Abstract
The purpose of this study was to characterize a large group of infants with complete DiGeorge anomaly and to evaluate the ability of thymus transplantation to reconstitute immune function in these infants. DiGeorge anomaly is characterized by varying defects of the heart, thymus, and parathyroid glands. Complete DiGeorge anomaly refers to the subgroup that is athymic (< 1%). The characteristics of 54 subjects at presentation and results from 44 consecutive thymus transplantations are reported. Remarkably, only 52% had 22q11 hemizygosity and only 57% had congenital heart disease requiring surgery. Thirty-one percent developed an atypical phenotype with rash and lymphadenopathy. To date, 33 of 44 subjects who received a transplant survive (75%) with post-transplantation follow-up as long as 13 years. All deaths occurred within 12 months of transplantation. All 25 subjects who were tested 1 year after transplantation had developed polyclonal T-cell repertoires and proliferative responses to mitogens. Adverse events developing after transplantation included hypothyroidism in 5 subjects and enteritis in 1 subject. In summary, diagnosis of complete DiGeorge anomaly is challenging because of the variability of presentation. Thymus transplantation was well tolerated and resulted in stable immunoreconstitution in these infants.
Introduction
DiGeorge anomaly is characterized by defects in the thymus and parathyroid glands as a result of malformation of the third and fourth pharyngeal pouches.1 A broad spectrum of abnormalities has been reported in this condition.2,3 Congenital heart disease, especially truncus arteriosus and interrupted aortic arch type B, results from defects in the fourth pharyngeal arch. No thymus is present in less than 1% of patients with DiGeorge anomaly.4–7 The term “complete DiGeorge anomaly” is used to describe the population of infants with DiGeorge anomaly who are athymic. The diagnosis of athymia is based on profound suppression of the numbers of recent thymic emigrants in the blood.8–10 The diagnosis of athymia cannot be made based on the gross appearance at cardiac surgery, by the absence of thymus on a chest radiograph, or by computerized tomogram because the thymus may be in an ectopic location or may be very small. Infants with complete DiGeorge anomaly have various genetic and syndromic associations,8,9 including 22q11 hemizygosity,11 CHARGE association (coloboma, heart defect, choanal atresia, growth or developmental retardation, genital hypoplasia, and ear anomalies, including deafness)12–15 and diabetic embryopathy.16,17
Infants with complete DiGeorge anomaly present with either a “typical” or “atypical” phenotype. Infants with typical complete DiGeorge anomaly have very low T-cell numbers and do not have a rash. At some point after birth, infants with typical complete DiGeorge anomaly may develop oligoclonal T-cell populations associated with rash and lymphadenopathy.18 These patients are described as having atypical complete DiGeorge anomaly. The oligoclonal T cells infiltrate the skin and other organs such as the liver.9,18,19 The T-cell numbers in peripheral blood can be low, normal, or elevated, with low or normal T-cell proliferative responses to the mitogen phytohemagglutinin. The presentation of infants with atypical complete DiGeorge anomaly may be confused with severe atopic dermatitis20,21 ; Omenn syndrome in RAG1, RAG2 deficiency or Artemis deficiency22 ; maternal T-cell engraftment in patients with severe combined immunodeficiency; or maternal T-cell engraftment in patients with complete DiGeorge anomaly.23–25 The diagnosis of atypical complete DiGeorge anomaly hinges on the infant having features of DiGeorge anomaly, profoundly depressed numbers of naive T cells, and absence of maternal T cells. The distinction between typical and atypical complete DiGeorge anomaly is important with respect to thymus transplantation because the T cells in atypical complete DiGeorge anomaly can reject transplants.
Our group has investigated transplantation of allogeneic postnatal cultured thymus as a therapy for infants with complete DiGeorge anomaly.8,9,26–29 We report results for 54 infants enrolled in thymus transplantation protocols. From 1993 through November 2006, 44 of the infants were treated with allogeneic postnatal cultured thymus transplantation. Twenty-six subjects who received a transplant are more than 1 year after transplantation. Our results support both the safety of thymus transplantation and efficacy reflected in a pronounced improvement in T-cell function and rates of infection.
