Abstract
CXCR4 chemokine receptors retain hematopoietic progenitors and leukemia cells within the marrow microenvironment. We prospectively evaluated the prognostic implication of CXCR4 in 90 consecutive patients with acute myelogenous leukemia (AML) by flow cytometry. Patients were divided into groups with low (n = 32), intermediate (n = 26), or high (n = 32) CXCR4 expression, as defined by CXCR4 mean fluorescence intensity ratio thresholds of less than 5, 5 to 10, or more than 10, respectively. We found that low CXCR4 expression on AML cells correlated with a better prognosis, resulting in a longer relapse-free and overall survival of 24.3 ± 2.9 months for low CXCR4-expressing patients, compared with 17.4 ± 3.4 months for intermediate and 12.8 ± 2 months (mean ± SEM) for patients with high expression. In univariate analyses, CXCR4 expression, cytogenetics, white blood cell count, and serum lactate dehydrogenase (LDH) predicted for shorter survival. Multivariate analysis revealed CXCR4 expression and unfavorable cytogenetics as independent prognostic factors. We conclude that CXCR4 expression in AML is an independent prognostic predictor for disease relapse and survival that can rapidly and easily be determined at disease presentation. These findings warrant further investigation into the role of CXCR4 in AML and suggest that CXCR4 should be incorporated into the risk assessment of AML patients.
Introduction
Despite major improvements in the understanding and treatment of certain leukemias during the past years, the overall survival (OS) of patients with acute myelogenous leukemia (AML) remains poor, and new prognostic markers and therapeutic strategies are urgently needed. Several prognostic markers have been described in AML, including age, performance status, karyotype, white blood cell (WBC) count, serum lactate dehydrogenase (LDH), presence or absence of an antecedent hematologic disorder (eg, myelodysplasia), and the presence of distinct cytogenetic abnormalities.1-3 Cytogenetic abnormalities can be detected in approximately 60% of AML patients and represent the most important predictor for responses to therapy and relapse probability. Depending upon cytogenetic characteristics, patients can be classified into low-risk, intermediate-risk, or high-risk groups.4,5 However, there is substantial heterogeneity within these groups. About 35% to 50% of AML patients display a normal karyotype, which can further be subdivided using predictive molecular markers, such as mutations in the fetal liver tyrosine kinase-3 (FLT3) gene and the mixed-lineage leukemia (MLL) gene.6,7
Several studies indicated that adhesion to marrow stromal cells affects the survival and proliferation of AML cells8,9 and protects AML cells from chemotherapy in vitro10 and in vivo.11 Adhesion molecules, particularly the very late antigen-4 (VLA-4) and VLA-5 integrins, are considered essential for AML cell adhesion to respective stromal ligands (fibronectin)12 and for protection of AML cells from spontaneous or drug-induced apoptosis.11,13 However, adhesion molecules (selectins, integrins) alone are responsible only for initial, transient adhesion events; a second step triggered by chemokine receptor activation is necessary to transform reversible into firm adhesion and directional migration.14,15
Stromal cell-derived factor-1 (SDF-1), which now is designated as CXCL12,16 is a homeostatic chemokine that is constitutively secreted by marrow stromal cells. CXCL12 signals through CXCR4, which in turn plays an important role in hematopoiesis, development, and organization of the immune system. CXCL12 and CXCR4 gene–deleted mice displayed an identical, lethal phenotype, characterized by deficient myelopoiesis and B lymphopoiesis, and abnormal neuronal and cardiovascular development.17-20 Chemotactic responsiveness of hematopoietic stem cells (HSCs) is restricted to CXCL12.21 This unique selectivity for CXCL12 may be necessary for retention of HSCs in the hematopoietic microenvironment and marrow-specific homing of circulating HSCs.22,23 Rapid mobilization of HSCs by CXCR4 antagonists alone or in synergy with granulocyte colony-stimulating factor (G-CSF) has been demonstrated in recent clinical trials and supports the hypothesis that CXCL12 is essential for HSC retention within the marrow.24
CXCR4 receptors are functional in AML25,26 even though expression levels are heterogeneous and the importance of CXCR4 for engraftment of AML cells in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice is controversial.27-29 Prognostic impact of CXCR4 expression levels on the neoplastic cells has been demonstrated in breast cancer30 and renal cell cancer.31,32 Recently, Rombouts and colleagues reported that expression of CXCR4 by CD34+ AML cells is related to Flt3 mutations and unfavorable prognosis.33 In this study, we examined the prognostic impact of CXCR4 surface expression by the CD34+ and/or CD117+ fraction of AML cells at the time of initial diagnosis in a series of 90 consecutive AML patients.
