L1 isoform (Figure 3B; Table 1). Under physiologic conditions (pH = 7.2), MIP-1α was detected as a single predominant peak of 13 830 ± 6 Da in unstimulated PBMC cultures of HTLV-2–infected subjects (Figure 3C). These observations suggest that an aggregation of native MIP-1α might be generated by electrostatic interactions of charged aa between highly similar monomeric structures of CCL3 and CCL3L1 isoforms. The Mr aggregate did not correspond quantitatively to the total amount of Mr isoforms. On the basis of the relative ion abundance ratio, the content of intact CCL3L1 was greater (about 60%) than that of truncated CCL3 and CCL3L1 isoforms (Table 1). These findings suggest that the high production of an intact form of CCL3L1 from HTLV-2/HIV-1MEU and HTLV-2/HIV-1 LTNP groups is directly related to HTLV-2 infection, despite the genotype of the chemokine. The greater levels of intact versus cleaved CCL3L1 protein secreted from PBMCs of HTLV-2–positive persons support the hypothesis of an inefficient processing by the serine protease CD26/DPP IV3 (Document S2, section 4).
MS analysis of MIP-1α isoforms and their interaction with CCR5 coreceptor. (A) Extract ion LC-ESI-MS chromatograms of CCL3 and CCL3L1 from recombinant isoforms mixture and from supernatants of PBMCs of HTLV-2/HIV-1MEU subjects. Retention peaks of the isoforms were extracted from chromatogram by processing data through known ions representative of MIP-1α isoforms and compared with those of rCCL3and rCCL3L1. In addition, MIP-1α cation exchange-positive fractions from HTLV-2/HIV-1MEU persons were immunoprecipitated with anti–MIP-1α or anti-CCL4 polyclonal Ab before MS analysis. (B) Deconvolution spectra of CCL3 and CCL3L1 isoforms from recombinant isoform mixture and from HTLV-2/HIV-1MEU subjects. Retention MIP-1α isoform peaks were subjected to MS analysis. The multiple-charged spectra represent the relative ion abundance of the various isoform charge states according to mass-charge ratio (m/z). (C) MS analysis of MIP-1α isolated from immunoreactive fractions of PBMCs of HTLV-2–infected persons eluted using physiologic conditions. (D) MS analysis of MIP-1α secreted by unstimulated PBMCs from HTLV-2/HIV-1–coinfected LTNPs. (E) Flow cytometric analysis of CCR5 expression on CCL3/CCL3L1 binding. The thin black line represents the background-negative control; thick black line, Hos-CCR5 cells (MFI = 19.97); gray line, Hos-CCR5 cells incubated with purified MIP-1α forms (MFI = 13.14).
MS analysis of MIP-1α isoforms and their interaction with CCR5 coreceptor. (A) Extract ion LC-ESI-MS chromatograms of CCL3 and CCL3L1 from recombinant isoforms mixture and from supernatants of PBMCs of HTLV-2/HIV-1MEU subjects. Retention peaks of the isoforms were extracted from chromatogram by processing data through known ions representative of MIP-1α isoforms and compared with those of rCCL3and rCCL3L1. In addition, MIP-1α cation exchange-positive fractions from HTLV-2/HIV-1MEU persons were immunoprecipitated with anti–MIP-1α or anti-CCL4 polyclonal Ab before MS analysis. (B) Deconvolution spectra of CCL3 and CCL3L1 isoforms from recombinant isoform mixture and from HTLV-2/HIV-1MEU subjects. Retention MIP-1α isoform peaks were subjected to MS analysis. The multiple-charged spectra represent the relative ion abundance of the various isoform charge states according to mass-charge ratio (m/z). (C) MS analysis of MIP-1α isolated from immunoreactive fractions of PBMCs of HTLV-2–infected persons eluted using physiologic conditions. (D) MS analysis of MIP-1α secreted by unstimulated PBMCs from HTLV-2/HIV-1–coinfected LTNPs. (E) Flow cytometric analysis of CCR5 expression on CCL3/CCL3L1 binding. The thin black line represents the background-negative control; thick black line, Hos-CCR5 cells (MFI = 19.97); gray line, Hos-CCR5 cells incubated with purified MIP-1α forms (MFI = 13.14).