Patients, materials, and methods
Subject inclusion criteria
All subjects were enrolled in protocols approved by the Duke Institutional Review Board. A total of 54 subjects with complete DiGeorge anomaly were enrolled from 1993 through November 2006; 44 received a transplant. Informed consent was obtained from the parents of all enrolled subjects, in accordance with the Declaration of Helsinki. Preliminary data were reported previously on 19 of these subjects.8,9,18
The subjects in this report were enrolled in 1 of 6 protocols. Three phase 1 protocols were descriptive only. Two are closed to enrollment, the third continues to investigate optimal immunosuppression regimens. Two other phase 1 protocols have as primary end points (1) naive T-cell counts and T-cell receptor repertoire variability at 1 year (looking for a dose effect) and (2) calcium supplementation after parathyroid transplantation. Both are open at this time. The final protocol is phase 2 and has the primary end point of survival.
To be considered athymic, subjects had either fewer than 50 naive T cells/mm3 or their naive T cells comprised less than 5% of total T cells by flow cytometry. Naive T cells were defined as those coexpressing CD45RA and CD62L.10 For the diagnosis of DiGeorge anomaly, subjects had at least one of the following: hypoparathyroidism, congenital heart disease, CHARGE association, or 22q11 hemizygosity. One person who met these criteria had ectodermal dysplasia with absence of T cells and B cells at birth. The T cells isolated from subjects with atypical complete DiGeorge anomaly were shown to be genetically host-derived prior to transplantation. This was done by a clinical molecular laboratory using multiplex polymerase chain reaction amplification for microsatellite markers with a sensitivity of 2%.
Thymus donor and tissue processing
Processing of the thymus tissue was done as described9,30,31 under an Investigational New Drug (IND) application with the Food and Drug Administration (FDA). In brief, thymus tissue was obtained as discarded tissue from pediatric cardiac surgery after informed consent of the donor's parents. All thymus donors were younger than 7 months. The donors and the mothers of the donors were screened for infectious diseases per FDA guidelines.32 In addition the presence of EBV and CMV was assessed because these viruses have resulted in lymphoproliferative disorders in infants treated in the past at other centers.33,34 As has been reported in the past,8,9 HLA matching was not required for transplantation.
Immunosuppression
Whether immunosuppression was used in the 44 subjects who received a transplant depended on the level of T-cell function of the recipient prior to transplantation. Twenty-two subjects with typical complete DiGeorge anomaly did not receive immunosuppression. They had fewer than 5000 counts per minute (cpm) of T-cell proliferative response to phytohemagglutinin (PHA) or less than a 20-fold response to PHA over background. Of the remaining 22 subjects, 8 had typical complete DiGeorge anomaly and received rabbit antithymocyte globulin. The usual dose was 2 mg/kg/d for 3 doses before transplantation. Methylprednisolone, 2 mg/kg, was given prior to the first dose of rabbit antithymocyte globulin followed by 0.5 mg/kg every 6 hours intravenously until 24 hours after finishing the rabbit antithymocyte globulin. These 8 subjects had a T-cell proliferative response to PHA of more than 5000 cpm or greater than 20-fold over background (3 subjects), enrolled in a protocol to receive parathyroid transplants (4 subjects), or had preexisting graft-versus-host disease (GVHD, 1 subject). This latter subject was also treated with cyclophosphamide and cyclosporine. The 14 subjects with atypical complete DiGeorge anomaly had varied proliferative responses to PHA, ranging from very low to more than 100 000 cpm. They received one of several immunosuppressive treatments. Deoxycoformycin was used to immunosuppress the first subject with atypical complete DiGeorge anomaly prior to transplantation. Four subjects with atypical complete DiGeorge received rabbit antithymocyte globulin (with concurrent steroids) before transplantation with no additional before or after transplantation immunosuppression. Nine additional subjects with atypical complete DiGeorge anomaly received rabbit antithymocyte globulin before transplantation plus various combinations of cyclosporine or tacrolimus (7 subjects), steroids (1 subject), and mycophenylate mofetil and daclizumab (1 subject who received cyclosporine and steroids as well) before and after transplantation.