Patients, materials, and methods
Patients and flow cytometry
Bone marrow aspirates of 90 consecutive patients diagnosed with AML between 2001 and 2004 at Freiburg University Hospital (Freiburg, Germany) were obtained after informed consent at the time of diagnosis and examined for CXCR4 expression during routine flow cytometry work-up, generally within 2 to 4 hours after the sample was drawn. The diagnosis of AML was established using standard morphology and cytochemistry according to the French-American-British (FAB) classification, immunophenotypic criteria, and cytogenetic evaluation. For immunodetection of CXCR4 on AML cells, a whole-blood lysis method was used with directly labeled primary antibodies following the manufacturer's instructions (Becton Dickinson, Heidelberg, Germany). Briefly, 100-μL aliquots of marrow aspirate containing EDTA were incubated for 15 minutes with appropriately diluted monoclonal antibody conjugates. For CXCR4 detection, 10 μL of PE-conjugated anti-CXCR4 (12G5) was used in combination with anti-CD34–FITC, anti-CD117–PC5, and anti-CD45–APC. A control sample was incubated with the appropriate isotype control antibodies. After incubation, 2 mL of fluorescence-activated cell sorter (FACS) lysing solution was added for 15 minutes, and cells were washed once and analyzed within 2 hours on a FACSCalibur (Becton Dickinson). All antibodies and FACS lysis solution were purchased from Becton Dickinson except for anti-CD117–PC5 (Beckman Coulter, Krefeld, Germany). Flow cytometry data were analyzed using the FlowJo software (version 2.7.4; Tree Star, Ashland, OR). AML cells were gated based upon their CD34 and/or CD117 expression (Figure 1). Mean fluorescence intensity ratios (MFIRs) were calculated by dividing the mean fluorescence intensity for CXCR4 by the mean fluorescence of the respective nonspecific isotype control. Based upon the distribution of CXCR4 expression in our patients' population, we defined groups with low CXCR4 expression as group A (MFIR less than 5), intermediate CXCR4 expression as group B (MFIR 5 to 10), and high CXCR4 expression as group C (MFIR more than 10).
Patients' characteristics
Forty-three patients presented with de novo AML (group A, n = 15; group B, n = 12; group C, n = 16), 35 with secondary AML and a history of myelodysplastic syndrome (MDS) (group A, n = 11; group B, n = 11; group C, n = 13) or with therapy-related AML (group A, n = 2; group B, n = 2; group C, n = 0), and 8 patients with MDS refractory anemia with excess blasts in transformation (RAEB-t) (group A, n = 4; group B, n = 1; group C, n = 3). Patients were treated at Freiburg University Hospital according to the following protocols: German Multicenter Study AML-01/99 SHG for patients 15 to 60 years except for 1 patient with AML M3 with t(15;17) who was treated in the European APL-Study 2000. According to the AML-01/99 SHG protocol, 24 patients received induction therapy (IVA-1/2) with cytarabine, etoposide, and idarubicin. Fifteen patients older than 60 years who were eligible for induction chemotherapy were treated with MICE (mitoxantrone, etoposide, cytarabine) or ICE (idarubicin, etoposide, cytarabine). Eleven patients received a cytarabine/anthracycline-based induction therapy off protocol, 13 patients were treated with decitabine as their initial therapy, and 18 patients received other cytoreductive therapies (hydroxyurea, mercaptopurine, all-trans retinoic acid, imatinib). Eight patients did not receive any cytoreductive therapies. The distribution of therapies among the different groups based on CXCR4 expression is displayed in Table 2. Forty-seven of all patients subsequently received an allogenic stem cell transplantation (group A, n = 17; group B, n = 17; group C, n = 13). Overall, 60 patients achieved a complete remission (CR) (66.6%) at some point during their treatment, as defined by the presence of less than 5% blast cells in a standardized bone marrow aspirate along with normalization of peripheral blood cell counts. Table 2 indicates the numbers and relative proportion of patients that achieved a CR after induction therapy, decitabine, or allogenic stem cell transplantation. Most patients with intermediate and high CXCR4 expression achieved a CR after allogenic stem cell transplantation and not after induction therapy (Table 2).
