Abstract
SHP-2 phosphatase forms a stable protein complex with and is heavily tyrosine-phosphorylated by the oncogenic tyrosine kinase Bcr-Abl. However, the role of SHP-2 in Bcr-Abl–mediated leukemogenesis is unclear. In the present report, we provide evidence that SHP-2 is required for hematopoietic cell transformation by Bcr-Abl. In vitro biological effects of Bcr-Abl transduction were diminished in SHP-2Δ/Δ hematopoietic cells, and the leukemic potential of Bcr-Abl–transduced SHP-2Δ/Δ cells in recipient animals was compromised. Further analyses showed that Bcr-Abl protein (p210) was degraded, and its oncogenic signaling was greatly decreased in SHP-2Δ/Δ cells. Treatment with proteasome inhibitors or reintroduction of SHP-2 restored p210 level in Bcr-Abl–transduced SHP-2Δ/Δ cells. Subsequent investigation revealed that SHP-2 interacted with heat shock protein 90, an important chaperone protein protecting p210 from proteasome-mediated degradation. The role of SHP-2 in the stability of p210 is independent of its catalytic activity. Blockade of SHP-2 expression in p210-expressing cells by antisense or small-interfering RNA approaches decreased p210 level, causing cell death. Inhibition of SHP-2 enzymatic activity by overexpression of catalytically inactive SHP-2 mutant did not destabilize p210 but enhanced serum starvation-induced apoptosis, suggesting that SHP-2 also plays an important role in downstream signaling of p210 kinase. These studies identified a novel function of SHP-2 and suggest that SHP-2 might be a useful target for controlling Bcr-Abl–positive leukemias.
Introduction
Chronic myeloid leukemia (CML) is a clonal hematopoietic cell malignancy associated with the reciprocal t(9;22)(q34;q11) chromosome translocation.1,2 Translocation of c-Abl located on chromosome 9 to the breakpoint-cluster region (Bcr) on chromosome 22 generates a fusion oncogene Bcr-Abl, which primarily produces the chimeric tyrosine kinase p210.3 Because of fusion of the autoinhibitory SH3 domain of c-Abl to Bcr, autoinhibition of Abl kinase by its SH3 domain is disrupted. As a result, the chimeric kinase is constitutively activated.4-6 Constitutively active p210 kinase appears to play a fundamental role as the primary causative factor in CML. The presence of this kinase is essential and sufficient for malignant transformation of hematopoietic cells in culture,7,8 and expression of p210 in transgenic mice causes a CML-like myeloproliferative disease.9,10 Treatment of Bcr-Abl–transformed hematopoietic cells with potent inhibitors of the p210 kinase, such as Gleevec (also known as imatinib mesylate or STI-571)11-13 and BMS-354825,14 leads to growth inhibition and apoptosis. The therapeutic efficacy of imatinib mesylate has been demonstrated in patients with CML.12,13
Although biological effects mediated by Bcr-Abl tyrosine kinase have been extensively characterized, the molecular mechanisms by which the oncogenic kinase transforms hematopoietic cells are not fully understood. Bcr-Abl kinase induces hematopoietic cell transformation by activation of cell-signaling pathways and dysregulation of cell-cycle progression.15,16 It may induce transformation by inhibition of cell death as well as induction of cell proliferation. Intracellular signaling molecules, such as MAP kinases, PI3 kinase, Jak/STAT, NF-κB, PKC, and c-Myc, are activated by Bcr-Abl. Bcr-Abl kinase (p210) forms a large protein complex with a number of cell-signaling proteins, and targets of p210, such as STAT5, Grb2, CrkL, Dok, Cbl, Shc, Gab2, and SHP-2, have been identified.15,16 These targets are heavily tyrosine-phosphorylated in Bcr-Abl–transformed cells. Of these targets, some, such as Gab2,17 play essential roles in Bcr-Abl–induced hematopoietic cell transformation and leukemogenesis, whereas others, such as Cbl, may not be required.18
SHP-2, a ubiquitously expressed SH2 domain–containing tyrosine phosphatase, has been implicated in diverse signaling pathways induced by a number of stimuli, including growth factors, cytokines, extracellular matrix,19-21 and even cellular stress.22-24 In many cases, especially in receptor tyrosine kinase–initiated intracellular signaling, SHP-2 enhances signal transmission. SHP-2 is highly expressed in hematopoietic cells. Our previous studies have shown that SHP-2 plays a critical role in hematopoietic cell development and function.25-27 To study signaling mechanisms of SHP-2 in hematopoietic cells, we generated SHP-2Δ/Δ hematopoietic cell lines/pools28 by immortalization of yolk sac cells from the gene-targeted mutant embryos with an amino acid 46 to 110 deletion mutation in SHP-2 (SHP-2Δ).25,29 Using these cell lines, we showed that SHP-2 played an indispensable role in IL-3–induced hematopoietic cell responses and that it functioned in both catalytic-dependent and -independent manners.28,30 Genetic lesions in the SHP-2 gene (PTPN11) causing hyperactivation of its catalytic activity have been identified in juvenile myelomonocytic leukemia, myelodysplastic syndromes, acute myeloid leukemia,31,32 as well as in sporadic solid tumors.33 Moreover, single gain-of-function mutations of SHP-2 enhance GM-CSF or IL-3–activated cellular responses in hematopoietic progenitor cells and induce myeloproliferative diseases in mice.34-38 These new findings further emphasize the importance of the role of SHP-2 in hematopoietic cell processes, in particular, its relevance to leukemogenesis.
The role of SHP-2 in Bcr-Ab–mediated hematopoietic cell transformation and leukemogenesis of CML has not been defined. SHP-2 is present in a protein complex with p210, and it is heavily tyrosine-phosphorylated.39-41 However, the exact function of SHP-2 in Bcr-Abl–mediated cellular effects remains unclear. We took advantage of IL-3–dependent SHP-2Δ/Δ hematopoietic cell lines28 to examine a role for SHP-2 in Bcr-Abl–mediated biological effects. We found that SHP-2 was required for hematopoietic cell transformation by Bcr-Abl, and this function of SHP-2 was attributed to its role in stability of p210 as well as in downstream signal transduction of p210 kinase.
Materials and methods
Mice, cell lines, and reagents
SHP-2+/Δ mice were bred in house. Wild-type (WT) C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All animal procedures complied with the NIH Guideline for the Care and Use of Laboratory Animals and were approved by the Case Western Reserve University Institutional Animal Care and Use Committee. Ba/F3, an IL-3–dependent murine pro-B lymphoma cell line, was maintained in RPMI-1640 medium with 10% fetal bovine serum (FBS) and 10% conditioned medium produced by murine IL-3 cDNA-transfected X63 AG8-653 myeloma cells. K562, a CML blast crisis leukemia cell line expressing Bcr-Abl (p210), was maintained in RPMI-1640 medium with 10% FBS. Anti–SHP-2, anti-Erk, anti–phospho-Erk, anti–c-Abl, and anti-GFP antibodies (Abs) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Hsp90 Ab was provided by Stressgen Biotechnologies (Victoria, BC, Canada). Anti–phospho-tyrosine (pY) (4G10) and anti–SHP-1 Abs were obtained from Upstate Biotechnology (Lake Placid, NY). Anti–phospho-Akt and anti-Akt Abs were purchased from Cell Signaling Technology (Beverly, MA). Lactacystin was supplied by Calbiochem (La Jolla, CA). The MTS cell proliferation assay (a colormetric method for determining numbers of viable cells) kit was obtained from Promega Life Science (Madison, WI).