Thymus transplantation
Immune phenotype and function
Standard flow cytometry was done on whole blood.9 Percentages of positive cells were recorded, and absolute numbers were calculated based on the absolute lymphocyte count. Standard tests of T-cell function were performed,9 including proliferative responses to PHA (3 concentrations, with cells harvested on days 3 and 4), concanavalin A (3 concentrations, with cells harvested on days 4 and 5), soluble and immobilized CD3 (cells harvested on day 4), and tetanus antigen and Candida skin test antigen (4 concentrations of each antigen, cells harvested on day 6).
Spectratyping to assess T-cell receptor diversity was performed as described, usually on isolated CD4 T cells.8,9 All panels were inspected to ensure that they met acceptable technical criteria in terms of sufficient height of the peaks above background and absence of overloading. Technically adequate panels were analyzed via computation of their Kullback-Leibler divergences (DKL).35 The DKL generically measures the divergence of 2 probability functions; in this case, it measures the divergence of each spectratype profile against the corresponding reference profile based on the average of 7 healthy adult volunteers. The normal range was determined by trimming the top and bottom 2.5% from the historical DKLs observed in healthy adult volunteers. The higher the DKL, the greater the divergence from the normal profile and the more oligoclonal the distribution of peaks.
Immunohistochemistry of graft biopsy
Biopsies of the thymus allograft were conducted at 2 to 3 months after transplantation if the medical condition of the subject was stable.8,28 Frozen and paraffin-embedded sections were reacted with a panel of antibodies, including cytokeratin (clones AE1/AE3; Dako, Carpinteria, CA) CD3 (polyclonal; Dako), CD1a (clone 010; Beckman Coulter, Marseille, France), and Ki-67 (clone mib-1; Beckman Coulter).8,28 Histologic evidence of thymopoiesis in biopsies was defined as the presence of a lacy pattern of cytokeratin-positive thymic epithelial cells and the presence of CD3+CD1a+Ki-67+ cells. The latter is the phenotype of cortical thymocytes.
Surveillance for adverse events
Infections were defined per the Blood and Marrow Transplant Clinical Trials Network.36 Any ongoing bacterial pulmonary infection or viral infection before transplantation was counted as a single infection because these infections usually persist until T cells develop. Except for the first 12 subjects, all recipients were screened prior to transplantation for the presence of Epstein Barr virus (EBV), cytomegalovirus (CMV), respiratory syncytial virus (RSV), parainfluenza virus, adenovirus, and enterovirus. Screening for HIV-1, human herpes virus type 6 (HHV6), and West Nile virus was added in the past 2 years. Stool, nasopharyngeal, and urine viral cultures were repeated after the initial screening based on clinical symptoms. Since 2001, all subjects have been screened for thyroid disease.
Results
Subject characteristics
During the study period from 1993 to November 2006, 54 infants were enrolled as subjects in thymus transplantation protocols. Two subjects were withdrawn by their parents prior to transfer to Duke University Medical Center (DUMC) because of the severity of other anomalies in the infants. Fifty-one were admitted to DUMC and one is awaiting transfer to DUMC. Three infants were withdrawn after admission (without transplantation), one by the parents because of the severity of other anomalies and 2 by the investigator because the infants were not medically stable enough for surgery. Three infants died of infection and one from respiratory arrest prior to transplantation. Forty-four infants underwent transplantation.