Statistical analysis
Statistical analysis was performed using the Solaris SAS 8.2 software (SAS, Heidelberg, Germany). Survival times and relapse-free survival (RFS) calculated from the time of diagnosis were plotted with the use of the Lifetest procedure for Kaplan-Meier estimates. For Kaplan-Meier estimates graphs, the GraphPadPrism version 3.0 (GraphPad Software, San Diego, CA) software package for Windows was used. The OS was calculated from the date of first diagnosis to date of death. The RFS was calculated from the date of first diagnosis to date of relapse and included antecedent CR. The significance of difference between survival curves was calculated by log-rank test. Groupwise comparisons of the distributions of variables were performed with the generalized Wilcoxon test. Cox regression was used to identify differences in survival due to prognostic factors. P values below .05 were considered significant. As possible prognostic factors, we included age, sex, WBC count, cytogenetics, serum lactate dehydrogenase (LDH), platelets, and CXCR4 expression levels as MFIRs.
Results
Detection of CXCR4 by flow cytometry
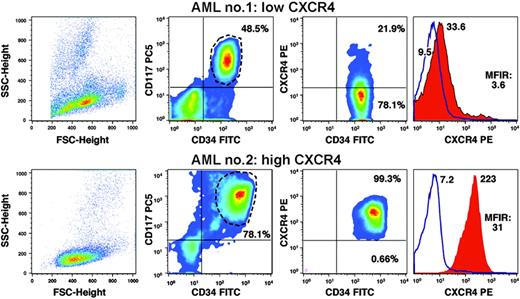
We examined for CXCR4 surface expression in the CD34+ and/or CD117+ fraction of AML cells. Representative samples of high or low CXCR4 expression by AML cells are displayed in Figure 1. Using flow cytometry, we observed a continuum in the levels of CXCR4 expressed by AML cells in the 90 samples, ranging from MFIR values of 1.2 to 73.4 (Table 1) In 32 samples (36%), the CD34+ and/or CD117+ AML cells displayed CXCR4 expression levels below the MFIR threshold of 5; 26 samples (29%) had an intermediate CXCR4 expression with MFIR values between 5 and 10; and 32 patients (36%) exceeded the MFIR threshold of 10. As such, we assigned 3 groups with low, intermediate, or high CXCR4 expression to these threshold levels.
Determination of CXCR4 expression by flow cytometry. The graph displays pseudocolor dot plots of 2 different AML samples with either low CXCR4 surface expression on CD34+/CD117+ AML cells (top row) or high CXCR4 expression (bottom row). The left-hand boxes display the relative size (forward scatter) and granularity (sideward scatter) characteristics of the patients' samples. The second column of boxes displays the relative fluorescence intensities of the AML cell samples stained with fluorescence-labeled anti-CD34 and anti-CD117 antibodies, as displayed on the horizontal and vertical axes, respectively. This allows for gating on the CD34+/CD117+ AML cells, as indicated by the gate drawn around the CD34+/CD117+ population. The percentage of CD34+/CD117+ cells is indicated above each of the populations. The third column of boxes displays relative fluorescence intensities for CD34 and CXCR4, as indicated on the horizontal and vertical axes. The relative proportion of CXCR4-positive and CXCR4-negative cells is displayed next to each of these populations. The right-hand boxes display overlay histogram plots that depict the relative CXCR4 fluorescence intensity of the CD34+/CD117+ AML cells (red-shaded histogram) in comparison with the isotype control stain (blue line). The mean fluorescence intensities are displayed next to each histogram. Moreover, the mean fluorescence intensity ratio (MFIR) for CXCR4 is displayed.
Determination of CXCR4 expression by flow cytometry. The graph displays pseudocolor dot plots of 2 different AML samples with either low CXCR4 surface expression on CD34+/CD117+ AML cells (top row) or high CXCR4 expression (bottom row). The left-hand boxes display the relative size (forward scatter) and granularity (sideward scatter) characteristics of the patients' samples. The second column of boxes displays the relative fluorescence intensities of the AML cell samples stained with fluorescence-labeled anti-CD34 and anti-CD117 antibodies, as displayed on the horizontal and vertical axes, respectively. This allows for gating on the CD34+/CD117+ AML cells, as indicated by the gate drawn around the CD34+/CD117+ population. The percentage of CD34+/CD117+ cells is indicated above each of the populations. The third column of boxes displays relative fluorescence intensities for CD34 and CXCR4, as indicated on the horizontal and vertical axes. The relative proportion of CXCR4-positive and CXCR4-negative cells is displayed next to each of these populations. The right-hand boxes display overlay histogram plots that depict the relative CXCR4 fluorescence intensity of the CD34+/CD117+ AML cells (red-shaded histogram) in comparison with the isotype control stain (blue line). The mean fluorescence intensities are displayed next to each histogram. Moreover, the mean fluorescence intensity ratio (MFIR) for CXCR4 is displayed.
