Abstract
Chronic graft-versus-host disease (cGvHD) is associated with functional immunodeficiency and an increased risk of opportunistic infections in allogeneic bone marrow transplantation (BMT). We used a parent to F1 model of allogeneic BMT to test the hypothesis that cGvHD leads to impaired antigen-specific antiviral immunity and compared BM transplant recipients with cGvHD to control groups of allogeneic BM transplant recipients without GvHD. Mice with and without cGvHD received a nonlethal dose of murine cytomegalovirus (MCMV) +100 days after transplantation. Recipients with cGvHD had more weight loss and higher viral loads in the spleen and liver. MCMV infection led to greater than 25-fold expansion of donor spleen–derived MCMV peptide–specific tetramer-positive CD8+ T cells in blood of transplant recipients with and without cGvHD, but mice with cGvHD had far fewer antigen-specific T cells in peripheral tissues and secondary lymphoid organs. The immunosuppression associated with cGvHD was confirmed by vaccinating transplant recipients with and without cGvHD using a recombinant Listeria expressing MCMV early protein (Lm-MCMV). Secondary adoptive transfer of lymphocytes from donor mice with or without cGvHD into lymphopenic congenic recipients showed that cGvHD impaired tissue-specific homing of antigen-specific T cells. These results indicate that cGvHD causes an intrinsic immunosuppression and explain, in part, the functional immunodeficiency in allogeneic transplant recipients.
Introduction
Allogeneic bone marrow transplantation (BMT) can cure patients with relapsed or refractory hematologic malignancies through establishment of a functional immune system from a genetically dissimilar donor with resultant graft-versus-tumor (GvT) effect. Donor T cells are typically responsible for the beneficial GvT effect and protect against opportunistic posttransplantation infections1 but are also responsible for acute and chronic graft-versus-host disease (cGvHD).2–5 cGvHD occurs in approximately 60% to 80% of long-term survivors of allogeneic BMT5 and is a major cause of morbidity and mortality.6 The development of cGvHD has a negative influence on reconstitution of T-cell function that contributes to severe posttransplantation immunodeficiency.7 The detailed mechanisms responsible for immunodeficiency associated with cGvHD, including the relative paucity of T cells in lymphoid organs, are still poorly understood
Previously, several strategies have been described to prevent and treat cGvHD complications in allogeneic BMT patients by depleting or impairing the function of donor T cells.8–10 The depletion of T cells from the donor graft reduces the incidence of both acute and cGvHD but increases the risk of opportunistic infection and the rates of posttransplantation relapse. Among patients receiving the T-cell depleted (TCD) graft, cytomegalovirus (CMV) pneumonia may develop in up to 50% of BM transplant recipients with virus reactivation.11 Polymerase chain reaction (PCR)–guided pre-emptive ganciclovir (GCV) therapy reduces viral load and the frequency of CMV disease12 but progressive CMV disease may develop in patients with severely impaired immune function. Furthermore, prophylactic and pre-emptive GCV therapy is associated with myelosuppression, bacterial and fungal infections, as well as late CMV disease.12,13 Therefore, to improve transplantation outcomes for patients with cGvHD, who are at risk for posttransplantation CMV and other opportunistic infections, a better understanding of the relationship among the opportunistic infections, cGvHD, and immunodeficiency is needed.
In this study, we used a murine model of MHC-mismatched allogeneic BMT coupled with a late posttransplantation CMV infection to investigate the relationship between cGvHD and antiviral immunity. We established a clinically relevant experimental allogeneic BMT model in which recipients of a sublethal dose of untreated donor splenocytes (3 × 106/mouse) developed clinical signs compared with control transplant recipients treated with ex vivo amotosalen-treated donor splenocytes (10 × 106/mouse) and recipients of TCD BM alone, which developed no GvHD.14,15 We found that recipients with cGvHD had impaired immune response to murine CMV (MCMV) with higher viral loads, impaired homing of antigen-specific antiviral T cells to target tissues, and fewer numbers of antigen-specific T cells.
Materials and methods
Mice
CB6F1 (C57BL/6 X BALB/c)(H-2b/d, CD45.2/Thy1.2) mice and PepBoy (B6.SJL-PtprcaPep3b/BoyJ)(H-2b, CD45.1/Thy1.2) mice on the C57BL/6 background were obtained from Jackson Laboratories (Bar Harbor, ME). BA mice (H-2b, CD45.2/Thy1.1) on the C57BL/6 background were bred at Emory.16 Procedures conformed to National Institutes of Health (NIH) animal care guidelines and were approved by the Emory University Institutional Animal Care and Use Committee (IACUC).
Preparation of lymphocytes for adoptive immunotherapy
Donor splenocytes from previously MCMV-infected PepBoy mice were harvested as previously described and enriched by removing plastic adherent cells.14,15 A dose of 3 × 106/0.2 mL/mouse nonadherent untreated live nucleated cells was used to induce cGvHD. Control mice with donor splenocyte–derived T-cell chimerism but lacking cGvHD received 10 × 106 nonadherent donor splenocytes treated with 2 nM amotosalen (S-59 psoralen; Cerus, Concord, CA).15
Irradiation, cell transfer, and MCMV infection of BM transplant recipients
On day −1, CB6F1 mice received a total of 11 Gy irradiation divided into 2 doses, 5.5 Gy 3 hours apart.17 The following day, BM was flushed from femora and tibiae of naive BA donor mice and CD3+ T cells were depleted (TCD) as described.17 TCD BM cells (5 × 106) were transplanted alone or with 3 × 106 untreated (+GvHD) and 10 × 106 amotosalen-treated (non-GvHD control) donor splenocytes via tail vein injection into irradiated CB6F1 recipient mice. Recipient mice were infected with 2.5 × 104 plaque-forming units (PFUs) MCMV intraperitoneally or vehicle between 100 and 133 days after transplantation (no major differences were observed in the physical and immunologic parameters among the mice with cGvHD when assessed at 100 to 150 days after transplantation). The development of clinical cGvHD was monitored twice weekly by measuring weight loss and clinical signs of hair loss, ruffled fur, diarrhea, and decreased activity.17 According to our IACUC protocol, moribund mice and animals with greater than 25% weight loss were euthanized and considered to have died on the day following euthanasia for analysis of posttransplantation survival.
Lymphocytes isolation from organs of BM transplant recipients
Blood samples were collected in tubes containing 50 μL heparin from the tail vein of transplant recipients with and without GvHD on days 0, 3, 10, 22, and 40 after MCMV infection, and red blood cells (RBCs) were lysed.18 Splenocytes, liver-cell suspensions, and thymocytes were harvested and single-cell suspensions were prepared from transplant recipients as previously described.18,19
Determination of viral load per liver and spleen
Liver and spleen viral loads were determined as described by Hossain et al.20 Briefly, liver and spleen were aseptically collected from the MCMV-infected recipients, homogenized, and centrifuged. Serial diluted supernatants were added on a 3T3 confluent monolayer of 24 tissue culture plates and incubated for 90 minutes at 37°C overlayered with 1 mL 2.5% methylcellulose in DMEM. MCMV PFUs were directly counted after 4 days under a light microscope (Nikon, Melville, New York) after staining the 3T3 confluent monolayer with methylene blue.