Characteristics of the entire group of 54 enrolled subjects and of the 44 subjects who received a transplant were similar and are shown in Table 1. Athymia was confirmed in all subjects by the presence of very low naive T-cell numbers or percentages. The initial absolute lymphocyte count was greater than the 10th percentile in 13% (7 of 54) of the subjects. Thirty-one percent (17 of 54) of the subjects presented with the atypical phenotype with rash and lymphadenopathy associated with oligoclonal T cells. The atypical subjects comprised 36% (10 of 28) of subjects with 22q11 hemizygosity as compared with 27% (7 of 26) normal at 22q11. Unexpectedly, the rate of conotruncal congenital heart disease was relatively low at 31% overall (Table 1) compared with approximately 60% in the literature.2,4
Patient characteristics
| Demographics . | Infants who received a transplant . | Infants enrolled . |
|---|---|---|
| n | 44 | 54 |
| Sex, no. | ||
| Male | 26 | 32 |
| Female | 18 | 22 |
| Race, no. | ||
| White | 32 | 40 |
| Black | 9 | 10 |
| Asian | 3 | 4 |
| Hispanic ethnicity, no. | 8 | 10 |
| Median age at time of consent, mo (range) | 3 (0.8-12.6) | 2.3 (0.8-12.6) |
| Genetic or syndromic associations, no./total (%) | ||
| 22q11 hemizygosity | 21/44 (48) | 28/54 (52) |
| CHARGE | 11/44 (25) | 14/54 (26) |
| Infant of diabetic mother | 8/44 (18) | 8/54 (15) |
| Ectodermal dysplasia | 1/44 (2) | 1/54 (2) |
| No genetic or syndromic associations,* no./total (%) | 3/44 (7) | 3/54 (6) |
| Type of complete DiGeorge anomaly, no./total (%) | ||
| Typical† | 30/44 (68) | 37/54 (69) |
| Atypical (with rash and lymphadenopathy) | 12/44 (32) | 17/54 (31) |
| Other clinical conditions, no./total (%) | ||
| Hypocalcemia requiring supplementation | 36/44 (82) | 44/54 (81) |
| Heart defect requiring surgery‡ | 22/44 (50) | 31/54 (57) |
| Conotruncal heart defects | ||
| In all subjects | 12/44 (27) | 17/54 (31) |
| In subjects with 22q11 hemizygosity | 8/21 (38) | 13/28 (46) |
| In subjects without 22q11 hemizygosity | 4/23 (17) | 4/26 (15) |
| Demographics . | Infants who received a transplant . | Infants enrolled . |
|---|---|---|
| n | 44 | 54 |
| Sex, no. | ||
| Male | 26 | 32 |
| Female | 18 | 22 |
| Race, no. | ||
| White | 32 | 40 |
| Black | 9 | 10 |
| Asian | 3 | 4 |
| Hispanic ethnicity, no. | 8 | 10 |
| Median age at time of consent, mo (range) | 3 (0.8-12.6) | 2.3 (0.8-12.6) |
| Genetic or syndromic associations, no./total (%) | ||
| 22q11 hemizygosity | 21/44 (48) | 28/54 (52) |
| CHARGE | 11/44 (25) | 14/54 (26) |
| Infant of diabetic mother | 8/44 (18) | 8/54 (15) |
| Ectodermal dysplasia | 1/44 (2) | 1/54 (2) |
| No genetic or syndromic associations,* no./total (%) | 3/44 (7) | 3/54 (6) |
| Type of complete DiGeorge anomaly, no./total (%) | ||
| Typical† | 30/44 (68) | 37/54 (69) |
| Atypical (with rash and lymphadenopathy) | 12/44 (32) | 17/54 (31) |
| Other clinical conditions, no./total (%) | ||
| Hypocalcemia requiring supplementation | 36/44 (82) | 44/54 (81) |
| Heart defect requiring surgery‡ | 22/44 (50) | 31/54 (57) |
| Conotruncal heart defects | ||
| In all subjects | 12/44 (27) | 17/54 (31) |
| In subjects with 22q11 hemizygosity | 8/21 (38) | 13/28 (46) |
| In subjects without 22q11 hemizygosity | 4/23 (17) | 4/26 (15) |
The diagnosis in these cases was made based on cardiac, parathyroid, and T-cell findings.
One of these infants presented with graft-versus-host disease from a blood transfusion.
Of 31 infants requiring heart surgery, 17 had conotruncal defects as follows: 3 with truncus arteriosus, 3 with interrupted aortic arch type B, 2 with both truncus arteriosus and interrupted aortic arch type B, 8 with tetralogy of Fallot, and 1 with a conoventricular ventricular septal defect (VSD). Of the 31 infants requiring heart surgery, the 14 non-conotruncal defects included 2 patent ductus arteriosus (PDAs), 1 PDA with atrial septal defect (ASD), 1 PDA with VSD, 1 PDA with a vascular ring, 1 AV, 1 vascular ring, 1 AV canal with pulmonary atresia, 2 VSDs, 1 VSD with pulmonary atresia, 1 VSD with a vascular ring, 1 VSD with an ASD, and 1 VSD with a coarctation.