Clinical and laboratory characteristics of the patients
| Characteristic . | Value . |
|---|---|
| No. of patients | 90 |
| Sex, no. | |
| Male | 54 |
| Female | 36 |
| Median age, y (range) | 62.5 (18-93) |
| FAB classification, no. | |
| M0 | 7 |
| M1 | 10 |
| M2 | 12 |
| M3 | 1 |
| M4 | 27 |
| M5 | 7 |
| M6 | 0 |
| M7 | 2 |
| MDS | 7 |
| Undefined by FAB | 17 |
| Cytogenetic abnormality, no. | |
| Favorable | 7 |
| Intermediate | 47 |
| Unfavorable | 26 |
| Unknown | 10 |
| Median WBC count, × 109/L (range) | 13.5 (0.5-300) |
| Median LDH level, U/L (range) | 328 (140-2760) |
| Median platelet count, × 109/L (range) | 70 (4-265) |
| Median % of CXCR4+ blasts (range) | 61.2 (4.7-100) |
| Median CXCR4, MFIR (range) | 6.9 (1.2-73.4) |
| Median time to follow-up, mo (range) | 13 (0-55) |
| Characteristic . | Value . |
|---|---|
| No. of patients | 90 |
| Sex, no. | |
| Male | 54 |
| Female | 36 |
| Median age, y (range) | 62.5 (18-93) |
| FAB classification, no. | |
| M0 | 7 |
| M1 | 10 |
| M2 | 12 |
| M3 | 1 |
| M4 | 27 |
| M5 | 7 |
| M6 | 0 |
| M7 | 2 |
| MDS | 7 |
| Undefined by FAB | 17 |
| Cytogenetic abnormality, no. | |
| Favorable | 7 |
| Intermediate | 47 |
| Unfavorable | 26 |
| Unknown | 10 |
| Median WBC count, × 109/L (range) | 13.5 (0.5-300) |
| Median LDH level, U/L (range) | 328 (140-2760) |
| Median platelet count, × 109/L (range) | 70 (4-265) |
| Median % of CXCR4+ blasts (range) | 61.2 (4.7-100) |
| Median CXCR4, MFIR (range) | 6.9 (1.2-73.4) |
| Median time to follow-up, mo (range) | 13 (0-55) |
Favorable includes t(8;21), t(15,17), inv 16; intermediate, normal karyotype, other; and unfavorable, −5, −7, t(6;9), (11q23) abn., complex.
Association between CXCR4 expression and clinical data
The characteristics of all AML patients are displayed in Table 1. Compared with the AML patient population evaluated by Rombouts et al,33 our patient population was older and had a higher proportion of patients with unfavorable cytogenetics. In Table 2, we display the patients' characteristics for each of the 3 different CXCR4 expression groups (low, intermediate, high). We noticed higher WBC counts and LDH and a higher frequency of blast persistence, relapses, and deaths in patients with higher CXCR4 expression (groups B, C) and a shorter overall and relapse-free survival when compared with patients with low CXCR4 expression (group A).
Clinical and laboratory characteristics of the AML patients when categorized by CXCR4 expression according to low (group A), intermediate (group B), or high (group C) CXCR4 expression
| . | Group A . | Group B . | Group C . | Groups B and C . |
|---|---|---|---|---|
| No. of patients | 32 | 26 | 32 | 58 |
| Prognostic markers | ||||
| Median CXCR4, MFIR (range) | 2.8 (1.2-4.9) | 6.9 (5.1-9.4) | 17.2 (10.3-73.4) | 11.29 (5.1-73.4) |
| Median age, y (range) | 62.0 (21-89) | 59.5 (18-81) | 65.0 (32-93) | 62.5 (18-93) |
| Median WBC count, × 109/L (range) | 4.0 (0.5-124.0) | 14.9 (0.8-300) | 25.2 (0.6-240) | 24.4 (0.8-300) |
| Median platelet count, × 109/L (range) | 56.0 (8-211) | 81.0 (10.0-265) | 82.5 (4-218) | 82 (4-265) |
| Median LDH level, U/L (range) | 251.5 (140-2637) | 380.0 (187-1816) | 357.5 (144-2760) | 376.5 (144-2760) |
| Unfavorable cytogenetics (%) | 6 of 28 (21.43) | 12 of 25 (48.0) | 8 of 27 (29.63) | 20 of 52 (38.46) |
| Variable, no. (%) | ||||
| Cytarabine ± anthracycline-based induction | 18 (56.25) | 19 (73.08) | 14 (43.75) | 33 (56.90) |
| Decitabine | 6 (18.75) | 2 (7.69) | 5 (16.63) | 7 (12.07) |
| Other cytoreductive therapy | 5 (16.63) | 4 (15.38) | 9 (28.13) | 13 (22.41) |
| No therapy | 3 (9.38) | 1 (3.85) | 4 (12.50) | 5 (8.62) |
| Allogenic SCT | 17 (53.13) | 17 (65.38) | 13 (40.63) | 30 (51.72) |