Flow cytometry
The origin of CD8+ and CD4+ T cells in BM transplant recipients was determined by staining with monoclonal antibodies (mAbs) specific for donor BM (Thy1.1+, CD45.2+, Thy1.2−), donor spleen (Thy1.2+, CD45.2−, CD45.1+), or host T cells (CD45.2+, Thy1.2+) in combination with mAbs to CD8 and CD4 and other surface activation markers (Pharmingen, San Jose, CA) as previously described.17 Anti-MCMV–specific CD8+ T cells were counted using an APC-conjugated HGIRNASFI-H-2Db tetramer (NIAID Tetramer Core Facility, Atlanta, GA).21 Intracellular IFN-γ– and TNF-α–producing CD8+ T cells were determined by using a Cytofix/Cytoperm kit purchased from BD Pharmingen (San Jose, CA). The cells were acquired by FACS Aria (Becton Dickinson, San Jose, CA) and analyzed by FlowJo software (Ashland, OR).
Assessment of cGvHD
Transplant recipients were monitored for weight loss, hair loss, eczematoid rash and dry skin in tail and ears, and motor activity twice weekly for the first 30 days, and then weekly. Additionally, cGvHD was scored through histologic analysis of tissue sections as described.17,22 Three to 5 mice from each treatment group were killed at predetermined time points and skin, liver, and intestine were collected in formalin. Chronic GvHD scores were assigned by trained pathologists unaware of the treatment group (B.P.P. and D.L.J.) using previously reported criteria17,22 and × 40 microscopy of formalin-fixed histologic tissue sections. The microscope was a Nikon Eclipse E400, with a 20×/0.75 Plan Apo objective lens. The camera was a SPOT Insight 3.2.0, and we acquired images with SPOT Advanced 4.1 software. The slides were stained with hematoxylin and eosin, and no imaging medium or solution was used. Scores (0-5) were based on the presence of lymphocyte infiltration in the liver periportal region, the presence of apoptotic cells in the intestinal epithelium along with foci of epithelial ulceration, and the presence of sclerodermoid sclerosis or fibrosis in the skin. The results for each mouse were tabulated and averaged by the first author (M.S.S.).
Assessment of the ability of antiviral T cells to home to sites of MCMV infection
Donor lymphocytes were harvested, using Lympholyte-Mammal (Cedarlane, Hornby, ON, Canada), from the pooled blood of 2 groups of 5 mice with or without cGvHD: either CB6F1 mice that had previously received transplants of 3 × 106 allogeneic Thy1.2+CD45.1+ C57BL/6 splenocytes and 5 × 106 TCD BM (cGvHD+ lymphocytes) or C57BL/6 mice that had previously received transplants of 10 × 106 Thy1.2+ CD45.1+ congenic donor splenocytes and 5 × 106 TCD BM (cGvHD− lymphocytes). Both groups of lymphocyte donors were infected with 2.5 × 104 PFUs MCMV intraperitoneally 10 days before the lymphocytes were collected (day 100 after transplantation). Secondary recipients (congenic Thy1.2+ CD45.2+ C57BL/6 mice) were irradiated and received transplants of 5 × 106 TCD BM syngenic BM cells in combination with 3 × 106 congenic blood lymphocytes from either cGvHD+ or cGvHD− donors. The secondary recipients were then infected with 3 × 103 PFUs MCMV intraperitoneally the following day. Nucleated cells from peripheral-blood mononuclear cells (PBMCs) and spleen and liver were harvested from the secondary recipients on 3 and 10 days after viral infection, as described previously.19 T cells derived from the transferred cGvHD+ or cGvHD− lymphocytes (Thy1.2+ CD45.2−) and donor lymphocyte–derived MCMV peptide–specific tetramer-positive CD8+ T cells per mL blood, gram of liver, or spleen were counted using flow cytometry.19
Immunization with an MCMV vaccine
Mice were vaccinated intraperitoneally with 1 × 106 CFUs Lm-MCMV, a Listeria monocytogenesis that has been rendered nonpathogenic by knockout of bacterial genes associated with virulence23 and engineered to express the MCMV H-2b–immunodominant peptide HGIRNASFI.21 The vaccine is prepared and supplied by Cerus. Nonvaccinated mice were killed for baseline data; vaccinated mice were killed 7 days after vaccination. Lymphocytes were harvested from blood, liver, and spleen, and vaccine-induced MCMV-specific CD8+ T cells were assayed using MCMV-HGIRNASFI peptide–specific tetramer and fluorescence-activated cell sorter (FACS) analysis.
Statistical analyses
Student t test was used to compare mean values for parameters of mice. Differences were considered significant when P values below .05 were obtained.
Results
The effects of MCMV infection on transplant recipients with cGvHD
Our overall goal was to investigate the mechanism of immune deficiency observed in allogeneic BM transplant recipients with cGvHD in the context of CMV infection. We established an experimental mouse allogeneic BMT model of parent to F1 transplant recipients in which lethally irradiated CB6F1 recipients received transplants of 3 × 106 allogeneic splenocytes from MCMV-immunized donors along with 5 × 106 TCD BM. These mice developed cGvHD that has a similar clinical presentation to that which develops in BMT patients more than 100 days after transplantation. Greater than 80% of mice with this form of cGvHD typically survived for more than 100 days after transplantation; mice with severe cGvHD were euthanized per IACUC guidelines.14,15 While the severity of cGvHD in mice surviving to day +100 was generally not life threatening, these mice nonetheless had developed hair loss and eczematoid rash and dry skin in tail and ears with decreased general activity compared with control recipients of amotosalen-treated splenocytes or TCD BM alone that did not develop GvHD. Mice with cGvHD surviving to day +100 had a mean weight of 20.3 ± 3.1 g (baseline 27.5 ± 2.1 g) compared with recipients of TCD BM alone (day +100 weight 26.6 ± 1.2 g; baseline weight 28.1 ± 1.2 g) and control mice without GvHD that had received amotosalen-treated donor splenocytes (day +100 weight 30.0 ± 1.7 g; baseline weight 29.6 ± 1.9 g). These differences were highly significant, comparing the day +100 weights between mice with cGvHD to recipients of TCD BM alone or BM plus treated splenocytes (P < .001).
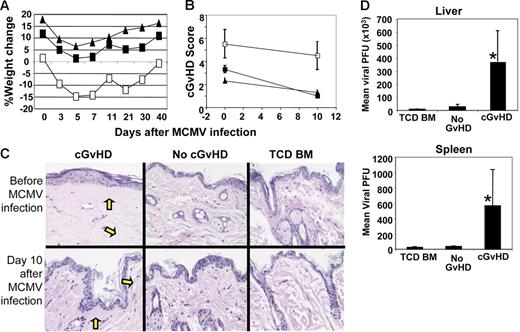
To investigate the antiviral immune responses in recipients with or without cGvHD, mice were infected with a sublethal dose (5 × 104 PFU/mouse) of MCMV between day 100 and day 133 after transplantation. While all recipients challenged with this dose of MCMV survived until 40 days after infection, recipients with cGvHD suffered greater weight loss than the recipients without cGvHD (Figure 1A). To test the effects of CMV infection on the pathophysiology of cGvHD, transplant recipients with cGvHD were killed on the day of MCMV infection and recipients without cGvHD were killed 10 days later. The sum of average cGvHD scores obtained from all 3 organs of recipients with cGvHD was significantly higher (P < .05) in both uninfected and MCMV-infected mice compared with the recipients without cGvHD (Figure 1B). The presence of sclerodermoid sclerosis and fibrosis was found only in the skin of recipients with cGvHD and not in recipients without cGvHD (Figure 1C). Comparing cGvHD histologic scores before and 10 days after MCMV infection, there was no significant exacerbation of cGvHD induced by MCMV infection (Figure 1B).