Survival
Survival of the entire group of 54 subjects is 64% (35 of 54). Currently, 75% of subjects who received a transplant (33 of 44) survive (Figure 1).
Kaplan-Meyer plot of survival of recipients of a thymus transplant. (A) Survival of 35 of 54 subjects who were enrolled. (B) Survival of 33 of 44 subjects who received a transplant.
Kaplan-Meyer plot of survival of recipients of a thymus transplant. (A) Survival of 35 of 54 subjects who were enrolled. (B) Survival of 33 of 44 subjects who received a transplant.
Of the 11 deaths after transplantation, none of the subjects died secondary to thymus transplantation. Two were due to RSV, 2 to CMV, 1 from CMV and GVHD from unirradiated blood transfusions given prior to transfer to Duke, 1 from a complication of calcium therapy, and 5, all in subjects with tracheostomies or on ventilators, from sepsis or progressive bronchopulmonary dysplasia. Although no subjects have died after 12 months after their transplantation, 2 of the infants have had more morbidity than others. One subject had a stroke secondary to sickle cell disease 2 years after transplantation and is significantly disabled. The infant with ectodermal dysplasia has had life-threatening problems with thrombosis and infections before and after thymus transplantation. Although T cells developed that respond to mitogens, they do not respond to antigens. These morbid events may yet lead to the death of some subjects later than 12 months after transplantation.
We examined the effect of 5 factors on survival: 22q11 hemizygosity, presentation with atypical complete DiGeorge anomaly, use of immunosuppression, age at transplantation, and experience of the medical and surgical transplantation team in managing these medically fragile subjects. We evaluated survival of the 54 subjects who were consented with the intent to treat and the 44 subjects who received a transplant. These analyses were done post hoc and do not have statistical power. Of the 54 enrolled subjects, we did not observe significant differences in survival rates between the 57% (16 of 28) of subjects with 22q11 hemizygosity who survive and the 73% (19 of 26) of subjects without 22q11 hemizygosity who survive (P = .347 by a chi-square goodness-of-fit test). Ten (59%) of 17 subjects with atypical DiGeorge survive versus 25 (68%) of 37 typical subjects (P = .75). Seventeen (77%) of 22 subjects who received a transplant without immunosuppression survive compared with 16 (73%) of 22 subjects who received a transplant with immunosuppression (P = .99). We did not find significant differences in survival rates for these comparisons. Regarding the effect of age, of the 22 subjects who received a transplant younger than the median age of 4.25 months, 14 survive (64%), whereas 19 (86%) of 22 subjects who received a transplant at the median age of 4.25 months or older survive. This difference likely relates to comorbid conditions leading to death of some children in the first few months of life. A fifth factor evaluated was whether improvements in medical care led to improved survival. Of the initial 27 subjects, 16 (59%) survive. Of the subsequent 27 subjects, 19 survive (70%). It is likely that 2 additional subjects consented in the past year may die (one with significant morbidity, one who did not receive a transplant and withdrawn from the protocol), so these percentages will likely be similar. The similar survival for the 2 groups of subjects suggests that experience in medical and surgical management of these subjects did not improve survival. Infants with complete DiGeorge anomaly continue to die of cardiac complications and infections (if T cells have not been reconstituted). The survival curve shows that the death rate only levels off after T-cell function develops after approximately 9 months.
Tlymphocyte phenotype, numbers, and function
Testing of peripheral blood samples showed that phenotypically normal T cells could be detected in the blood between 3 and 6 months after thymus transplantation. Specifically, the subjects developed more CD4 than CD8 single-positive T cells and had low levels of double negative (CD4−CD8−) T cells. The CD4 TCR repertoire normalized (see data under “Spectratyping”). Circulating naive T cells could be detected by 6 months.