| CR | 25 (78.13) | 19 (73.08) | 16 (50) | 35 (60.34) |
| CR after induction | 13 (40.63) | 7 (26.92) | 4 (12.5) | 11 (18.97) |
| CR after double induction | 0 (0) | 0 (0) | 1 (3.13) | 1 (1.72) |
| CR after low-dose decitabine | 1 (3.13) | 0 (0) | 1 (3.13) | 1 (1.72) |
| CR after allogenic SCT | 11 (34.38) | 12 (46.15) | 10 (31.25) | 22 (37.93) |
| Primary refractory | 5 of 18 (27.78) | 12 of 19 (63.16) | 10 of 14 (71.43) | 22 of 33 (66.66) |
| Relapses | 4 of 25 (16.00) | 12 of 19 (63.16) | 8 of 16 (50) | 20 of 35 (57.14) |
| Total deaths | 10 (31.25) | 17 (65.38) | 20 (62.50) | 37 (63.79) |
| Survival | ||||
| Mean overall survival, mo (SEM) | 24.3 (2.9) | 17.4 (3.4) | 12.8 (2.0) | 15.5 (2.2) |
| Mean relapse-free survival, mo (SEM) | 24.2 (2.4) | 18.7 (3.8) | 11.5 (1.4) | 19.3 (3.0) |
| . | Group A . | Group B . | Group C . | Groups B and C . |
|---|---|---|---|---|
| No. of patients | 32 | 26 | 32 | 58 |
| Prognostic markers | ||||
| Median CXCR4, MFIR (range) | 2.8 (1.2-4.9) | 6.9 (5.1-9.4) | 17.2 (10.3-73.4) | 11.29 (5.1-73.4) |
| Median age, y (range) | 62.0 (21-89) | 59.5 (18-81) | 65.0 (32-93) | 62.5 (18-93) |
| Median WBC count, × 109/L (range) | 4.0 (0.5-124.0) | 14.9 (0.8-300) | 25.2 (0.6-240) | 24.4 (0.8-300) |
| Median platelet count, × 109/L (range) | 56.0 (8-211) | 81.0 (10.0-265) | 82.5 (4-218) | 82 (4-265) |
| Median LDH level, U/L (range) | 251.5 (140-2637) | 380.0 (187-1816) | 357.5 (144-2760) | 376.5 (144-2760) |
| Unfavorable cytogenetics (%) | 6 of 28 (21.43) | 12 of 25 (48.0) | 8 of 27 (29.63) | 20 of 52 (38.46) |
| Variable, no. (%) | ||||
| Cytarabine ± anthracycline-based induction | 18 (56.25) | 19 (73.08) | 14 (43.75) | 33 (56.90) |
| Decitabine | 6 (18.75) | 2 (7.69) | 5 (16.63) | 7 (12.07) |
| Other cytoreductive therapy | 5 (16.63) | 4 (15.38) | 9 (28.13) | 13 (22.41) |
| No therapy | 3 (9.38) | 1 (3.85) | 4 (12.50) | 5 (8.62) |
| Allogenic SCT | 17 (53.13) | 17 (65.38) | 13 (40.63) | 30 (51.72) |
| CR | 25 (78.13) | 19 (73.08) | 16 (50) | 35 (60.34) |
| CR after induction | 13 (40.63) | 7 (26.92) | 4 (12.5) | 11 (18.97) |
| CR after double induction | 0 (0) | 0 (0) | 1 (3.13) | 1 (1.72) |
| CR after low-dose decitabine | 1 (3.13) | 0 (0) | 1 (3.13) | 1 (1.72) |
| CR after allogenic SCT | 11 (34.38) | 12 (46.15) | 10 (31.25) | 22 (37.93) |
| Primary refractory | 5 of 18 (27.78) | 12 of 19 (63.16) | 10 of 14 (71.43) | 22 of 33 (66.66) |
| Relapses | 4 of 25 (16.00) | 12 of 19 (63.16) | 8 of 16 (50) | 20 of 35 (57.14) |
| Total deaths | 10 (31.25) | 17 (65.38) | 20 (62.50) | 37 (63.79) |
| Survival | ||||
| Mean overall survival, mo (SEM) | 24.3 (2.9) | 17.4 (3.4) | 12.8 (2.0) | 15.5 (2.2) |
| Mean relapse-free survival, mo (SEM) | 24.2 (2.4) | 18.7 (3.8) | 11.5 (1.4) | 19.3 (3.0) |
Because patients with intermediate and high CXCR4 expression did not significantly differ in terms of OS and RFS, we combined the data for these patients in the right-hand column labeled “B + C.” Unless stated otherwise, the percentages refer to the absolute number of patients in the respective groups that are displayed in the top row. Cytogenetic results were not available for all patients (only for 28 of 32 patients in group A, 25 of 26 in group B, and 27 or 32 in group C), and the percentages are adjusted accordingly. Patients were considered primary refractory only if they had received cytarabine/anthracycline-based inducion therapy (18 of 32 in group A, 19 of 26 in group B, and 14 of 32 in group C), and the percentages refer to these numbers. Moreover, patients were considered to be relapsed only if they had achieved a prior CR (25 of 32 in group A, 19 of 26 in group B, and 16 of 32 in group C).