Evidence of cGvHD and antiviral immune response in recipients with untreated splenocytes. Irradiated CB6F1 recipients of 3 × 106 untreated donor splenocytes (cGvHD), 10 × 106 amotosalen-treated donor splenocytes (no-GvHD–treated splenocyte recipients), or TCD BM alone (no GvHD) were infected with MCMV (5 × 104 PFU intraperitoneally) on 100+ days after transplantation. (A) Percentage weight loss was calculated from the initial weight measured on day 0 of transplantation. Here, day 0 represents the percentage weight loss measured on day 133 after transplantation (□, cGvHD, splenocytes recipients; ▪, no GvHD; and ▴, TCD BM). (B) Recipients with cGvHD, no-GvHD–treated splenocyte recipients, and TCD BM were killed on day 100 after transplantation (day 0 after MCMV infection) and day 10 after 5 × 104 PFU MCMV infection. Skin, liver, and small intestine were collected in formalin solution and cGvHD scores were assessed from the histologic tissue sections of skin, liver, and small intestines. The average mean value and standard deviation of cGvHD scores obtained from the 3 organs of recipients with cGvHD (□), no GvHD (▪), and TCD BM (▴) on days 0 and 10 after MCMV infection. (C) The presence of sclerodermoid sclerosis and fibrosis (yellow arrows) is shown in the histologic slides of skin tissue sections collected from the recipients with cGvHD, no GvHD, and TCD BM under uninfected conditions (day 100 after transplantation) and day 10 after MCMV infection (day 110 after transplantation). The data are representative of 1 of 2 similar experiments and 4 to 5 mice were used per group in each time point. (D) Recipients with cGvHD, no GvHD, and TCD BM alone were infected with MCMV (5 × 104 PFU intraperitoneally) at 100 days after transplantation. Liver and spleen were aseptically collected on day 3 after infection and viral load per organs was determined by spreading the tissue homogenates on a 3T3-cell confluent monolayer as described in “Materials and methods.” Mean viral PFU and standard deviations obtained from each of the infected organs of 5 to 6 mice per group were used (*P < .001, Student t test).
Evidence of cGvHD and antiviral immune response in recipients with untreated splenocytes. Irradiated CB6F1 recipients of 3 × 106 untreated donor splenocytes (cGvHD), 10 × 106 amotosalen-treated donor splenocytes (no-GvHD–treated splenocyte recipients), or TCD BM alone (no GvHD) were infected with MCMV (5 × 104 PFU intraperitoneally) on 100+ days after transplantation. (A) Percentage weight loss was calculated from the initial weight measured on day 0 of transplantation. Here, day 0 represents the percentage weight loss measured on day 133 after transplantation (□, cGvHD, splenocytes recipients; ▪, no GvHD; and ▴, TCD BM). (B) Recipients with cGvHD, no-GvHD–treated splenocyte recipients, and TCD BM were killed on day 100 after transplantation (day 0 after MCMV infection) and day 10 after 5 × 104 PFU MCMV infection. Skin, liver, and small intestine were collected in formalin solution and cGvHD scores were assessed from the histologic tissue sections of skin, liver, and small intestines. The average mean value and standard deviation of cGvHD scores obtained from the 3 organs of recipients with cGvHD (□), no GvHD (▪), and TCD BM (▴) on days 0 and 10 after MCMV infection. (C) The presence of sclerodermoid sclerosis and fibrosis (yellow arrows) is shown in the histologic slides of skin tissue sections collected from the recipients with cGvHD, no GvHD, and TCD BM under uninfected conditions (day 100 after transplantation) and day 10 after MCMV infection (day 110 after transplantation). The data are representative of 1 of 2 similar experiments and 4 to 5 mice were used per group in each time point. (D) Recipients with cGvHD, no GvHD, and TCD BM alone were infected with MCMV (5 × 104 PFU intraperitoneally) at 100 days after transplantation. Liver and spleen were aseptically collected on day 3 after infection and viral load per organs was determined by spreading the tissue homogenates on a 3T3-cell confluent monolayer as described in “Materials and methods.” Mean viral PFU and standard deviations obtained from each of the infected organs of 5 to 6 mice per group were used (*P < .001, Student t test).
To determine whether the presence of cGvHD interfered with antiviral immunity, we next determined the viral loads in the liver and spleen of transplant recipients following intraperitoneal infection with a lethal dose of MCMV. As reported previously, viral loads in the liver and spleen peak 3 days after MCMV infection in normal C57BL/6 mice20 and in the irradiated recipients of allogeneic BM transplants (our unpublished data, October 2004). Tissue homogenates of liver and spleen were prepared on day 3 after infection. Viral loads were 15-fold higher in the spleen and 13-fold higher in the liver among recipients with cGvHD compared with recipients of treated splenocytes without cGvHD (Figure 1D; P < .001). We also found significantly higher viral loads in the lungs of recipients with cGvHD compared with recipients without GvHD 10 days after MCMV infection (data not shown). Based upon the increased viral loads among mice with cGvHD, we then explored potential mechanisms for the relative lack of in vivo antiviral immunity including (1) the lack of antigen-specific T cells; (2) the lack of organ-specific T-cell expansions after MCMV infection; (3) the lack of functional antiviral T cells; and (4) the lack of tissue-specific homing of T cells.
Delayed MCMV infection resulted in extensive peripheral expansion of donor T cells in the blood of recipients with cGvHD
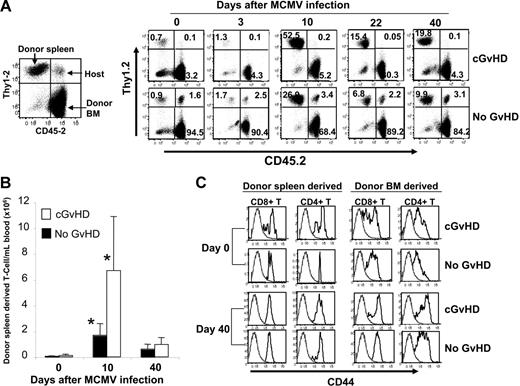
We tested whether increased MCMV viral loads in mice with cGvHD were due to lack of peripheral expansion of antigen-specific donor T cells. Following MCMV infection, antiviral T cells in the blood of mice were enumerated using MCMV peptide–specific class I H-2b tetramer. Surprisingly, more than 50-fold expansion of lymphocyte-gated donor spleen–derived T cells occurred in the blood of recipients with cGvHD and over 25-fold expansion occurred in mice without GvHD that received transplants 10 days after MCMV infection followed by a contraction phase during which T-cell populations in blood returned to baseline levels by day 40 after infection (Figure 2A). Accordingly, the absolute number of donor spleen–derived T cells per mL of blood increased significantly (P < .005) 10 days after MCMV infection, in recipients both with and without cGvHD compared with the uninfected mice. Of note, the number of donor spleen–derived T cells in blood of mice with cGvHD was significantly higher (P < .05) compared with recipients without cGvHD 10 days after infection (Figure 2B). However, in both types of recipient, donor T-cell levels returned to similar baselines by day 40 after MCMV infection (Figure 2B).