Cell transformation assay
WT and SHP-2Δ/Δ hematopoietic cells transduced with Bcr-Abl or the vector control were sorted by fluorescence-activated cell sorting (FACS). Sorted cells were seeded (1 × 103 cells/mL) in 0.9% methylcellulose RPMI-1640 medium containing 15% FBS, glutamine (10−4 M), β-mecaptoethanol (3.3 × 10−5 M) without hematopoietic growth factors. After 7 days of culture at 37°C in a humidified 5% CO2 incubator, cell colonies consisting of more than 50 cells were counted under an inverted microscope. To test for the transformation of Bcr-Abl– and vector-transduced WT and SHP-2Δ/Δ embryonic fibroblasts, cells were seeded (0.5 × 104 cells/mL) into DMEM with 15% FBS and 0.3% low melting point agarose (GIBCO BRL, Gaithersburg, MD). After 10 days of culture at 37°C in a humidified 5% CO2 incubator, colonies consisting of more than 50 cells were scored.
Migration assay
Transwells (8 μm in pore size, 6.5 mm in diameter; Corning Costa, Cambridge, MA) were coated on both sides with fibronectin (10 μg/mL) at 4°C overnight. WT and SHP-2Δ/Δ hematopoietic cells transduced with Bcr-Abl or the vector were sorted with FACS. Sorted cells were seeded into upper chambers (2 × 105 cells/100 μL) with RPMI-1640 medium without serum and allowed to migrate to lower chambers containing 600 μL RPMI-1640 medium with 10% FBS and 5% IL-3–conditioned medium in a 37°C, 5% CO2 incubator. Six hours later, transwell membranes were fixed in methanol, and cells on the upper surface were mechanically removed. Migrated cells adhering to the lower side of the membranes were stained with Giemsa and enumerated under a microscope at ×200 magnification.
Cell-cycle analysis
Cells were harvested and fixed in 70% ethanol. Fixed cells were treated with RNase A (20 μg/mL) at 37°C for 30 minutes, washed with phosphate-buffered saline (PBS), and then stained with propidium iodide (50 μg/mL in PBS). Cellular DNA content was analyzed with FACS using BD-LSR flow cytometry (BD Biosciences, San Jose, CA). Cell-cycle profiles were determined using CELLQuest software (BD Biosciences).
Immunoprecipitation and immunoblotting
Cells were lysed in RIPA buffer (50 mM Tris-HCl pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM NaF, 2 mM Na3VO4, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1 mM PMSF). Whole-cell lysates (500 μg) were immunoprecipitated with 1 to 2 μg purified Abs or 2 μL antiserum Abs. Immunoprecipitates were washed 3 times with HNTG buffer (20 mM HEPES pH 7.5, 150 mM NaCl, 1% glycerol, 0.1% Triton X-100, and 1 mM Na3VO4) and resolved by SDS–polyacrylamide gel electrophoresis (PAGE) followed by immunoblotting with the indicated Abs.
In vitro kinase assay
WT and SHP-2Δ/Δ cells transduced with Bcr-Abl or the vector control were sorted and lysed in RIPA buffer. Cell lysates (500 μg) were immunoprecipitated with anti–c-Abl Ab. Immunocomplexes were washed 3 times with RIPA buffer, one time with kinase buffer (50 mM Tris-HCl, pH 7.5, 10 mM MnCl2, and 0.1 mM sodium orthovanadate), and were finally resuspended in 30 μL kinase buffer containing GST-Crk (5 μg), 10 μM ATP, and 5 μCi (0.185 MBq) γ-32P-ATP. All reactions were incubated at room temperature for 30 minutes. Proteins in whole-reaction systems were resolved by SDS-PAGE, and phosphorylated GST-Crk was visualized by autoradiography.
Northern blotting
Total RNA was extracted from WT and SHP-2Δ/Δ cells transduced with Bcr-Abl or the vector control. RNA (30 μg) was then resolved in 1% agarose formaldehyde gels and transferred onto nylon membranes. Membranes were hybridized with α-32P-deoxycytidine triphosphate (dCTP)–labeled Bcr-Abl cDNA probe, and blots were visualized using a Storm 860 phosphorimmager (Molecular Dynamics, Sunnyvale, CA). Blots were stripped and reprobed with the GAPDH-positive control probe to monitor RNA loading.
SHP-2 antisense oligodeoxynucleotides and siRNA
Three 20-mer oligodeoxynucleotides were synthesized by Sigma Genosys (The Woodlands, TX) based on sequences reported.42 SHP-2 antisense oligo is 5′-CTC CGC GAT GTC ATG TTC, CT-3′. SHP-2 sense oligo is 5′-GAG GAA CAT GAC ATC GCG GA-3′. The nonsense control is TGG GTG TGT CCA AGA GAA CT-3′. The oligodeoxynucleotides were phosphorothioate modified and resuspended in sterile H2O. Human SHP-2 siRNA duplex was purchased from Dharmacon (Lafayette, CO). The sequence is
GAA UCC UAU GGU GGA AAC AdTdT
dTdT CUU AGG AUA CCA CCU UUG U.
Antisense oligo nucleotides and siRNA were transfected into p210-expressing cells using Fugene transfection reagent and by electroporation, respectively.
Hematopoietic cell transduction and transplantation
Bcr-Abl cDNA was cloned into the MSCV-IRES-GFP retroviral vector43 containing an internal ribosomal entry sequence (IRES) driving expression of a downstream green fluorescence protein (GFP) gene to facilitate tracking of transduced cells. High-titer and helper-free ecotropic retroviruses were generated by transient cotransfection of 293T cells with pQEPAM3 (Minus E) gag polymerase-expressing plasmid, pSrαG (VSV-G) envelope protein-expressing plasmid, and the recombinant Bcr-Abl retroviral plasmid. To transduce primary yolk sac hematopoietic cells with Bcr-Abl, yolk sac cells harvested from embryonic day 9.0 to 9.5 embryos were prestimulated in RPMI-1640 medium containing 10% FBS, SCF (50 ng/mL), IL-3 (20 ng/mL), and IL-6 (50 ng/mL) for 2 days and then infected with the retroviruses in the presence of polybrene (6 μg/mL) for 8 hours. After 2 rounds of hit, transduced cells were harvested and determined for transduction efficiencies by FACS based on GFP expression. Transduced yolk sac cells (2 × 103 cells with approximately 50% transduction efficiencies) were mixed with 5 × 105 WT bone marrow cells (carrier cells) freshly harvested from C57BL/6 mice and were injected through lateral tail veins into C57BL/6 recipient mice that had been irradiated with γ-radiation [11 Gy (1100 rad)]. Animals were monitored until 6 months after transplantation.