High and intermediate CXCR4 expression on AML cells is associated with a decreased overall survival and relapse-free survival
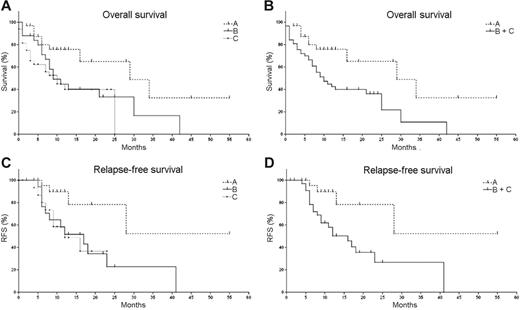
To determine the prognostic impact of CXCR4 expression on AML cells, we evaluated the overall survival (OS) (90 patients) and relapse-free survival (RFS) (60 patients) of all included prognostic parameters using the Kaplan-Meier procedure. Figure 2 displays Kaplan-Meier curves of overall and relapse-free survival estimates, respectively, for groups A through C based upon the CXCR4 expression by the AML cells. The significance of Kaplan-Meier curves was verified by the log-rank test and the generalized Wilcoxon test (Table 3) While sex, age, and platelet counts did not reach statistical significance, unfavorable cytogenetics, WBC, LDH, and CXCR4 MFIRs correlated with a reduced OS (Table 3). Despite differences in mean OS and RFS, patients with high or intermediate CXCR4 expression did not show significant differences in survival curves.
Impact of CXCR4 expression on overall survival (OS) and relapse-free survival (RFS) in AML patients. These graphs display Kaplan-Meier estimates of OS (A-B) and RFS (C-D) of AML patients based upon CXCR4 expression on the leukemia cells at time of diagnosis. OS and RFS were calculated for patients with low CXCR4 expression as defined by mean CXCR4 fluorescence intensity ratios (MFIRs) of less than 5 (group A, n=32), intermediate CXCR4 expression with MFIRs of 5 to 10 (group B, n=26), or high CXCR4 expression with MFIRs more than 10 (group C, n=32), as displayed in panels A and C, respectively. Because intermediate and high CXCR4 expression were not significantly different regarding OS and RFS, groups B and C were combined in panels B and D.
Impact of CXCR4 expression on overall survival (OS) and relapse-free survival (RFS) in AML patients. These graphs display Kaplan-Meier estimates of OS (A-B) and RFS (C-D) of AML patients based upon CXCR4 expression on the leukemia cells at time of diagnosis. OS and RFS were calculated for patients with low CXCR4 expression as defined by mean CXCR4 fluorescence intensity ratios (MFIRs) of less than 5 (group A, n=32), intermediate CXCR4 expression with MFIRs of 5 to 10 (group B, n=26), or high CXCR4 expression with MFIRs more than 10 (group C, n=32), as displayed in panels A and C, respectively. Because intermediate and high CXCR4 expression were not significantly different regarding OS and RFS, groups B and C were combined in panels B and D.
P values of all prognostic markers for overall survival as determined by log-rank test and generalized Wilcoxon test
| Prognostic marker . | P, log-rank . | P, generalized Wilcoxon . |
|---|---|---|
| Sex | .62 | .28 |
| Age | .45 | .19 |
| Cytogenetics | .04 | .23 |
| WBCs | .02 | .05 |
| LDH | .01 | .02 |
| Platelets | .66 | .97 |
| CXCR4, A/B/C, MFIR | .01 | .02 |
| CXCR4, A/B + C, MFIR | .00 | .01 |
| Prognostic marker . | P, log-rank . | P, generalized Wilcoxon . |
|---|---|---|
| Sex | .62 | .28 |
| Age | .45 | .19 |
| Cytogenetics | .04 | .23 |
| WBCs | .02 | .05 |
| LDH | .01 | .02 |
| Platelets | .66 | .97 |
| CXCR4, A/B/C, MFIR | .01 | .02 |
| CXCR4, A/B + C, MFIR | .00 | .01 |
In this analysis, the impact of CXCR4 expression on overall survival was analyzed for patients with low (group A), intermediate (group B), or high (group C) CXCR4 expression. In addition, we compared patients with low CXCR4 expression to patients with intermediate and high expression (B + C, bottom row).