Twenty-two typical subjects received a transplant without immunosuppression of whom 17 survive. Fourteen of these are past 1 year after transplantation. The immune results for all 14 typical subjects who were transplanted without immunosuppression and are more than 1 year from transplantation are shown in Figure 2A-D. (The 3 subjects who are under 1 year after transplantation are not shown. The other 5 subjects died.). Seven of these 14 subjects have previously been reported.8
T-cell development as determined by T-cell subset antibody staining and T-cell proliferative responses to mitogens for subjects past 1 year after thymus transplantation. Fourteen subjects who did not receive immunosuppression (A-C,G) are compared with 12 subjects who did receive immunosuppression (D-F,H); (A,D) CD3, (B,E) CD4, (C,F) CD8, (G,H) PHA responses. Each subject is represented by a separate line. The x-axis is years after transplantation. The y-axis for panels A to F is cells/mm3. The y-axis for panels G and H is counts per minute. Note the T-cell amplifications seen in panels A to C for 4 subjects who developed the atypical form of DiGeorge anomaly shortly after transplantation.
T-cell development as determined by T-cell subset antibody staining and T-cell proliferative responses to mitogens for subjects past 1 year after thymus transplantation. Fourteen subjects who did not receive immunosuppression (A-C,G) are compared with 12 subjects who did receive immunosuppression (D-F,H); (A,D) CD3, (B,E) CD4, (C,F) CD8, (G,H) PHA responses. Each subject is represented by a separate line. The x-axis is years after transplantation. The y-axis for panels A to F is cells/mm3. The y-axis for panels G and H is counts per minute. Note the T-cell amplifications seen in panels A to C for 4 subjects who developed the atypical form of DiGeorge anomaly shortly after transplantation.
In the group of 22 subjects who received a transplant and did not receive immunosuppression, 4 developed early T-cell amplifications (Figure 2A-C).
Two of these subjects developed a mild rash immediately prior to transplantation. One of these 2 subjects progressed to a full atypical phenotype with circulating CD3+CD4−CD8+ oligoclonal T cells. Two different subjects with typical complete DiGeorge anomaly developed transient oligoclonal T-cell proliferations associated with rash and lymphadenopathy within 2 months of transplantation. When naive T cells developed at 5 to 7 months after transplantation, the rash and lymphadenopathy spontaneously resolved and the oligoclonal T cells dramatically decreased in number. For 1 of the 2 latter subjects, the prominent oligoclonal T cells were CD3+CD4−CD8−. Normalization of the CD4/CD8 ratio occurred shortly before the appearance of naive T cells. We do not think that the development of oligoclonal T cells in these subjects was secondary to transplantation, but instead that the development was equivalent to development of the atypical phenotype in an infant with typical complete DiGeorge anomaly.
Of 22 subjects who received immunosuppression, 16 survive. The immune results for the subset of 12 subjects who are more than 1 year from transplantation are shown in Figure 2D-F,H. (The 4 subjects who are under 1 year after transplantation are not shown. The other 6 died.) T-cell numbers level off slightly below the 10th percentile for age. Seven of these infants had the atypical phenotype. Two of those had predominant CD3+CD4−CD8+ T-cell clones. The CD4/CD8 ratio in these subjects normalized coincident with disappearance of rash and lymphadenopathy and appearance of naive T cells around 4 to 5 months after transplantation. Seven of these 22 subjects have been reported previously.9 Similar results are seen in the groups with and without immunosuppression.
Of the 26 subjects 1 year after transplantation, 21 have had at least 2 immune studies completed after year 1. In comparing 13 subjects without immunosuppression and 8 subjects with immunosuppression, the CD3, CD4, and CD8 mean numbers for the first 2 studies after 1 year are slightly higher in the group with immunosuppression (CD3, 935/mm3 versus 804/mm3; CD4, 631/mm3 versus 554/mm3; and CD8, 215/mm3 versus 159/mm3). Naive CD4 and CD8 numbers also remain slightly below the 10th percentile (not shown) and are nearly identical in the 2 groups. T-cell proliferative responses to the mitogen PHA became normal by 6 to 12 months. The most recent mean proliferative responses after year 1 were somewhat higher in the subjects who received immunosuppression (221 702 cpm versus 177 515 cpm). Although not shown, antigen-specific T-cell proliferative responses have been documented in each of these subjects except the one who has ectodermal dysplasia. In summary, although the T-cell numbers are below normal, the T-cell proliferative function is robust.