Results
SHP-2 phosphatase forms a stable protein complex with Bcr-Abl (p210) and is heavily tyrosine phosphorylated by p210 kinase.39-41 To address whether and how SHP-2 is involved in p210-induced hematopoietic cell transformation, the SHP-2 mutant hematopoietic cell lines generated from yolk sac cells of SHP-2 knock-out embryos were used.28 Although the targeted SHP-2 mutation in these mutant cells encompasses amino acid 46 to 10 deletion, expression of the truncated form of SHP-2 (SHP-2Δ) is extremely low.28 This deletion mutation represents a loss-of-function mutation of SHP-2. Because both WT and SHP-2Δ/Δ cell lines are IL-3 dependent for survival and growth, these lines served as a good model for investigation of the role of SHP-2 in Bcr-Abl–mediated cellular effects.
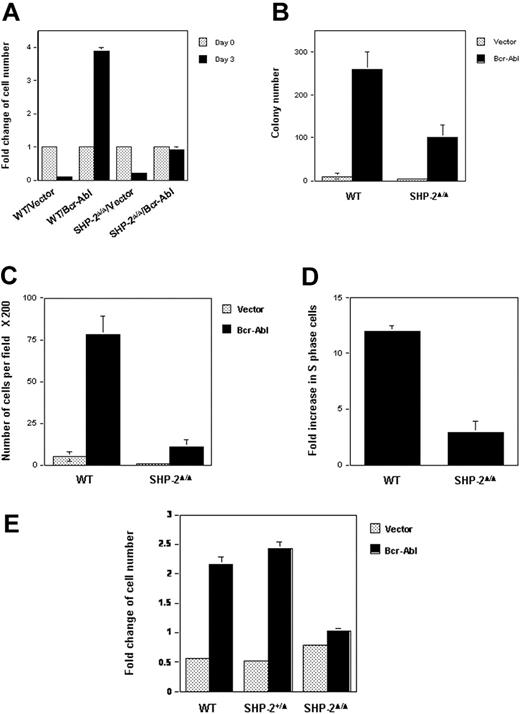
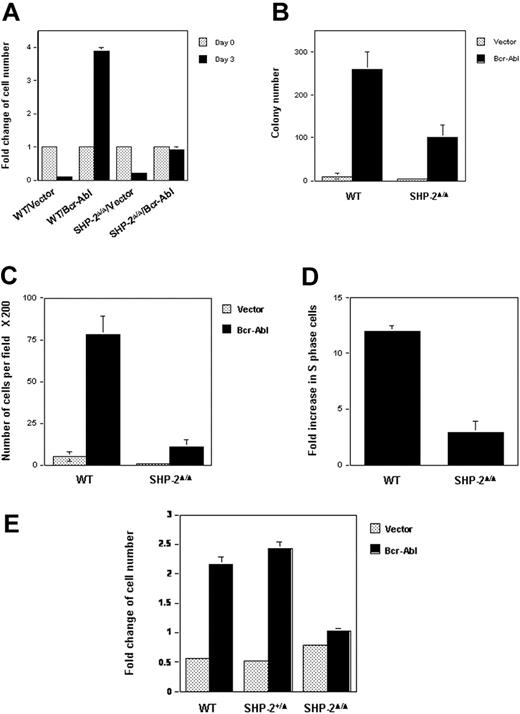
WT and SHP-2Δ/Δ cells were transduced with either Bcr-Abl or the vector control by retroviral-mediated gene transfer. Transduced cells were sorted by FACS, based on the expression of GFP, the independent marker gene contained in the retroviral vector. Biologic consequences of Bcr-Abl transduction were then examined using sorted cell pools. Parental hematopoietic cell lines depend on IL-3 for survival and growth. Transduction of Bcr-Abl in WT cells resulted in IL-3–independent growth, consistent with previous reports that p210 kinase confers growth factor independence in hematopoietic cells.15,16 Intriguingly, SHP-2Δ/Δ cells were no longer growth factor independent after Bcr-Abl transduction (Figure 1A). They stopped growing in IL-3–free medium, even though they were still alive, based on the trypan blue exclusion assay (data not shown). Similarly, colony formation of Bcr-Abl–transduced SHP-2Δ/Δ cells in methylcellulose medium without hematopoietic growth factors was also significantly decreased (Figure 1B). Moreover, the sizes of colonies derived from Bcr-Abl–transduced SHP-2Δ/Δ cells were greatly reduced (data not shown), indicative of slower cell growth. Consistent with previous observations that Bcr-Abl transduction enhances hematopoietic cell adhesion and migration,44 Brc-Abl–transduced WT cells showed strong adhesion in tissue culture plates. In contrast, adhesion of Bcr-Abl–transduced SHP-2Δ/Δ cells was diminished (data not shown), and their migration ability was also dramatically decreased (Figure 1C). Additionally, cell-cycle distributions of Bcr-Abl–transduced WT and SHP-2Δ/Δ cells cultured in IL-3–free medium were examined and compared with those of the vector-transduced cells. As shown in Figure 1D, Bcr-Abl transduction in WT hematopoietic cells greatly increased the percentage of S-phase cells (DNA synthesizing cells). In contrast, Bcr-Ab–induced S-phase increase in SHP-2Δ/Δ cells was markedly reduced. These results suggest a positive role for SHP-2 phosphatase in Bcr-Abl–mediated biological effects.
Biologic effects of Bcr-Abl transduction in SHP-2Δ/Δ cells are decreased. WT and SHP-2Δ/Δ hematopoietic cell lines were transduced with Bcr-Abl or the vector control by retroviral-mediated gene transfer. Transduced cells were sorted by FACS, based on expression of GFP. (A) Sorted cells were seeded into 96-well plates (1 × 104 cells/well) in RPMI-1640 medium with 10% FBS. Cells were quantitated on day 0 and day 3 by the MTS assay, a colorimetric method for detecting viable cells. Cell growth rates (fold changes) were determined by OD490 values and normalized to day 0. (B) WT/Bcr-Abl–, SHP-2Δ/Δ/Bcr-Abl–, and the vector control–transduced cells were plated into 0.9% RPMI-1640 methylcellulose medium containing 15% FBS (1000 cells/mL). After 7 days of incubation in a humidified 5% CO2 incubator, colonies consisting of more than 50 cells were scored under an inverted microscope. (C) Bcr-Abl– or vector-transduced WT and SHP-2Δ/Δ cells were examined by migration assay as described in “Materials and methods.” Migrated cells adhering to the lower side of trans-membranes were stained and counted under a microscope. (D) Bcr-Abl– and the vector control–transduced WT or SHP-2Δ/Δ cells were cultured in IL-3–free RPMI-1640 medium for 24 hours. Cells were then harvested and processed for cell-cycle analyses. Fold changes of the percentages of S-phase in Bcr-Abl–transduced cells over those of the vector–transduced cells were then determined. (E) Embryonic day 9.0 to 9.5 yolk sacs were dissected from timed pregnant SHP-2+/Δ female mice mated with SHP-2+/Δ male mice. Embryonic tissues were used for genomic DNA extraction and subsequent PCR genotyping. Yolk sac cells were dissociated, prestimulated, and transduced with Bcr-Abl or vector as described in “Materials and methods.” Transduced cells were sorted by FACS according to GFP expression. Sorted cells were seeded into 96-well plates in IL-3–free medium and were assayed for cell growth rates as described in Figure 1A. Two to 3 independent experiments were performed, and similar results were obtained in each. Results shown are the mean ± SD of triplicates from one experiment.