To define the prognostic significance of the observed correlation between disease outcome and CXCR4 expression as well as the prognostic relevance of other parameters including sex, age, WBC, LDH, and cytogenetic abnormalities, multivariate and univariate Cox regression analysis was performed, and the results are displayed in Table 4In univariate analysis, unfavorable cytogenetics and CXCR4 MFIR groups A through C were significant predictive factors resulting in a reduced OS and RFS, whereas LDH was a significant predictive factor only for OS and WBC only for RFS (Table 4). Cox proportional hazards analysis of CXCR4 expression (as numerical MFIR values) and other prognostic markers as predictors of the time from diagnosis to relapse or death revealed that the hazard ratio associated with CXCR4 was 2.699 (95% confidence interval, 1.327 to 5.489) for OS and 3.853 (95% confidence interval, 1.298 to 11.442) for RFS in univariate analysis. In multivariate Cox regression analysis, only unfavorable cytogenetics and CXCR4 expression by AML cells were significant predictive factors for OS. Considering CXCR4 expression as a risk factor for relapse (P = .01), the presence of other risk factors including cytogenetics lost their impact for risk of relapse (Table 4).
Prognostic impact of CXCR4 and other prognostic variables on overall survival and relapse-free survival
| Variable . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Overall survival* | ||||||
| CXCR4 MFIR | 2.699 | 1.327-5.489 | .006 | 2.007 | 1.044-3.858 | .037 |
| LDH level | 2.436 | 1.152-5.151 | .02 | — | — | — |
| Unfavorable cytogenetics | 2.182 | 1.146-4.154 | .018 | 2.478 | 1.129-5.441 | .024 |
| Relapse-free survival† | ||||||
| CXCR4 MFIR | 3.853 | 1.298-11.442 | .015 | 3.853 | 1.298-11.442 | .015 |
| WBC count | 0.315 | 0.124-0.802 | .015 | — | — | — |
| Unfavorable cytogenetics | 2.283 | 0.986-5.286 | .054 | — | — | — |
| Variable . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Overall survival* | ||||||
| CXCR4 MFIR | 2.699 | 1.327-5.489 | .006 | 2.007 | 1.044-3.858 | .037 |
| LDH level | 2.436 | 1.152-5.151 | .02 | — | — | — |
| Unfavorable cytogenetics | 2.182 | 1.146-4.154 | .018 | 2.478 | 1.129-5.441 | .024 |
| Relapse-free survival† | ||||||
| CXCR4 MFIR | 3.853 | 1.298-11.442 | .015 | 3.853 | 1.298-11.442 | .015 |
| WBC count | 0.315 | 0.124-0.802 | .015 | — | — | — |
| Unfavorable cytogenetics | 2.283 | 0.986-5.286 | .054 | — | — | — |
In this analysis, CXCR4 MFIR values were analyzed as a numeric variable without grouping into low, intermediate, or high expression (groups A to C).
HR indicates hazard ratio; CI, confidence interval; —, not applicable.
n = 90.
n = 60.