Extensive expansion of peripheral donor T cells in recipients with cGvHD after viral infection. Recipients with cGvHD and no GvHD were bled on 133 days after transplantation (day 0 prior to infection) and mice were infected with 5 × 104 PFUs MCMV intraperitoneally. Panel A represents the kinetics of peripheral expansion of donor spleen–derived (Thy1.2+ CD45.1+), host-derived (Thy1.2+ CD45.2+) T cells, as well as a mixture of BM-derived (Thy1.1+ CD45.2+) T cells and host-derived non-T cells. The FACS data shown here represent the same mouse in each group that is representative of 4 to 5 mice in each group. Panel B represents the total number of donor spleen–derived T cells per mL of blood. Data from an average of 4 to 5 mice per group are shown for each time point (*P < .001 for uninfected data vs infected data; *P < .05 for cGvHD vs no GvHD on day 10 after infection). Panel C represents the surface expression of CD44 antigen on the donor spleen– and BM-derived CD8+ and CD4+ T cells that were measured prior to MCMV infection (133 days after transplantation) as shown in top 2 panels and 40 days after MCMV infection as shown in bottom 2 panels from the same mouse of each group. Dotted histogram plot represents the isotype control mAb used for CD44. The data are representative from 1 mouse of each group out of 4 to 5 mice used per group.
Extensive expansion of peripheral donor T cells in recipients with cGvHD after viral infection. Recipients with cGvHD and no GvHD were bled on 133 days after transplantation (day 0 prior to infection) and mice were infected with 5 × 104 PFUs MCMV intraperitoneally. Panel A represents the kinetics of peripheral expansion of donor spleen–derived (Thy1.2+ CD45.1+), host-derived (Thy1.2+ CD45.2+) T cells, as well as a mixture of BM-derived (Thy1.1+ CD45.2+) T cells and host-derived non-T cells. The FACS data shown here represent the same mouse in each group that is representative of 4 to 5 mice in each group. Panel B represents the total number of donor spleen–derived T cells per mL of blood. Data from an average of 4 to 5 mice per group are shown for each time point (*P < .001 for uninfected data vs infected data; *P < .05 for cGvHD vs no GvHD on day 10 after infection). Panel C represents the surface expression of CD44 antigen on the donor spleen– and BM-derived CD8+ and CD4+ T cells that were measured prior to MCMV infection (133 days after transplantation) as shown in top 2 panels and 40 days after MCMV infection as shown in bottom 2 panels from the same mouse of each group. Dotted histogram plot represents the isotype control mAb used for CD44. The data are representative from 1 mouse of each group out of 4 to 5 mice used per group.
Donor spleen– and BM–derived T cells in the blood of recipients with and without cGvHD had differential expression of CD44 surface markers
Previous reports claimed that reactivity of T cells depends on the surface expression of CD62L24 and CD44.25 As most of the antiviral effector T cells undergo apoptosis during the contraction phase after 10 days after infection, the persistence of activated (effector or allo-reactive) T cells and MCMV-specific memory T cells was measured at day 40 after MCMV infection based upon the expression of CD62L and CD44 surface antigens on donor spleen– and BM-derived CD4+ and CD8+ T cells. The expression of CD62L at baseline and at day 40 after infection was similar among CD4+ and CD8+ T cells of either donor spleen or donor BM origins in recipients with and without GvHD (data not shown). In recipients with cGvHD, few donor spleen–derived and a majority of the BM-derived CD8+ and CD4+ T cells were CD44lo on day 0 of viral infection (133 days after transplantation), whereas donor spleen–derived CD8+ and CD4+ T cells in transplant recipients without GvHD were almost all CD44hi. Unlike the donor spleen–derived T cells, donor BM–derived CD8+ and CD4+ T cells were mostly CD44med, a pattern consistent with the naive T-cell phenotypes in recipients both with and without cGvHD (Figure 2C top panels). At 40 days following MCMV infection, following the contraction phase of the immune response, all donor spleen–derived CD8+ and CD4+ T cells were CD44hi (memory phenotype) in recipients both with and without cGvHD. Of note, recipients with cGvHD had a fraction of donor BM–derived CD44lo CD8+ T cells, a type of T cell that may be responsible for cGvHD (Figure 2C, bottom panels).
Anti-CMV tetramer-positive CD8+ T cells increased to equivalent degrees in the blood of recipients with and without cGvHD
To investigate overall T-cell immune reconstitution and antiviral T cells in the peripheral blood of BM transplant recipients with cGvHD, we next determined the absolute numbers of CD4+ T and CD8+ T cells as well as MCMV peptide–specific tetramer-positive CD8+ T cells in the blood following MCMV infection. Interestingly, CD8+ T cells of donor origin significantly expanded in response to MCMV infection in recipients both with and without cGvHD, but no differences were found in the number of host-derived T cells or donor CD4+ T cells between the infected and uninfected recipients (Table 1). Similarly, the absolute number of donor spleen– and BM-derived MCMV peptide–specific tetramer-positive CD8+ T cells per mL of blood was significantly increased in recipients both with and without cGvHD 10 days after MCMV infection compared with the uninfected mice. Moreover, expansion of donor spleen–derived MCMV peptide–specific tetramer-positive CD8+ T cells predominated over BM-derived antiviral T cells in recipients with cGvHD, whereas expansion of donor BM–derived MCMV peptide–specific tetramer-positive CD8+ T cells predominated over donor spleen–derived tetramer-positive T cells in recipients without cGvHD (Table 1). Thus, MCMV infection resulted in significant expansion of CD8+ T cells as well as MCMV peptide tetramer–specific CD8+ T cells in the blood of transplant recipients with and without cGvHD, whereas there was limited expansion of the CD4+ T cells.
Atrophy of primary and secondary lymphoid organs in cGvHD
The spleens of mice with cGvHD (day +100 after transplantation) were atrophic, and splenocytes isolated from these organs failed to proliferate in response to MCMV infection in contrast to brisk proliferation of splenic T cells in response to MCMV infection in recipients without cGvHD (Figure 3A). The thymus was atrophic in recipients with cGvHD, and the number of thymocytes decreased significantly in all mice after MCMV infection compared with uninfected mice (Figure 3B). On the other hand, no differences were found in the number of lymphocytes isolated from the liver of recipients with and without cGvHD both before and after MCMV infection (Figure 3C). Accordingly, the numbers of donor spleen– and BM-derived T-cell populations in the spleen and thymus of recipients with cGvHD were significantly less than in the recipients without GvHD (P < .001; Figure 3D-E,G-H). Of note, donor spleen– and BM-derived T cells were significantly increased in the spleen and decreased in the thymus on day 10 after MCMV infection in recipients without GvHD compared with the uninfected mice (Figure 3D-E,G-H). Again, there were no differences in the numbers of donor spleen– and BM-derived T cells in livers between the recipients with and without cGvHD in both MCMV-infected and uninfected mice (Figure 3F,I).
Splenic and thymic functions were severely suppressed in recipients with cGvHD. Spleen, thymus, and liver were collected from the recipients with and without cGvHD and TCD BM alone prior to MCMV infection (day 100 after transplantation) and day 10 after MCMV infection (day 110 after transplantation). Lymphocytes were isolated and absolute numbers of nucleated cells per organ were measured by counting the live cells under fluorescence microscopy. Panels A, B, and C represent the absolute number of nucleated cells per spleen, thymus, and liver, respectively (▧, recipients with cGvHD; ▪, recipients with no GvHD; and □, recipients of TCD BM). Panels D, E, and F represent the absolute number of donor spleen T cells per spleen, thymus, and liver, respectively (▧, recipients with cGvHD; ▪, recipients with no GvHD). Panels G, H, and I represent the absolute number of donor BM–derived T cells per spleen, thymus, and liver, respectively (▧, mice with cGvHD; ▪, recipients with no GvHD) that were calculated from the data obtained from the FACS analysis. Data represent an average of 5 to 6 mice were used per group (*P < .05 [Student t test] is for the significant decrease compared with the cell numbers in the spleen and thymus of recipients with no GvHD and TCD BM alone).
Splenic and thymic functions were severely suppressed in recipients with cGvHD. Spleen, thymus, and liver were collected from the recipients with and without cGvHD and TCD BM alone prior to MCMV infection (day 100 after transplantation) and day 10 after MCMV infection (day 110 after transplantation). Lymphocytes were isolated and absolute numbers of nucleated cells per organ were measured by counting the live cells under fluorescence microscopy. Panels A, B, and C represent the absolute number of nucleated cells per spleen, thymus, and liver, respectively (▧, recipients with cGvHD; ▪, recipients with no GvHD; and □, recipients of TCD BM). Panels D, E, and F represent the absolute number of donor spleen T cells per spleen, thymus, and liver, respectively (▧, recipients with cGvHD; ▪, recipients with no GvHD). Panels G, H, and I represent the absolute number of donor BM–derived T cells per spleen, thymus, and liver, respectively (▧, mice with cGvHD; ▪, recipients with no GvHD) that were calculated from the data obtained from the FACS analysis. Data represent an average of 5 to 6 mice were used per group (*P < .05 [Student t test] is for the significant decrease compared with the cell numbers in the spleen and thymus of recipients with no GvHD and TCD BM alone).
Mice with cGvHD lack functional antiviral T cells in the spleen and liver
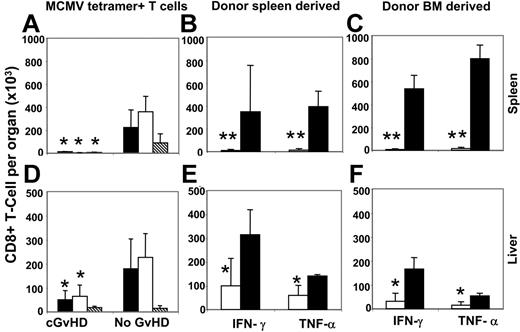
MCMV peptide–specific tetramer-positive donor antiviral CD8+ T cells increased considerably in blood 11 days after MCMV infection in recipients with cGvHD, but they failed to control viral replication in the liver and spleen (Table 1; Figure 1D). To investigate the possible causes of the failure of antiviral activities in these target organs, we estimated organ-specific antiviral T cells using tetramer staining on day 10 after viral infection. Significantly lower frequencies of both donor spleen– and BM-derived MCMV-specific antiviral CD8+ T cells were found in the spleen and liver of recipients with cGvHD compared with the recipients without cGvHD (Figure 4A,D).
Liver and spleen antiviral T-cell responses were significantly decreased in recipients with cGvHD. Lymphocytes harvested from the spleen and liver of recipients with and without cGvHD were stained with MCMV peptide–specific MHC class I tetramer, and tetramer-positive CD8+ T cells were measured by FACS analysis. Panels A and D represent the absolute number of donor spleen– (▪), donor BM– (□), and host-derived (▧) MCMV peptide–specific (HGIRNASFI-H2b) tetramer-positive CD8+ T cells detected from the spleen and liver, respectively. Panels B, C and E, F represent the total frequency of donor spleen– and donor BM–derived intracellular IFN-γ– and TNF-α–producing CD8+ T cells per spleen and liver, respectively, on day 10 after MCMV infection as measured after 4 hours in vitro stimulation of total lymphocytes with HGIRNASFI MCMV peptide (□, cGvHD; ▪, no GvHD). *P < .05 and **P < .01 (Student t test) are for the decreased absolute numbers of MCMV peptide–specific tetramer-positive IFN-γ+ and TNF-α+ CD8+ T cells per organ in recipients with cGvHD compared with no GvHD.
Liver and spleen antiviral T-cell responses were significantly decreased in recipients with cGvHD. Lymphocytes harvested from the spleen and liver of recipients with and without cGvHD were stained with MCMV peptide–specific MHC class I tetramer, and tetramer-positive CD8+ T cells were measured by FACS analysis. Panels A and D represent the absolute number of donor spleen– (▪), donor BM– (□), and host-derived (▧) MCMV peptide–specific (HGIRNASFI-H2b) tetramer-positive CD8+ T cells detected from the spleen and liver, respectively. Panels B, C and E, F represent the total frequency of donor spleen– and donor BM–derived intracellular IFN-γ– and TNF-α–producing CD8+ T cells per spleen and liver, respectively, on day 10 after MCMV infection as measured after 4 hours in vitro stimulation of total lymphocytes with HGIRNASFI MCMV peptide (□, cGvHD; ▪, no GvHD). *P < .05 and **P < .01 (Student t test) are for the decreased absolute numbers of MCMV peptide–specific tetramer-positive IFN-γ+ and TNF-α+ CD8+ T cells per organ in recipients with cGvHD compared with no GvHD.
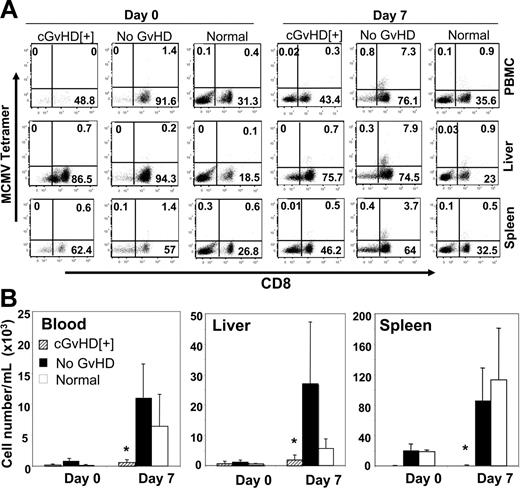
To investigate the functional status of anti-CMV T cells, we stimulated lymphocytes harvested from the spleen and liver in vitro with the HGIRNASFI MCMV peptide21 (same peptide used as in tetramer) for 4 hours in presence of Brefeldin A and measured the frequency of intracellular IFN-γ– and TNF-α–producing CD8+ T cells by multicolor FACS analysis. Significantly lower levels of both donor spleen– and BM-derived IFN-γ+ and TNF-α+ CD8+ T cells were detected in the spleen and liver of recipients with cGvHD compared with recipients without GvHD (Figure 4B,E, and 4C,F). The reduced antiviral T-cell response in recipients with cGvHD could be due to an immunosuppressive effect of higher viral loads in the infected organs or the results of cGvHD itself. To clarify this issue we used a nonpathogenic, highly immunogeneic recombinant Listeria vaccine (Lm-MCMV) that expresses the early MCMV peptide to immunize allogeneic transplant recipients with cGvHD, syngenic transplant recipients with no GvHD, and normal C57BL/6 mice. Vaccinated mice had normal weight gain and activity, with no signs of illness. We measured the frequency of antiviral T cells in the blood, liver, and spleen on 0 and 7 days after vaccination. Significantly lower frequencies of MCMV peptide–specific tetramer-positive CD8+ T cells were found on day 7 after inoculation in organs collected from mice with cGvHD compared with syngenic transplant recipients with no GvHD or control normal mice (Figure 5A). The absolute numbers of antiviral T cells were comparable between syngenic recipients and control mice that did not receive transplants and were markedly reduced in the in blood (P < .05), liver (P < .05), and spleen (P < .001) of mice with cGvHD (Figure 5B).
Immunosuppression associated with cGvHD responsible for the deficit antiviral T-cell responses. Allogeneic transplant recipient with cGvHD, syngenic transplant with no GvHD, and normal C57BL/6 mice were intraperitoneally vaccinated with 1 × 106 CFUs Lm-MCMV. Lymphocytes were harvested from the blood, liver, and spleen collected on days 0 and 7 after vaccination, and MCMV peptide–specific tetramer-positive CD8+ T cells were measured by FACS analysis. (A) Representative dot plots of donor spleen–derived tetramer-positive CD8+ T cells in the blood, liver, and spleen of recipients with cGvHD and no GvHD and CD3+-gated populations of normal C57BL/6 mice. (B) Absolute number of tetramer-positive CD8+ T cells per organ measured from the recipients with cGvHD, no GvHD, and normal C57BL/6 mice. *P < .05 in blood and liver and *P < .001 in spleen (Student t test) are for the decreased absolute numbers of MCMV peptide–specific tetramer-positive CD8+ T cells per organ in recipients with cGvHD compared with without GvHD or normal mice. Three to 5 mice were used per group in each time point.
Immunosuppression associated with cGvHD responsible for the deficit antiviral T-cell responses. Allogeneic transplant recipient with cGvHD, syngenic transplant with no GvHD, and normal C57BL/6 mice were intraperitoneally vaccinated with 1 × 106 CFUs Lm-MCMV. Lymphocytes were harvested from the blood, liver, and spleen collected on days 0 and 7 after vaccination, and MCMV peptide–specific tetramer-positive CD8+ T cells were measured by FACS analysis. (A) Representative dot plots of donor spleen–derived tetramer-positive CD8+ T cells in the blood, liver, and spleen of recipients with cGvHD and no GvHD and CD3+-gated populations of normal C57BL/6 mice. (B) Absolute number of tetramer-positive CD8+ T cells per organ measured from the recipients with cGvHD, no GvHD, and normal C57BL/6 mice. *P < .05 in blood and liver and *P < .001 in spleen (Student t test) are for the decreased absolute numbers of MCMV peptide–specific tetramer-positive CD8+ T cells per organ in recipients with cGvHD compared with without GvHD or normal mice. Three to 5 mice were used per group in each time point.
T cells from mice with cGvHD lack homing ability
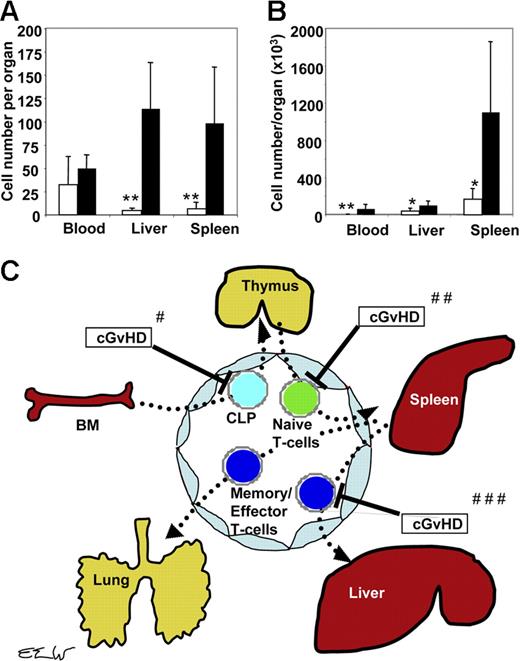
Following MCMV infection, the number of antigen-specific antiviral CD8+ T cells in the liver of mice with cGvHD was markedly reduced compared with the recipients without GvHD, although there were similar numbers of tetramer-positive CD8+ T cells in the blood (Table 1). The lack of antiviral T cells in specific tissues could be due to the defects of T-cell homing or a failure of antigen-specific T cells to expand in infected tissues in mice with cGvHD. To address this issue, we next determined the ability of antiviral CD8+ T cells to home to the spleen and liver by transferring antigen-specific T cells from donor mice with or without cGvHD to syngenic recipients without cGvHD. Three million blood lymphocytes were prepared from cGvHD+ CB6F1 and cGvHD− C57BL/6 mice containing 9210 and 25 900 CMV tetramer–positive CD8+ T cells, respectively. The tetramer-positive CD8+ T cells were derived, in both cases, from previously transplanted Thy1.2+ CD45.1+ immune splenocytes. To induce peripheral lymphopenia and facilitate expansion of adoptively transferred T cells, Thy 1.2+ CD45.2+ secondary recipients were irradiated and received TCD BM. To study the homing ability and functional antiviral activity of the adoptively transferred MCMV tetramer–positive CD8+ T cells, we infected the secondary recipients with MCMV the day following secondary transfer. Surprisingly, while equivalent numbers of Thy 1.2+ CD45.2− tetramer-positive CD8+ T cells were present in the blood of secondary transplant recipients on day 3 after transplantation, greater than 20-fold fewer tetramer-positive CD8+ T cells had homed to the liver and greater than 10-fold fewer tetramer-positive CD8+ T cells had homed to spleen in mice that received PBMCs obtained from the cGvHD+ donor mice compared with recipients of PBMCs from cGvHD− donor mice (Figure 6A). Accounting for the 2-fold difference in the absolute numbers of antigen-specific T cells transferred from cGvHD− and cGvHD+ donors, there still were significantly more antigen-specific T cells in peripheral tissues among secondary recipients in the former group (>10-fold difference in the liver, and > 5-fold difference in the spleen). Peripheral expansion of antigen-specific T cells from cGvHD+ donors did not appear to be significantly impaired, as the difference in absolute numbers of antigen-specific T cells in the liver and spleen of recipients of cGvHD− donor lymphocytes versus cGvHD+ lymphocytes on day 10 after transfer had decreased to 3-fold and 7-fold, respectively (Figure 6B).
Homing defects of donor T cells to sites of CMV infection. Blood lymphocytes were harvested from 2 groups of donors: cGvHD+ CB6F1 mice that previously received transplants of C57Bl/6 donor cells, and cGvHD− C57BL/6 recipients of syngenic transplants. Both groups of recipients were infected with 2.5 × 104 PFUs MCMV intraperitoneally on day 100 after transplantation, and PBMCs were harvested from the pooled blood of 5 infected mice on day 10 after viral infection as described in “Materials and methods.” Three million PBMCs from mice with or without cGvHD were adoptively transferred to 2 groups of irradiated (11 Gy) C57BL/6 mice that were then infected with 3 × 103 PFUs MCMV intraperitoneally the next day. Nucleated cells were harvested on days 3 and 10 after infection from the blood and spleen (as described in “Materials and methods”) from the infected secondary recipients. The numbers of donor-derived lymphocytes per gram of liver tissue are shown. Donor-derived T cells and MCMV peptide–specific tetramer-positive CD8+ T cells per mL blood or gram liver or spleen were counted by using multicolor FACS analysis. (A) MCMV peptide–specific tetramer-positive CD8+ T cells per organ measured from the recipients of lymphocytes harvested from cGvHD+ donors (□) and from recipients of lymphocytes harvested from no-GvHD donors (▪) on day 3 after MCMV infection. (B) MCMV peptide–specific tetramer-positive CD8+ T cells per organ measured on day 10 after MCMV infection. Values represent an average of data from 4 to 5 mice per group (*P < .05 and **P < .005; Student t test). (C) Schematic diagram for the inhibitory effects of cGvHD on the migration and homing of donor T cells to different lymphoid and nonlymphoid organs. #cGvHD interferes with homing of T-cell progenitors to the thymus. ##cGvHD interferes with homing of donor antiviral T cells from blood to lymphoid organs. ###cGvHD interferes with homing of antigen-specific T cells to target organs infected by CMV (eg, lung and liver).
Homing defects of donor T cells to sites of CMV infection. Blood lymphocytes were harvested from 2 groups of donors: cGvHD+ CB6F1 mice that previously received transplants of C57Bl/6 donor cells, and cGvHD− C57BL/6 recipients of syngenic transplants. Both groups of recipients were infected with 2.5 × 104 PFUs MCMV intraperitoneally on day 100 after transplantation, and PBMCs were harvested from the pooled blood of 5 infected mice on day 10 after viral infection as described in “Materials and methods.” Three million PBMCs from mice with or without cGvHD were adoptively transferred to 2 groups of irradiated (11 Gy) C57BL/6 mice that were then infected with 3 × 103 PFUs MCMV intraperitoneally the next day. Nucleated cells were harvested on days 3 and 10 after infection from the blood and spleen (as described in “Materials and methods”) from the infected secondary recipients. The numbers of donor-derived lymphocytes per gram of liver tissue are shown. Donor-derived T cells and MCMV peptide–specific tetramer-positive CD8+ T cells per mL blood or gram liver or spleen were counted by using multicolor FACS analysis. (A) MCMV peptide–specific tetramer-positive CD8+ T cells per organ measured from the recipients of lymphocytes harvested from cGvHD+ donors (□) and from recipients of lymphocytes harvested from no-GvHD donors (▪) on day 3 after MCMV infection. (B) MCMV peptide–specific tetramer-positive CD8+ T cells per organ measured on day 10 after MCMV infection. Values represent an average of data from 4 to 5 mice per group (*P < .05 and **P < .005; Student t test). (C) Schematic diagram for the inhibitory effects of cGvHD on the migration and homing of donor T cells to different lymphoid and nonlymphoid organs. #cGvHD interferes with homing of T-cell progenitors to the thymus. ##cGvHD interferes with homing of donor antiviral T cells from blood to lymphoid organs. ###cGvHD interferes with homing of antigen-specific T cells to target organs infected by CMV (eg, lung and liver).
Discussion
In this study, we have investigated cGvHD-induced immunodeficiency using a posttransplantation CMV infection in a murine model of allogeneic BMT. Mice that developed cGvHD after transplantation with 3 × 106 untreated allogeneic donor splenocytes were compared with control allogeneic transplant recipients lacking cGvHD or mice that received TCD BM alone.14,15 We challenged BM transplant recipients with a sublethal dose of MCMV on days 100 to 133 after transplantation in order to study immune responses to a clinically relevant opportunistic infection. We found several significant differences in the antiviral T-cell responses between recipients with and without cGvHD. First, although both groups showed significant increases in the number of donor spleen–derived T cells in the blood 10 days after MCMV infection, mice with cGvHD failed to control viral replication in the spleen and liver. Secondly, splenic and thymic T-cell numbers were significantly reduced in mice with cGvHD compared with mice without cGvHD. Thirdly, significantly lower numbers of MCMV peptide–specific tetramer-positive antiviral CD8+ T cells were found in spleen and liver on day 10 after infection in recipients with cGvHD compared with mice without cGvHD. Vaccination with Lm-MCMV confirmed the lack of immune response in mice with cGvHD, and secondary transfer experiments documented a defect in homing and tissue expansion of lymphocytes from mice with cGvHD (Figure 6).
The mechanisms of cGvHD-induced immunosuppression remain to be fully defined but could include deficits in antigen presentation, homing, or expansion of antigen-specific donor T cells in MCMV-infected tissue. The results of this study suggest that cGvHD impairs primary immune responses as well as homing of T cells to lymphoid organs and sites of infection. Significantly higher viral loads on day 3 after MCMV infection in the livers and spleens of recipients with cGvHD compared with mice without cGvHD (Figure 1D) suggest a lack of early innate antiviral immune responses in recipients with cGvHD. Significantly lower levels of MCMV peptide–specific tetramer-positive IFN-γ+ and TNF-α+ CD8+ T cells in the spleen and liver on 10 days after viral infection in mice with cGvHD suggest the lack of adaptive, antigen-specific antiviral T-cell responses compared with mice without GvHD (Figure 4). In recipients with cGvHD, higher numbers of MCMV peptide–specific tetramer-positive CD8+ T cells were found in the blood on day 10 after infection with MCMV (Figure 2; Table 1), but the migration of these T cells to the liver and spleen were significantly impaired compared with the recipients without cGvHD (Figure 4). The lack of antiviral T cells in infected tissues may be due to defects in homing or chemokine receptor expression in antigen-specific T cells in the presence of cGvHD. Notably, the relative decrease in CD44 expression on T cells from mice with cGvHD may impair the rolling phase of lymphocyte adhesion26,27 and interfere with lymphocyte–endothelial cell (EC) adhesion.28 The lower levels of CD44 expression may also impair signaling through tyrosine kinases, indirectly inhibiting homing and activation.29
Since mice with cGvHD had higher viral loads following MCMV infection, it was possible that the presence of high levels of MCMV in the blood and tissues had a direct or indirect inhibitory effect on primary immune responses and/or T-cell homing to target tissues of MCMV. Alternatively, the lower antiviral T-cell response in recipients with cGvHD could be due to an intrinsic immunosuppression associated with the presence of cGvHD. We specifically addressed this issue by vaccinating mice with and without cGvHD using a nonviral Lm-MCMV vaccine. Marked anti-MCMV immune responses were observed 7 days after vaccination in normal mice and in syngenic transplant recipients without GvHD, whereas tetramer-positive antiviral T cells were nearly undetectable in the blood and organs of mice with cGvHD (Figure 5). Thus, we conclude that the deficit of antiviral T cells in recipients with cGvHD is due, in large part, to the immunosuppressive effects of cGvHD rather than an effect of the virus.
We specifically tested the effect of cGvHD on homing of T cells by transferring blood lymphocytes from MCMV-infected donors with and without cGvHD to irradiated naive recipient C57BL/6 mice. Although 3 × 106 PBMCs obtained from cGvHD+ donors contained about half of the amount of donor spleen–derived T cells than the 3 × 106 PBMCs obtained from the GvHD− donors, there was an average of more than 20-fold more donor spleen–derived tetramer-positive antiviral CD8+ T cells in the liver and more than 10-fold more tetramer-positive T cells in the spleen of secondary recipients that received cGvHD− lymphocytes compared with secondary transplant recipients that received cGvHD+ lymphocytes (Figure 6A; P < .005). The expansion of antigen-specific CD8+ T cells in the liver and spleen, between day 3 and day 10, was similar among recipients of cGvHD+ and cGvHD− lymphocytes, although the absolute numbers of antigen-specific T cells was higher in the latter group (Figure 6B; P < .05). Taken together, these data suggest that cGvHD acts at multiple levels to impair antigen-specific immunity including (a) an effect on the generation of new T cells from BM progenitors through the thymus; and (b) retarding the homing of antiviral T cells to the secondary lymphoid organs and target tissues infected with MCMV (Figure 6C).
This study may shed some light on the relationship between cGvHD and CMV infections in clinical BMT. While cGvHD and CMV infections are often seen together in allogeneic BM transplant recipients, the causal relationship between these 2 conditions is not clear. Söderberg et al30 reported that CMV infection in transplantation patients was associated with the development of autoantibodies to CD13, a marker expressed on granulocytes, monocytes, fibroblasts, endothelial cells, epithelial cells, smooth muscle cells, and certain neuronal cells. Through analysis of the immunoreactivity of serum samples on skin biopsies they showed that most patients with CMV disease and cGvHD also had CD13-specific antibodies that may contribute to the cutaneous manifestations of cGvHD as well as CMV-associated granulocytopenia in allogeneic BM transplant recipients.30 While Via et al31 found that MCMV infection induced synergistic effects on acute GvHD, previous reports using experimental murine BMT model systems have not described the effects of MCMV infection on cGvHD. The current study used a clinico-pathologic scoring system for cGvHD and found that MCMV infection did not exacerbate existing cGvHD when mice received sublethal injections of virus (Figure 1).
In conclusion, the functional deficiency of antiviral immunity observed in mice with cGvHD is consistent with the clinical reports that document an increased incidence of opportunistic viral infections in patients with cGvHD.32 Using a murine allogeneic BMT model, we found that peripheral expansions of donor T cells occurred in the blood in response to MCMV infection, but these T cells failed to efficiently home to MCMV-infected tissues and thus failed to mount effective antiviral immune responses in mice with cGvHD. In contrast, allogeneic transplant recipients without cGvHD successfully developed brisk and effective antiviral immune responses. These findings indicate that studying functional cellular immune responses of patients undergoing allogeneic BMT may require measurement of tissue-localized effectors in addition to monitoring antigen-specific cells in the blood. The failure of effective tissue-specific antiviral immune responses in transplant recipients with cGvHD indicates the need for adoptive cell therapies that do not cause GvHD.15,18 The impaired immune responses to the MCMV-Listeria vaccine in mice with cGvHD suggest that intrinsic immunosuppression associated with clinical cGvHD rather than immunosuppressive drugs limits the efficacy of vaccination in this setting.
Authorship
Conflict-of-interest disclosure: J.D.R. receives research grant support from Cerus Corporation, a company whose potential product was studied in the present work. J.D.R. and E.K.W. have a US patent pending related to the work described in the present study. The remaining authors declare no competing financial interests.
Correspondence: Edmund K. Waller, Department of Hematology and Oncology, Winship Cancer Institute, Emory University, 1365 Clifton Road, Building C, WCI 4th Floor, Room # C4032, Atlanta, GA 30322; e-mail: ewaller@emory.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors wish to thank Cerus for providing amotosalen (S-59 Psoralen.HCl) and Listeria-MCMV vaccine.
This work was supported by National Institutes of Health (NIH) grant HL70997 (J.D.R.) and by NIH grant CA-74364 (E.K.W.).

![Figure 3. Splenic and thymic functions were severely suppressed in recipients with cGvHD. Spleen, thymus, and liver were collected from the recipients with and without cGvHD and TCD BM alone prior to MCMV infection (day 100 after transplantation) and day 10 after MCMV infection (day 110 after transplantation). Lymphocytes were isolated and absolute numbers of nucleated cells per organ were measured by counting the live cells under fluorescence microscopy. Panels A, B, and C represent the absolute number of nucleated cells per spleen, thymus, and liver, respectively (▧, recipients with cGvHD; ▪, recipients with no GvHD; and □, recipients of TCD BM). Panels D, E, and F represent the absolute number of donor spleen T cells per spleen, thymus, and liver, respectively (▧, recipients with cGvHD; ▪, recipients with no GvHD). Panels G, H, and I represent the absolute number of donor BM–derived T cells per spleen, thymus, and liver, respectively (▧, mice with cGvHD; ▪, recipients with no GvHD) that were calculated from the data obtained from the FACS analysis. Data represent an average of 5 to 6 mice were used per group (*P < .05 [Student t test] is for the significant decrease compared with the cell numbers in the spleen and thymus of recipients with no GvHD and TCD BM alone).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-04-017442/4/m_zh80100701050003.jpeg?Expires=1769806123&Signature=HNAoAUTnNo2zz4uYKl3AGh3RKNDMdUu1oNdtojlUB2ZR-WxPfh~I9WvVaHj94o4YvPC0EBM9KMFCMPaLSQsF-IlEj41EyRSPF8VPrlUrUfHHkgAwLcDG-CRekV~8pBsbn3srhP8-D9Jw4k~r9xyBjb3EExIETgHIVmIMAuqIopmulF1fourNcqGVQHatBgzaeWGM1lra25VTrhLXjs~opB13ID3vORqEj2FzwVUvSzliUlCQ~7HdmnB60H1-Mrmew8LgSlPPrfTQvscwgeH72FSqCPNpPn5Lugw494UaSdzL3ZE3rHLAoJ8LQpG1~tfkhbYGtHqLhw4tevjMMwMP8g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)