Biologic effects of Bcr-Abl transduction in SHP-2Δ/Δ cells are decreased. WT and SHP-2Δ/Δ hematopoietic cell lines were transduced with Bcr-Abl or the vector control by retroviral-mediated gene transfer. Transduced cells were sorted by FACS, based on expression of GFP. (A) Sorted cells were seeded into 96-well plates (1 × 104 cells/well) in RPMI-1640 medium with 10% FBS. Cells were quantitated on day 0 and day 3 by the MTS assay, a colorimetric method for detecting viable cells. Cell growth rates (fold changes) were determined by OD490 values and normalized to day 0. (B) WT/Bcr-Abl–, SHP-2Δ/Δ/Bcr-Abl–, and the vector control–transduced cells were plated into 0.9% RPMI-1640 methylcellulose medium containing 15% FBS (1000 cells/mL). After 7 days of incubation in a humidified 5% CO2 incubator, colonies consisting of more than 50 cells were scored under an inverted microscope. (C) Bcr-Abl– or vector-transduced WT and SHP-2Δ/Δ cells were examined by migration assay as described in “Materials and methods.” Migrated cells adhering to the lower side of trans-membranes were stained and counted under a microscope. (D) Bcr-Abl– and the vector control–transduced WT or SHP-2Δ/Δ cells were cultured in IL-3–free RPMI-1640 medium for 24 hours. Cells were then harvested and processed for cell-cycle analyses. Fold changes of the percentages of S-phase in Bcr-Abl–transduced cells over those of the vector–transduced cells were then determined. (E) Embryonic day 9.0 to 9.5 yolk sacs were dissected from timed pregnant SHP-2+/Δ female mice mated with SHP-2+/Δ male mice. Embryonic tissues were used for genomic DNA extraction and subsequent PCR genotyping. Yolk sac cells were dissociated, prestimulated, and transduced with Bcr-Abl or vector as described in “Materials and methods.” Transduced cells were sorted by FACS according to GFP expression. Sorted cells were seeded into 96-well plates in IL-3–free medium and were assayed for cell growth rates as described in Figure 1A. Two to 3 independent experiments were performed, and similar results were obtained in each. Results shown are the mean ± SD of triplicates from one experiment.
Because the parental cell lines we used for the experiments described in Figure 1A-D are HOX11-immortalized cell lines,28 to rule out the possibility that HOX11 immortalization might affect the analyses, we transduced Bcr-Abl into primary yolk sac cells freshly isolated from embryonic day 9.0 to 9.5 embryos (SHP-2Δ/Δ embryos cannot survive beyond day 10.5). Transduced cells were sorted, based on GFP expression, and then cultured in growth factor–free medium. WT and heterozygous (SHP-2+/Δ) cells transduced with Bcr-Abl doubled after 3 days of culture; however, Bcr-Abl–transduced SHP-2Δ/Δ cells failed to grow (Figure 1E). These results obtained with primary cells are consistent with those from HOX11-immortalized cell lines, reaffirming the requirement of SHP-2 in hematopoietic cell transformation by Bcr-Abl.
Bcr-Abl–transduced primary WT and SHP-2Δ/Δ yolk sac cells were next assessed for their in vivo leukemic potential. Bcr-Abl– or vector-transduced cells (2 × 103 cells, with approximately 50% transduction efficiencies after 2 rounds of retrovirus hits) were, respectively, pooled and mixed with 5 × 105 WT bone marrow cells before transplantation into lethally irradiated recipient mice. WT carrier bone marrow hematopoietic cells were used to help reconstitute the hematopoietic system, because yolk sac stage hematopoietic precursor cells are not able to repopulate the hematopoietic system of adult recipient mice.45,46 Because availability of yolk sac cells was limited, 5 recipient mice were used for each group in 2 independent experiments. Recipient mice were monitored for 6 months. As summarized in Table 1, the survival rate of animals that received a transplant with SHP-2Δ/Δ/Bcr-Abl cells was higher than that of mice receiving WT/Bcr-Abl cells. Autopsies of dead animals showed that they manifested splenomegaly. Mice that received a transplant with SHP-2Δ/Δ/Bcr-Abl cells were followed up to 10 months; no significant signs of myeloproliferative disease were observed based on peripheral blood examination (white cell counts). These in vivo results suggest that the leukemic potential of Bcr-Abl transduction is compromised in SHP-2Δ/Δ cells, supporting a critical role of SHP-2 in Bcr-Abl–mediated hematopoietic cell transformation and leukemogenesis.
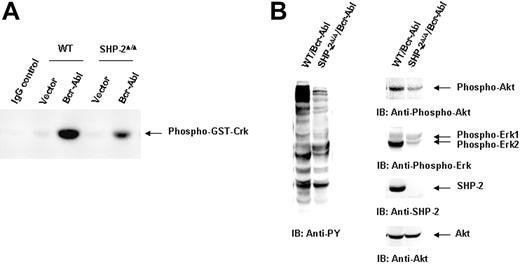
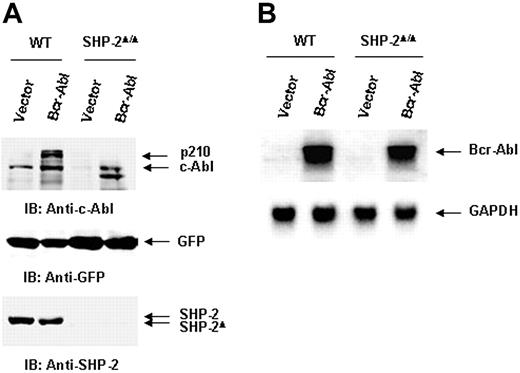
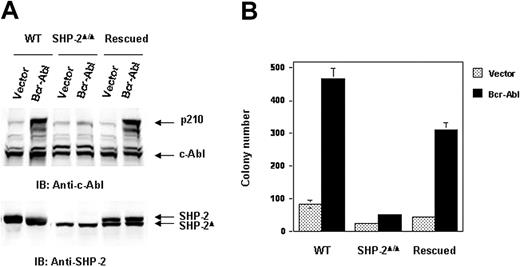
We next evaluated the molecular mechanisms by which SHP-2 phosphatase might regulate Bcr-Abl function. We first examined Bcr-Abl kinase activities in the transduced cells by immunocomplex kinase assay,47,48 using GST-Crk as the substrate. In agreement with the biological results shown in Figure 1, Bcr-Abl kinase activity in SHP-2Δ/Δ cells was much lower than that of Bcr-Abl–transformed WT cells (Figure 2A). We also analyzed whole-cell lysates prepared from Bcr-Abl–transduced cells with anti–phospho-tyrosine (pY) immunoblotting. Tyrosine phosphorylation of cellular proteins was greatly reduced in Bcr-Abl–transduced SHP-2Δ/Δ cells (Figure 2B). Activities of downstream Akt and Erk kinases important for cell survival and proliferation were also markedly decreased (Figure 2B). p210 expression was examined in Bcr-Abl–transduced WT and SHP-2Δ/Δ cells. Using anti–c-Abl Ab, we could easily detect both p210 and endogenous c-Abl in Bcr-Abl–transformed WT cells. Surprisingly, p210 protein was barely detected in Bcr-Abl–transduced SHP-2Δ/Δ cells (Figure 3A). Instead, several smaller proteins were recognized by anti–c-Abl Ab. These proteins were presumably degradation products (proteolytic fragments) from p210. In contrast, the expression levels of GFP, the marker protein expressed separately by the retroviral vector, were comparable in all transduced cell lines (Figure 3A). In addition, we noticed that the c-Abl level in SHP-2Δ/Δ hematopoietic cells was also lower than that in WT cells, unlike the stable expression of c-Abl in mutant embryonic fibroblasts with the same targeted deletion mutation in SHP-2 (Figure 4A). 24 Why c-Abl is destabilized in SHP-2Δ/Δ hematopoietic cells but not in mutant fibroblasts might reflect a difference of the 2 cell types or the higher level of truncated SHP-2 (SHP-2Δ) expressed in mutant fibroblasts24,49,50 that might still retain certain functions. To determine whether the decreased p210 level is caused by inefficient transcription, we performed Northern blotting analyses. As shown in Figure 3B, similar levels of mRNA were generated in Bcr-Abl–transduced WT and SHP-2Δ/Δ cells. Thus, it appears that p210 is degraded after transcription in SHP-2Δ/Δ hematopoietic cells. We also transduced WT and SHP-2Δ/Δ fibroblasts with Bcr-Abl. Likewise, p210 level in SHP-2Δ/Δ fibroblasts was also markedly decreased (Figure 4A). Notably, reintroduction of SHP-2 into Bcr-Abl–transduced SHP-2Δ/Δ cells restored the p210 level and thereby p210-induced transformation (Figure 4B), suggesting that the decreased p210 level in SHP-2Δ/Δ cells is directly attributed to loss of SHP-2 function.
Bcr-Abl kinase activity is diminished in Bcr-Abl–transduced SHP-2Δ/Δ cells. WT/Bcr-Abl–, SHP-2Δ/Δ/Bcr-Abl–, and the vector control–transduced cells were expanded in IL-3–containing medium for 5 days. (A) Whole-cell lysates were prepared and then immunoprecipitated with anti–c-Abl Ab followed by the kinase assay as described in “Materials and methods.” Lysates of Bcr-Abl–transduced WT cells were immunoprecipitated with rabbit IgG as the negative control. (B) Bcr-Abl–transduced WT and SHP-2Δ/Δ cells were cultured in IL-3–free medium for 24 hours. Whole-cell lysates were prepared and examined with anti-pY immunoblotting (IB). Activities of Akt and Erk kinases were also analyzed with IB using anti–phospho-Akt and anti–phospho-Erk Abs. Shown are representative results from 3 independent experiments.
Bcr-Abl kinase activity is diminished in Bcr-Abl–transduced SHP-2Δ/Δ cells. WT/Bcr-Abl–, SHP-2Δ/Δ/Bcr-Abl–, and the vector control–transduced cells were expanded in IL-3–containing medium for 5 days. (A) Whole-cell lysates were prepared and then immunoprecipitated with anti–c-Abl Ab followed by the kinase assay as described in “Materials and methods.” Lysates of Bcr-Abl–transduced WT cells were immunoprecipitated with rabbit IgG as the negative control. (B) Bcr-Abl–transduced WT and SHP-2Δ/Δ cells were cultured in IL-3–free medium for 24 hours. Whole-cell lysates were prepared and examined with anti-pY immunoblotting (IB). Activities of Akt and Erk kinases were also analyzed with IB using anti–phospho-Akt and anti–phospho-Erk Abs. Shown are representative results from 3 independent experiments.
Bcr-Abl oncoprotein (p210) is degraded at the protein level in SHP-2Δ/Δ cells. (A) Whole-cell lysates prepared from Bcr-Abl– or vector-transduced WT and SHP-2Δ/Δ cells were examined with anti–c-Abl IB. The blot was stripped and reimmunoblotted with anti–SHP-2 and anti-GFP Abs. (B) Total RNA was extracted from Bcr-Abl– or vector-transduced cells. RNA samples were examined by Northern blotting using Bcr-Abl cDNA as the probe. The blot was stripped and reprobed with the positive control GAPDH to monitor RNA loading. Shown are representative results of 3 independent experiments.
Bcr-Abl oncoprotein (p210) is degraded at the protein level in SHP-2Δ/Δ cells. (A) Whole-cell lysates prepared from Bcr-Abl– or vector-transduced WT and SHP-2Δ/Δ cells were examined with anti–c-Abl IB. The blot was stripped and reimmunoblotted with anti–SHP-2 and anti-GFP Abs. (B) Total RNA was extracted from Bcr-Abl– or vector-transduced cells. RNA samples were examined by Northern blotting using Bcr-Abl cDNA as the probe. The blot was stripped and reprobed with the positive control GAPDH to monitor RNA loading. Shown are representative results of 3 independent experiments.
p210 level is restored in the rescued SHP-2Δ/Δ cells transduced by Bcr-Abl. SV40 large T-antigen–immortalized WT, SHP-2Δ/Δ, and SHP-2–rescued embryonic fibroblasts were transduced with Bcr-Abl or the vector control. (A) Transduced cells were sorted and analyzed with anti–c-Abl IB. The blot was stripped and reprobed with anti–SHP-2 antibody. (B) Sorted cells were seeded into DMEM with 15% FBS and 0.3% low-melting point agarose (0.5 × 104 cells/mL). After 10 days of culture at 37°C in a humidified 5% CO2 incubator, colonies consisting of more than 50 cells were scored.
p210 level is restored in the rescued SHP-2Δ/Δ cells transduced by Bcr-Abl. SV40 large T-antigen–immortalized WT, SHP-2Δ/Δ, and SHP-2–rescued embryonic fibroblasts were transduced with Bcr-Abl or the vector control. (A) Transduced cells were sorted and analyzed with anti–c-Abl IB. The blot was stripped and reprobed with anti–SHP-2 antibody. (B) Sorted cells were seeded into DMEM with 15% FBS and 0.3% low-melting point agarose (0.5 × 104 cells/mL). After 10 days of culture at 37°C in a humidified 5% CO2 incubator, colonies consisting of more than 50 cells were scored.