Discussion
In this study we found a strong association between the surface expression of CXCR4 in CD34+ and/or CD117+ AML cells and OS and RFS. Patients who had a low CXCR4 expression (MFIR less than 5) had a significantly longer RFS and OS than patients who displayed intermediate or high CXCR4 levels. Multivariate analysis revealed that CXCR4 is a prognostic marker that is independent of other previously established prognostic markers, such as cytogenetic abnormalities, LDH, leukocytosis, or age. These findings confirm the study by Rombouts and colleagues that demonstrated a correlation between CXCR4 expression on AML cells, Flt3 mutational status, and poor outcome.33 However, in contrast to this study, which used frozen AML cell samples that previously had been enriched for AML cells by Ficoll separation and T-cell depletion, we used fresh marrow aspirates and a whole blood lysis method during preparation for flow cytometry. The conditions used in our study are commonly used during routine immunophenotyping of leukemias, demonstrating that CXCR4 staining of AML cells can be incorporated into routine flow cytometry analysis using PE-conjugated 12G5 anti-CXCR4 antibodies. 12G5 is the initially described and most widely used antibody clone to detect CXCR4.34 The data analysis approach is also different between both studies: Rombouts et al33 defined 1% or 20% of CXCR4-positive AML cells coexpressing CD34 as cut-points to define poor prognostic subgroups, whereas this study uses MFIRs to define 3 groups: low, intermediate, or high CXCR4-expressing AMLs. This approach is not affected by differences in gating by individual investigators or by background staining between different AML samples and thus may represent a more standardized approach that should easily be transferable to different laboratories. Furthermore, our patient population had a median age of 62.5 years, and 26 patients had unfavorable cytogenetics, whereas Rombouts et al's patient population was younger (median age, 44 years) and displayed fewer patients with unfavorable cytogenetics (n = 7).33
The cutoff MFIR levels of 5 and 10 to define low, intermediate, and high CXCR4 expression initially were based on the distribution of CXCR4 MIFRs among the samples. No significant difference in OS or RFS was noticed between intermediate or high CXCR4-expressing AML patients by Kaplan-Meier analysis (Figure 2 A,C), and therefore it is questionable whether there is a need for distinguishing between intermediate and high CXCR4. As displayed in Table 2, groups A through C had a clear trend for higher WBCs and shorter OS and RFS, and we therefore recommend the use of these 3 groups for a subsequent, larger study to determine if intermediate versus high CXCR4 expression has a statistically significant prognostic impact in a larger series of patients.
Because of its powerful prognostic value, CXCR4 expression by AML cells may have an important function in the biology of this disease. CXCR4 is functional in AML cells,25,26,35,36 and surface CXCR4 expression, which generally is low compared with lymphoid cells, appears to correlate with functional responses such as chemotaxis.37 CXCR4-dependent engraftment of AML cells in NOD/SCID mice was demonstrated by Tavor and colleagues,28 and this group also reported that the proteolytic enzyme elastase is involved in regulating SDF-1–dependent migration and proliferation of AML cells in vitro and in vivo.38 We recently reported that CXCR4, in cooperation with VLA-4 integrins, mediates spontaneous migration of AML cells beneath marrow stromal cells.26 In addition, we noticed a decreased proliferation of migrated AML cells within the stromal layer, as demonstrated by cell cycle analysis and cell division tracking.26 This suggests that CXCR4 expression by the AML cells favors the enrichment of a more primitive, noncycling subpopulation of AML cells within the stromal layer. These cells may be less susceptible to cytotoxic treatments, and they may represent a reservoir for minimal residual disease (MRD) and/or dormant leukemia progenitors.39 Further experiments are needed to experimentally prove this hypothesis.
CXCR4 antagonists could mobilize AML cells from the protective stromal microenvironments and make them more susceptible to conventional therapy. The general feasibility and safety of such an approach has been demonstrated by recent studies in which CXCR4 antagonists were administered to patients or volunteers.24,40,41 Stroma-mediated AML cell protection from chemotherapy can partially be reversed by CXCR4 antagonists in vitro.42 As such, a clinical trial with administration of a CXCR4 antagonist in an attempt to mobilize AML cells from their protective environment prior to conventional therapy may be feasible in the near future, even though an increased toxicity to normal hematopoiesis due to simultaneous progenitor cell mobilization is a concern.
Collectively, our data indicate that CXCR4 surface expression, as determined by flow cytometry, is an important prognostic marker in AML. CXCR4 expression therefore should be incorporated into the initial diagnostic work-up of AML patients treated within clinical trials. Confirmation of these data in a larger series of patients may lead to the incorporation of this marker into the routine diagnostic work-up and into risk-stratified treatment strategies for patients with AML.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: A.C.S. collected and analyzed data and prepared the tables; M.L. contributed patient data and data analysis; W.G.W. contributed to data analysis and review of the manuscript; and J.A.B. designed and supervised the study and wrote the manuscript.
Acknowledgments
We thank Jürgen Schulte-Mönting, Institute of Medical Biometry and Informatics (IMBI), Albert Ludwigs University, Freiburg, for statistical analysis of the data and thank U. Habig-Buchwald, R. Fröhlich, M. Riedl, and C. Ströhlein for expert technical assistance with flow cytometry. We are also grateful to Hans Schall for outstanding support with the analysis of treatment data and to Elihu Estey for valuable suggestions and critically reviewing the manuscript.
This work was supported by Deutsche Krebshilfe grant no. 10-1688-Bu (J.A.B.) and Deutsche José Carreras Leukämiestiftung grant no. DJCLS R02/08 (J.A.B.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal