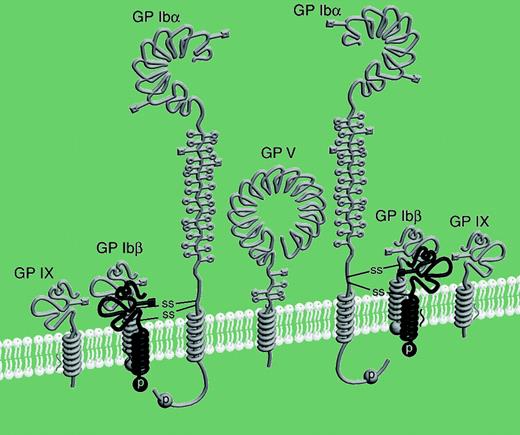
In this issue, Luo and colleagues show that the platelet GP Ib complex (GP Ib-V-IX) has a GP Ibα/GP Ibβ stoichiometry of 1:2 rather than 1:1 as previously thought, a result that has consequences for GP Ib complex structure and its role in platelet function.
Blood rarely publishes an article on the stoichiometry of the subunits in a membrane receptor complex, but it is particularly justified in doing so in this case because ideas dating back around 20 years have been successfully challenged. There are good reasons why a 1:1 model for GP Ibα/GP Ibβ should have stood for so long, mainly because monoclonal antibodies recognizing the extracellular domain of GP Ibβ were missing, making quantitation difficult. Size assessed on gels was not sufficiently precise to differentiate the 2 possibilities. The second, neighboring, Cys residue on GP Ibα (C484,C485) was explained away as being buried in the outer zone of the membrane lipids and therefore not accessible. Now that there is convincing evidence for 2 GP Ibβs and for a reactive second Cys on GP Ibα, the question of how this may affect the interpretation of the GP Ib complex's function can be addressed. The GP Ib complex (GP Ib-V-IX) is one of the major receptors on platelets (see figure) and has a role in many aspects of platelet function, particularly platelet activation by von Willebrand factor and thrombin. While the ligand-binding sites lie on the outer domains of GP Ibα, the other subunits, such as GP Ibβ, and the cytoplasmic domain of GP Ibα anchor the complex to the cytoskeleton and, when clustered, activate signaling pathways. It remains unclear whether all GP Ib complexes are identical and behave and signal in the same way. Some evidence for differences comes from studies of cytoskeletal association that indicate that only about 75% is anchored, whereas about 25% is free in the membrane.1 Studies on high-affinity thrombin receptors, in which GP Ib is implicated, suggest that only a few dozen receptors are involved.2 These new data about the structure of the complex provide possible mechanisms for these differences that need to be followed up. Thus, there may be small populations containing disulfide-linked GP Ibα dimers via the second Cys residue, as suggested by autoantibodies in a patient that detect a 210-kDa band containing GP Ibα.3 The presence of disulfide isomerases in platelet membranes might also offer the possibility of disulfide switching as a mechanism following platelet activation.4
Schematic drawing of the GP Ib-IX-V complex with the revised stoichiometry. See the complete figure in the article beginning on page 603.
Schematic drawing of the GP Ib-IX-V complex with the revised stoichiometry. See the complete figure in the article beginning on page 603.
Although deletion or mutation of both Cys484 and Cys485 in GP Ibα would lead to Bernard-Soulier syndrome, a natural mutation of only one of these would not. Whether it would cause a clinical problem is an interesting question and would depend on the validity of some of the just-mentioned speculation. However, the issue could be investigated by expression of mutated GP Ibα in a GP Ibα knock-out mouse.
The presence of 2 GP Ibβ subunits rather than 1 may also help to explain how this complex is able to withstand the forces involved in platelet rolling. When platelets interact with exposed subendothelium or inflamed endothelium, they need to be slowed by formation of membrane tethers that extend from the platelet surface and either break off or pull on the platelet cytoskeleton, bringing the platelet into intimate contact with the subendothelium/inflamed endothelium and allowing activated integrins to ensure strong binding.5 The receptor on the platelet tether is GP Ib, and it needs to be securely anchored in both the cytoskeleton and the membrane to withstand the forces applied to it without being pulled out of the platelet. Having 2 GP Ibβ subunits attached undoubtedly makes the anchoring more secure and probably facilitates signaling via associated molecules such as 14.3.3ζ. Just as an aircraft landing on an aircraft carrier needs a hook (GP Ib) and a catch wire (von Willebrand factor) to stop in an appropriately short distance, it is probably more effective if the hook is held on with 3 bolts rather than 2! Until we have some sort of high-resolution picture of the whole GP Ib-V-IX complex as it exists and functions in the platelet membrane, there are all sorts of difficult problems pertaining to how the individual components fit together. Developing a better idea of the stoichiometry of the complex is one step along the way.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ▪