Previous studies have shown that p210 protein is normally degraded by proteasome-mediated proteolysis.51,52 To investigate whether the decreased p210 protein level in SHP-2Δ/Δ/Bcr-Abl cells is attributed to enhanced proteasome-mediated degradation, we treated Bcr-Abl–transduced SHP-2Δ/Δ hematopoietic cells with proteasome inhibitors and then examined p210. Treatment of lactacystin, a highly specific inhibitor targeting the catalytic β-subunit of the 20S proteasome, partially restored protein levels of both p210 and c-Abl (Figure 5). Similar results were obtained with treatment using another proteasome inhibitor MG132 (data not shown).
p210 is degraded through the proteasome-mediated pathway in SHP-2Δ/Δ cells. Bcr-Abl–transduced SHP-2Δ/Δ cells were treated with proteasome inhibitor lactacystin at the indicated concentrations for 8 hours (A) or with lactacystin (10 μM) for the indicated time periods (B). Cells were lysed in RIPA lysis buffer supplemented with 0.1% SDS. Cell lysates were prepared and then examined with anti–c-Abl IB. Blots were stripped and reimmunoblotted with anti–SHP-1 Ab to monitor protein loading. Shown are representative results of 3 independent experiments.
p210 is degraded through the proteasome-mediated pathway in SHP-2Δ/Δ cells. Bcr-Abl–transduced SHP-2Δ/Δ cells were treated with proteasome inhibitor lactacystin at the indicated concentrations for 8 hours (A) or with lactacystin (10 μM) for the indicated time periods (B). Cells were lysed in RIPA lysis buffer supplemented with 0.1% SDS. Cell lysates were prepared and then examined with anti–c-Abl IB. Blots were stripped and reimmunoblotted with anti–SHP-1 Ab to monitor protein loading. Shown are representative results of 3 independent experiments.
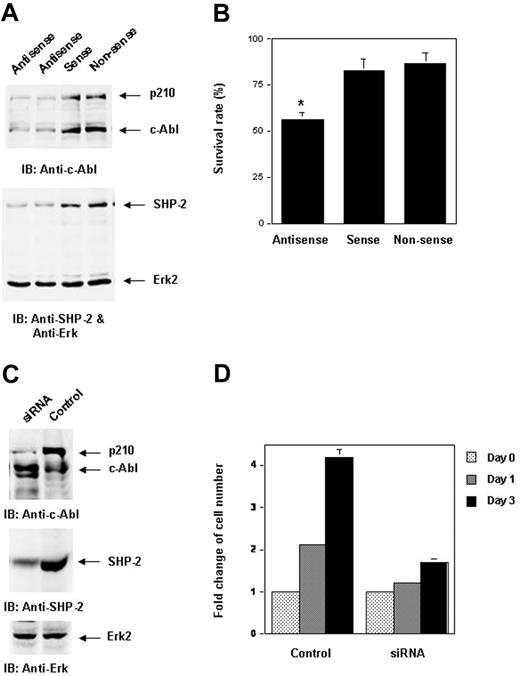
We next tested whether reduced SHP-2 expression by SHP-2 antisense oligonucleotide or small-interfering RNA (siRNA) transfection would decrease p210 level. K562 cells, a p210-expressing human CML blast crisis cell line, were used. As illustrated in Figure 6A, transfection of SHP-2 antisense but not sense or nonsense oligonucleotides inhibited SHP-2 expression. Consequently, p210 and c-Abl levels were also decreased. As a control, Erk kinase expression was not affected. The decrease in p210 level reduced survival of the transfected K562 cells relative to 2 counterparts (Figure 6B), consistent with previous observations that inhibition of p210 expression results in apoptosis.15,16 We also used SHP-2 siRNA to inhibit SHP-2 expression in WT/Bcr-Abl cells. The p210 level was markedly decreased by knockdown of SHP-2 (Figure 6C). As a result, the cell growth was attenuated (Figure 6D). These data further verified the requirement for SHP-2 in p210 stability.
Inhibition of SHP-2 expression by SHP-2 antisense oligonucleotides or siRNA decreases p210 level. (A) p210-expressing K562 leukemia cells were transfected with SHP-2 antisense, sense, and nonsense oligonucleotides (7.5 μM) using the Fugene transfection reagent. Cells were harvested 8 hours after transfection and examined for p210 with anti–c-Abl IB. The blot was stripped and reimmunoblotted with anti–SHP-2 and anti-Erk Abs. (B) Cell-survival rates were determined by trypan blue exclusion assay. The asterisk indicates significant differences between antisense versus sense or nonsense (P < .01 by Student t test). (C) WT/Bcr-Abl cells were transfected with SHP-2 siRNA by electroporation (25 μg for 1 × 107 cells). Cells were harvested 12 hours after transfection and analyzed for p210 level with anti–c-Abl IB. The blot was stripped and reimmunoblotted with anti–SHP-2 and anti-Erk Abs. (D). Fold changes in cell numbers of siRNA-transfected cells over 3 days of culture were determined by the MTS assay as described in Figure 1A. Results displayed are representative of 2 to 3 independent experiments. Results shown on panels B and D are the mean ± SD of triplicates from one experiment.
Inhibition of SHP-2 expression by SHP-2 antisense oligonucleotides or siRNA decreases p210 level. (A) p210-expressing K562 leukemia cells were transfected with SHP-2 antisense, sense, and nonsense oligonucleotides (7.5 μM) using the Fugene transfection reagent. Cells were harvested 8 hours after transfection and examined for p210 with anti–c-Abl IB. The blot was stripped and reimmunoblotted with anti–SHP-2 and anti-Erk Abs. (B) Cell-survival rates were determined by trypan blue exclusion assay. The asterisk indicates significant differences between antisense versus sense or nonsense (P < .01 by Student t test). (C) WT/Bcr-Abl cells were transfected with SHP-2 siRNA by electroporation (25 μg for 1 × 107 cells). Cells were harvested 12 hours after transfection and analyzed for p210 level with anti–c-Abl IB. The blot was stripped and reimmunoblotted with anti–SHP-2 and anti-Erk Abs. (D). Fold changes in cell numbers of siRNA-transfected cells over 3 days of culture were determined by the MTS assay as described in Figure 1A. Results displayed are representative of 2 to 3 independent experiments. Results shown on panels B and D are the mean ± SD of triplicates from one experiment.
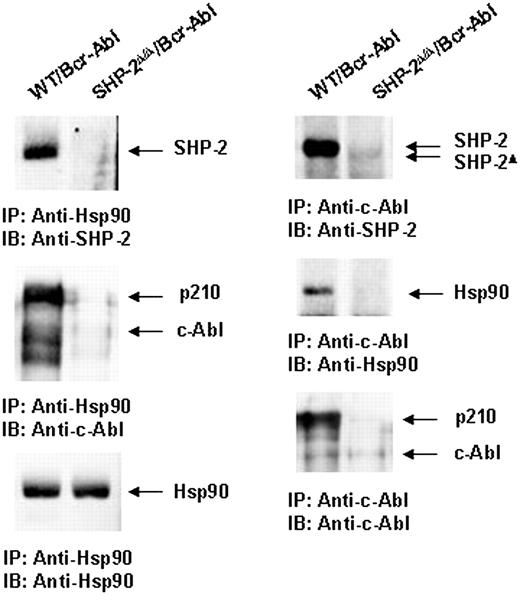
Because p210 protein is destabilized in SHP-2Δ/Δ cells (Figure 3A), we attempted to define the mechanisms by which loss of SHP-2 function accelerates proteasome-mediated p210 degradation. p210 and c-Abl are stabilized and are protected from proteasome-mediated degradation by association with chaperone proteins, in particular, heat shock protein 90 (Hsp90).51,52 We examined a potential interaction between SHP-2 and Hsp90. Anti-Hsp90 immunoprecipitation followed by anti–SHP-2 and anti–cAbl immunoblottings clearly showed that SHP-2 and p210 were present in the Hsp90 protein complex in Bcr-Abl–transformed WT but not SHP-2Δ/Δ cells (Figure 7). Reciprocally, Hsp90 and SHP-2 were detected in the p210 immunocomplex in Bcr-Abl–transduced WT cells (Figure 7). These results suggest that SHP-2 may promote p210 stability by interacting with Hsp90.
SHP-2 is associated with chaperone protein Hsp90. Whole-cell lysates prepared from Bcr-Abl–transduced WT and SHP-2Δ/Δ cells were immunoprecipitated (IP) with anti-Hsp90 or anti–c-Abl Abs followed by anti–SHP-2, anti-Hsp90, or anti–c-Abl IBs. Blots were stripped and reimmunoblotted with anti-Hsp90 or anti–c-Abl Abs. Results shown are representative of 3 experiments.
SHP-2 is associated with chaperone protein Hsp90. Whole-cell lysates prepared from Bcr-Abl–transduced WT and SHP-2Δ/Δ cells were immunoprecipitated (IP) with anti-Hsp90 or anti–c-Abl Abs followed by anti–SHP-2, anti-Hsp90, or anti–c-Abl IBs. Blots were stripped and reimmunoblotted with anti-Hsp90 or anti–c-Abl Abs. Results shown are representative of 3 experiments.
Because SHP-2 is a phosphatase that possesses catalytic activity, it was important to test whether the role of SHP-2 in p210 stability is associated with its catalytic activity. To address this question, we transduced Bcr-Abl into the catalytically inactive SHP-2 C459S-overexpressing Ba/F3 cells that we previously generated.28,30 Transduced cells were sorted and then examined for p210. As seen in Figure 8A, inhibition of the catalytic activity of endogenous SHP-2 by overexpression of SHP-2 C459S mutant did not change p210 or c-Abl levels. The cell growth was not affected either (data not shown). We also cotransfected SHP-2 C459S with Bcr-Abl into 293T cells. No changes in p210 level were observed (data not shown). Thus, the role of SHP-2 in p210 stability is independent of its phosphatase activity. These results suggest that SHP-2 protein per se but not its catalytic activity is important for maintenance of p210 stability.
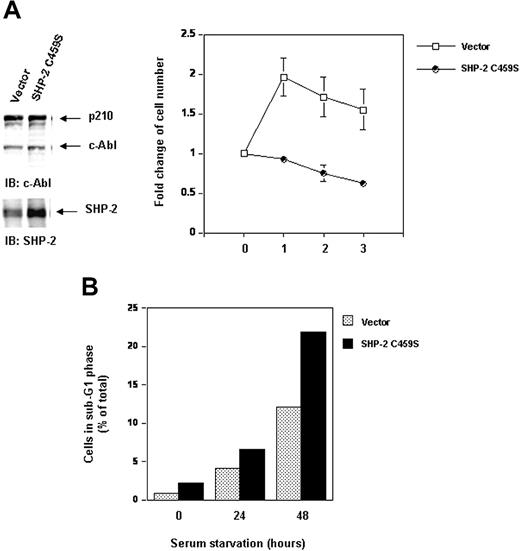
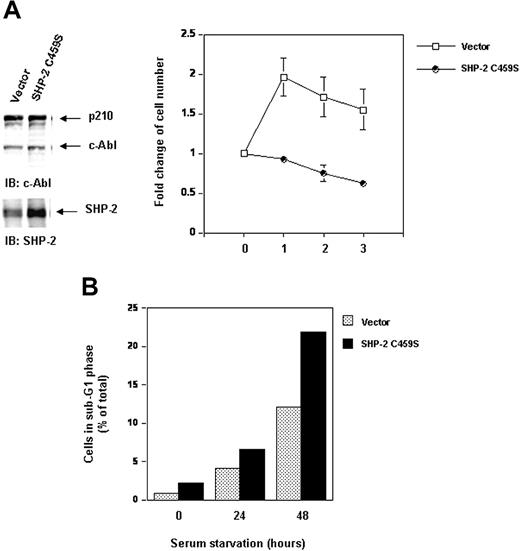
Inhibition of SHP-2 catalytic activity does not destabilize p210 but attenuates cell survival and enhances serum starvation-induced apoptosis. Ba/F3 cells overexpressing catalytically inactive SHP-2 C459S and the vector control cells were transduced with Bcr-Abl as described in “Materials and methods.” (A) Whole-cell lysates prepared from the cell lines were examined for p210 and SHP-2 with IB using anti–c-Abl or anti–SHP-2 Abs. Exponentially growing cells were seeded into serum-free RPMI-1640 medium. Cell-survival rates at the indicated time points were determined by the MTS assay as described in Figure 1A. (B) Exponentially growing cells were starved of serum for 24 and 48 hours. Cells were harvested and processed for cell-cycle analyses. Percentages of apoptotic cells with sub-G1 DNA content were determined. Results shown are representative of 3 experiments.
Inhibition of SHP-2 catalytic activity does not destabilize p210 but attenuates cell survival and enhances serum starvation-induced apoptosis. Ba/F3 cells overexpressing catalytically inactive SHP-2 C459S and the vector control cells were transduced with Bcr-Abl as described in “Materials and methods.” (A) Whole-cell lysates prepared from the cell lines were examined for p210 and SHP-2 with IB using anti–c-Abl or anti–SHP-2 Abs. Exponentially growing cells were seeded into serum-free RPMI-1640 medium. Cell-survival rates at the indicated time points were determined by the MTS assay as described in Figure 1A. (B) Exponentially growing cells were starved of serum for 24 and 48 hours. Cells were harvested and processed for cell-cycle analyses. Percentages of apoptotic cells with sub-G1 DNA content were determined. Results shown are representative of 3 experiments.
Even though p210 protein stability is not affected in SHP-2 C459S-overexpressing cells, interestingly, inhibition of SHP-2 catalytic activity decreased cell survival in serum-free medium (Figure 8A). Serum starvation resulted in apoptosis in Bcr-Abl–transformed cells. Bcr-Abl–transduced Ba/F3 cells overexpressing SHP-2 C459S were more susceptible to serum starvation-induced apoptosis. In response to serum starvation, the percentage of the cells with sub-G1 DNA content, known to be apoptotic cells, was higher than that of the control cells (Figure 8B). These data suggest that, although the catalytic activity of SHP-2 is dispensable for stability of p210, it may play a positive role in p210-triggered signaling for cell survival.
Discussion
In this report we provide evidence that SHP-2 phosphatase is required for hematopoietic cell transformation by Bcr-Abl oncogenic kinase (p210). The role of SHP-2 in this capacity is 2-fold. First, SHP-2 plays an important role in the stability of p210, and this function appears to be independent of its catalytic activity. Loss of functional SHP-2 protein but not inhibition of its catalytic activity resulted in destabilization and degradation of p210. Second, SHP-2 also functions in signaling processes downstream of p210 kinase. It promotes p210-triggered signal transduction in a catalytic-dependent manner. Inhibition of the catalytic activity of SHP-2 phosphatase significantly decreases cell survival of p210-transformed hematopoietic cells following serum starvation. Thus, our studies have identified a novel function of SHP-2 phosphatase. This work also suggests that SHP-2 might be a useful target for controlling Bcr-Abl–positive leukemias.
SHP-2 phosphatase is a multifunctional protein, involved in multiple cellular processes. SHP-2 has been recognized for its role in a variety of signaling pathways induced by growth factor/cytokine receptors, other cell-surface molecules19-21 and even genotoxic stress (DNA damage).22-24 Studies in this report suggest that SHP-2 is required for leukemogenesis by Bcr-Abl. Transformation signs, such as growth factor–independent growth in both liquid and semisolid cultures, in SHP-2Δ/Δ cells transduced with Bcr-Abl were attenuated (Figure 1). Furthermore, the in vivo leukemic potential of Bcr-Abl–transduced SHP-2Δ/Δ cells in recipient mice was compromised (Table 1). This is apparently because of the low-protein level of Bcr-Abl kinase (p210) in SHP-2 mutant cells. Although SHP-2Δ/Δ cells were well transduced by the Bcr-Abl retroviral vector, and the marker gene (GFP) contained in the retroviral vector was expressed equally in these cells, p210 protein level was dramatically decreased (Figure 3A). This is also the case with SHP-2Δ/Δ fibroblasts (Figure 4A). Notably, reintroduction of SHP-2 into SHP-2Δ/Δ cells restored the p210 level and thereby p210-induced transformation (Figure 4B), suggesting that the decrease in p210 is attributable to loss of SHP-2 function. In support of this notion, inhibition of SHP-2 expression by SHP-2 antisense or siRNA transfection resulted in reduction in p210 level (Figure 6). As Bcr-Abl mRNA expression levels were comparable in Bcr-Abl–transduced WT and SHP-2Δ/Δ cells (Figure 3B), it is likely that p210 protein in SHP-2Δ/Δ cells was degraded. Because treatment of Bcr-Abl–transduced mutant cells with proteasome inhibitors partially restored the p210 level in the mutant cells (Figure 5), it seems that SHP-2 normally protects p210 from proteasome-mediated degradation.
It remains to be determined exactly how SHP-2 protects p210 oncoprotein from proteasome-mediated degradation. Several mechanisms may be involved. We showed that SHP-2 formed a stable protein complex with Hsp90 (Figure 7), an important molecular chaperone required for stability of p210.51,52 Chaperone proteins such as Hsp90 help other proteins avoid misfolding pathways that produce inactive or aggregated states. Hsp90 acts in concert with other chaperones and partners to provide maturation and folding, as well as trafficking and function of its client proteins such as steroid hormone receptors, growth factor receptors, and a number of protein kinases, including p210.53,54 Because SHP-2 has a strong interaction with Hsp90, SHP-2 may be one of these partners, helping Hsp90 protect p210 from proteasome-mediated degradation. Inhibition of the catalytic activity of endogenous SHP-2 by overexpression of SHP-2 C459S mutant did not decrease p210 level (Figure 8A), suggesting that the role of SHP-2 in stability of p210 is independent of its catalytic activity. Previous studies have suggested that Hsp90 associates with many client proteins to maintain stability and function of these proteins.53,54 Whether SHP-2 is also involved in stabilization of other proteins as well remains to be determined. A comprehensive examination of the role of SHP-2 in stability of other Hsp90 client proteins is being undertaken in the laboratory. Nevertheless, the possibility that the diminished level of p210 in SHP-2Δ/Δ/Bcr-Abl cells may be also caused by other unknown mechanisms cannot be excluded. We previously showed that SHP-2Δ/Δ cells responded poorly to IL-3. IL-3 signaling in these mutant cells is essentially blocked.28 As a result, cellular activities of SHP-2Δ/Δ cells in IL-3–containing culture medium are markedly decreased. This may somehow reduce synthesis efficiencies of some proteins such as p210 or destabilize these proteins. However, this seems not to be likely because many proteins examined in our previous studies28,38 were not decreased in SHP-2Δ/Δ cells. Moreover, GFP, the marker contained in the Bcr-Abl expression vector, was comparably expressed in SHP-2Δ/Δ cells. Further studies are needed to define the underlying mechanisms of p210 destabilization in SHP-2Δ/Δ cells.
In addition to the role in maintaining p210 stability, SHP-2 appears to also function in its downstream oncogenic signaling. p210 induces leukemogenesis through constitutive activation of multiple intracellular signaling pathways. Previous studies showed that SHP-2 phosphatase was present in the protein complex of p210.39-41 However, the role of SHP-2 in p210-triggered signaling and cellular effects has not been characterized. Our results in this report suggest that SHP-2 promotes p210-triggered signaling and that this role depends on its catalytic activity. Inhibition of the catalytic activity of endogenous SHP-2 by overexpression of catalytically inactive SHP-2 C459S mutant in Bcr-Abl–transformed Ba/F3 cells enhanced serum starvation-induced apoptosis (Figure 8B). It remains to be further determined how SHP-2, a tyrosine phosphatase, promotes cellular effects of p210 kinase. Studies using a murine bone marrow transduction/transplantation model demonstrated that mutations at the Grb2-binding site (Tyr177) in the coiled-coil domain of p210 or at the Grb2-interacting scaffolding adaptor Gab2 impair Bcr-Abl ability to induce CML-like myeloproliferative disease.17,55-57 Because SHP-2 closely interacts with Grb2 and Gab2 in transducing signals to Erk and PI3 kinase pathways, it may function in the Bcr-Abl signal transduction through interactions with these adaptor proteins. However, the direct biochemical significance of the catalytic activity of SHP-2 in this context remains to be characterized. The catalytic activity of SHP-2 is also recognized for its positive role in receptor tyrosine kinase-induced signaling, in particular, the Erk pathway.19-21 The underlying mechanisms of this positive role are also unclear.19-21 Even though a number of SHP-2–interacting proteins and targets have been discovered, none of the putative substrates identified to date can fully account for the overall positive signaling effects of SHP-2 on the many pathways with which it has been implicated. Additionally, because the catalytic activity of SHP-2 is generally important for cell survival and growth, the possibility that the effect of SHP-2 C459S on survival of Bcr-Ab–transduced Ba/F3 cells may be nonspecific cannot be excluded. Further studies are required to explore this possibility.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: J.C., W.-M.Y., and H.D. conducted the research and summarized the data; H.E.B. and B.J.D. provided critical reagents, discussed the work, and proofedited the manuscript; C.-K.Q. designed the experiments and provided technical training to the first 3 authors.
J.C. and W.-M.Y. contributed equally to this work. J.C. is currently affiliated with the National Cancer Center, National Institutes of Health.
Acknowledgments
We thank Drs Benjamin G. Neel, Robert Zhao, and Mark Williams for reagents, technical assistance, helpful discussions, and critical reading of the manuscript.
This work was supported by the National Institutes of Health (grant R01 HL68212) (C.-K.Q.).