MIP-1α isoforms, either recombinant or purified from PBMCs of HTLV-2/HIV-1MEU persons, were tested for their capacity of inducing CCR5 down-regulation in Hos-CCR5–transfected cells. Purified CCL3/CCL3L1 aggregates, at the peak concentration of 0.09 nM, induced a 40% decrease in CCR5 expression that was comparable to that induced by 5 nM rCCL3L1 (Figure 3E). In contrast, no evidence of CCR5 down-regulation was observed by rCCL3 at concentrations up to 100 nM (E.P., unpublished observations, July 2004). These findings suggest that the enhanced anti-HIV potency of natural CCL3L1 secreted by PBMCs of HTLV-2–infected persons correlates to a significantly higher CCR5 binding affinity than that of CCL3, as previously indicated.18
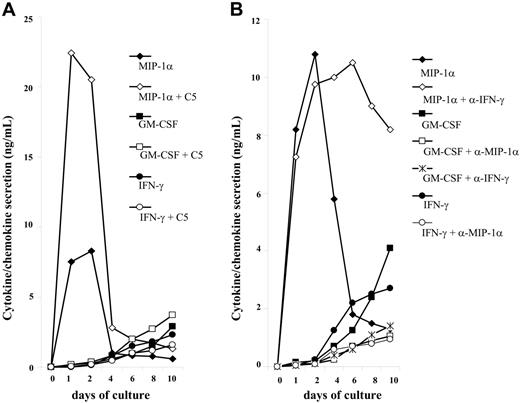
CCL3/CCL3L1 controls IFN-γ and GM-CSF secretion in PBMCs from HTLV-2/HIV-1MEU subjects. The HTLV-2 transactivating protein Tax is known to enhance transcription of various cytokines.19 In addition, several studies have demonstrated that HTLV-2–infected PBMCs undergo spontaneous proliferation in short-term cultures in association with the secretion of several cytokines, including GM-CSF and IFN-γ.20–22 Both anti-inflammatory cytokines and IFNs can suppress both transcriptional and posttranscriptional steps in the HIV life cycle.23 Of interest, putative regulatory sequences of CCL3 and CCL3L1 genes have been detected in the promoter regions of human GMCSF and IFNG,24 which code for 2 cytokines that can strongly modulate the susceptibility of macrophages to HIV-1 infection. In this regard, IFN-γ is a potent inducer of MIP-1α and CCL4 in monocyte-derived macrophages, and, conversely, MIP-1α is known to enhance IFN-γ production from Ag-stimulated T cells in vitro.25–27 Therefore, the possibility of a reciprocal correlation between MIP-1α and IFN-γ or GM-CSF production in HTLV-2–activated PBMC cultures was investigated, both in the presence and absence of HIV Env C5 peptide (see Document S2, section 2; Table S2). The kinetics of GM-CSF and IFN-γ secretion was similar and occurred later than that of MIP-1α in unstimulated PBMC cultures (Figure S2A). In this regard MIP-1α was already secreted at high (about 8 ng/mL) levels after 24 hours of culture, whereas cytokine secretion usually occurred after 48 hours of cultivation. MIP-1α production was increased in C5-stimulated PBMC cultures by 3.5-fold with respect to controls, whereas either a modest or lack of increase of GM-CSF or IFN-γ secretion was observed in this experimental condition (Figure 4A).
Kinetics of MIP-1α, IFN-γ, and GM-CSF secretion in control and C5-stimulated PBMC culture supernatants from HTLV-2/HIV-1MEU persons. (A) PBMCs were seeded at 2 × 105 cells/well and cultured in medium supplemented with 5% human serum in the presence or absence of 30 μg/mL C5-peptide. Supernatants were harvested at regular intervals and then tested for chemokine/cytokine concentrations. Results are representative of 5 independent experiments. (B) Correlation between MIP-1α and IFN-γ or GM-CSF production in unstimulated cultures of PBMCs from HTLV-2/HIV-1MEU subjects. PBMCs were cultivated in the presence or absence of anti–MIP-1α or anticytokine NmAb (2 μg/mL each) that were added a single time at the beginning of the culture. Neutralization of MIP-1α resulted in a significant reduction of GM-CSF and IFN-γ secretion.
Kinetics of MIP-1α, IFN-γ, and GM-CSF secretion in control and C5-stimulated PBMC culture supernatants from HTLV-2/HIV-1MEU persons. (A) PBMCs were seeded at 2 × 105 cells/well and cultured in medium supplemented with 5% human serum in the presence or absence of 30 μg/mL C5-peptide. Supernatants were harvested at regular intervals and then tested for chemokine/cytokine concentrations. Results are representative of 5 independent experiments. (B) Correlation between MIP-1α and IFN-γ or GM-CSF production in unstimulated cultures of PBMCs from HTLV-2/HIV-1MEU subjects. PBMCs were cultivated in the presence or absence of anti–MIP-1α or anticytokine NmAb (2 μg/mL each) that were added a single time at the beginning of the culture. Neutralization of MIP-1α resulted in a significant reduction of GM-CSF and IFN-γ secretion.
Addition of anti–GM-CSF NmAb did not alter either IFN-γ or MIP-1α production over time (C.C., unpublished data, June 14, 2004). Interestingly enough, addition of anti–IFN-γ NmAb did not impair MIP-1α secretion at day 6 but inhibited the production of GM-CSF (from 4.1 ng/mL to 1.4 ng/mL; Figure 4B). However, anti–MIP-1α NmAb reduced the level of both GM-CSF (from 4.1 ng/mL to 1.1 ng/mL) and of IFN-γ (from 2.7 ng/mL to 0.9 ng/mL). These results demonstrate that MIP-1α acts as a master controller of cytokine secretion, such as GM-CSF and IFN-γ that can contribute to CCR5 down-modulation from the cell surface of PBMCs from HTLV-2/HIV-1MEU subjects.
Discussion
Independent studies have shown that an increased copy number of CCL3L1 results in the enhanced secretion of CCL3L1 by activated leukocytes.5,16 An inverse association between CCL3L1 copy number and CCR5 expression on T-cell surface suggested that either chemokine binding or CCR5 signaling in the presence of an elevated CCL3L1 copy number causes receptor internalization.5 Our current findings provide strong evidence that the cell activation state induced by HTLV-2 infection, rather than gene copy number, drives CCL3L1 expression and, consequently, CCR5 down-regulation. In addition, PBMCs from HTLV-2–infected persons show an up-regulation of GM-CSF and IFN-γ secretion that may also inhibit CCR5 expression,28 and this effect was abrogated by MIP-1α neutralization.
HTLV-2, a virus that has not yet been linked to a specific human disease, may be a natural inhibitor of HIV-1 infection and replication. Our findings underscore 2 important functional aspects related to the role of CCL3L1 in HIV infection. First, the high CCL3L1/CCL3 mRNA ratio observed in PBMCs of HIV-monoinfected LTNPs is likely to be related to their CCL3L1 genotype. In contrast, the increased expression of CCL3L1 mRNA observed in PBMCs of HTLV-2/HIV-1MEU subjects and HTLV-2/HIV-1–coinfected LTNPs is independent of the gene copy number, and it is likely to be caused by HTLV-2 infection. We hypothesize that a Tax2 specific/preferential induction of the CCL3L1 promoter might occur in these persons,12 in contrast to the PHA-mediated PBMC activation that preferentially activates CCL3 expression. With respect to this preferential induction, different regulatory sequences are present in the 5′-flanking regions of CCL3 and CCL3L1 genes, whereas similar sequences present in the mouse GMCSF promoter mediate an HTLV-1 Tax1-inducible response.29 In addition to a direct antiviral mechanism provided by the epigenetic up-regulation of CCL3L1, the pattern of chemokines and cytokines induced by HTLV-2 infection (ie, GM-CSF and IFN-γ) may indeed contribute to induce a “protective” phagocyte-dependent Th1 response involving development of cytotoxic T lymphocytes against invading pathogens,30 whereas a dominant Th2 response has been implicated in the progression of HIV infection.31 The relevance of CCR5-binding chemokines as correlates of protective innate immune mechanisms against R5 HIV-1 infection, which have been here analyzed in the context of HTLV-2 infection or coinfection, has been previously highlighted in studies of experimental infection of macaques by Lehner et al32 and Bogers et al.33
Protective effects against HIV-1 disease progression by other coinfections have been described. Induction of HIV-inhibitory chemokines and reduction of CCR5 expression that may improve the course of HIV disease have been reported after in vitro infection of PBMCs with hepatitis GB virus C.34,35 Unlike these studies, our experimental system is based on unstimulated PBMCs obtained from HTLV2/HIV-1–coinfected persons. Other viruses, such as cytomegalovirus, have an accelerating effect on HIV disease progression,36 whereas human herpesvirus 6 can exert both protective and accelerating effects as a function of the experimental conditions.7
The selective up-regulation of CCL3L1 isoform expression by HTLV-2 infection adds novel importance to this chemokine variant that has already been implicated as a protective mechanism against HIV-1 infection because of its in vitro potency18 and in vivo gene duplication.5 The postgenomic mechanism triggered by HTLV-2 infection or coinfection and leading to CCL3L1 up-regulation is likely to be transcriptional. These observations are also relevant to the development of innovative strategies of immunologic stimulation of protective innate immune responses.
Authorship
Contribution: E.P. designed and performed the research and analyzed data; L.E., E.V., M.C.R., and S.A. performed the research; U.B. and G.P. analyzed data and participated in writing of the manuscript; C.C. designed the research, analyzed data, and wrote the paper; and all authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claudio Casoli, Department of Clinical Medicine, Nephrology, and Health Sciences, School of Medicine, University of Parma, Via Gramsci 14, 43100 Parma, Italy; e-mail: claudio.casoli@unipr.it.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Prof Lucy Rasmussen (Stanford University, CA) for discussion and for assistance in preparation of the manuscript. We also thank Prof Andrea Mozzarelli and Dr Barbara Campanini for advice and discussion regarding chemokine purification and Drs Rocco Caccavari, Francesca Berghenti, Giacomo Magnani, Daria Sacchini, and Antonio Boschini for providing the clinical history and follow-up information on patients included in this study. The University of Parma participates in the City-AIDS Program supported by the UNAIDS.
This work was supported by the Istituto Superiore di Sanità (ISS) National Research Project on AIDS (grant 40D.14, Evaluation of LNTP: Viroimmunological Italian Study [ELVIS]), and the Cariparma Banking Foundation (grant 2004.0190) (C.C.).