Spectratyping
TCRBV repertoire diversity is assessed by spectratyping. The Kullback-Leibler divergence (DKL) statistic is used to assess quantitatively the diversity of the TCRBV repertoire as compared with normal diversity.34 (The higher the DKL, the more the repertoire diverges from a normal diverse repertoire.) When T cells first emerge from the thymus after thymus transplantation, they are quite oligoclonal with high DKL values. The TCRBV repertoire rapidly diversifies and the DKL drops to the normal range. Prior to transplantation, subjects with atypical complete DiGeorge anomaly have high numbers of oligoclonal T cells (with high DKL values). The DKL drops (indicating less divergence from a normal repertoire), entering the normal range by 9 months after transplantation in both typical and atypical complete DiGeorge anomaly subjects (Figure 3A-B). The current CD4 spectratyping data for one subject who is now 7 years after transplantation is shown as an example of long-term maintenance of T-cell diversity (Figure 3C).
CD4 TCRBV repertoire diversity as assessed by spectratyping. (A) DKL values for 16 subjects with typical complete DiGeorge anomaly. (B) DKL values for 7 subjects with atypical complete DiGeorge anomaly. (C) CD4 spectratyping profiles for subject DIG005 at 7 years after transplantation. The Jurkat control panel is in the lower right. The DKL value for this spectratype is 0.11.
CD4 TCRBV repertoire diversity as assessed by spectratyping. (A) DKL values for 16 subjects with typical complete DiGeorge anomaly. (B) DKL values for 7 subjects with atypical complete DiGeorge anomaly. (C) CD4 spectratyping profiles for subject DIG005 at 7 years after transplantation. The Jurkat control panel is in the lower right. The DKL value for this spectratype is 0.11.
Thymus biopsy results
Biopsies of the thymus grafts were done in 30 of the 44 subjects who underwent transplantation. Biopsies were not performed in infants with pulmonary infections or unstable heart disease. The graft (keratin-positive material) was identified in 25 of the 30 biopsies. In the other 5 biopsies, only fat or muscle was obtained. Finding only fat or muscle does not rule out engraftment because the transplanted tissue may not have been sampled. Examples of biopsies showing thymopoiesis have been published.9,27–29 Thymopoiesis was found in 23 of the 25 biopsies in which keratin-positive epithelial cells were identified. Two biopsies detected viable epithelium but no thymopoiesis. One of these 2 subjects had GVHD and CMV infection from unirradiated blood transfusions before transplantation. The second subject had atypical complete DiGeorge anomaly and was suppressed before transplantation with rabbit anti–human thymocyte globulin. The oligoclonal T cells increased dramatically by day 13 after transplantation at which time cyclosporine and steroids were initiated. This subject eventually developed naive T cells with a normal TCRBV repertoire; however, the percentage of naive T cells is lower than in most subjects. Because of the findings in that one subject and to protect the thymus graft from oligoclonal T cells after transplantation, subsequent atypical subjects have been started on cyclosporine before transplantation and are maintained on this treatment until naive T cells develop.
B-cell function
All subjects received intravenous immunoglobulin (IVIG) infusions until 2 years after transplantation. After the IVIG was stopped, antibody responses to tetanus toxoid and pneumococcal immunizations were checked (Tables 2–3).
Immunoglobulin evaluations in children more than 2 years after transplantation (n = 21)
| . | Normal for age n* . | Low, n . | High, n . |
|---|---|---|---|
| IgG levels for infants off IVIG† | 13 | 1 | 1 |
| IgA levels | 15 | 2 | 4 |
| IgM levels | 17 | 4 | 0 |
| IgE levels‡ | 15 | 3 | 2 |
| . | Normal for age n* . | Low, n . | High, n . |
|---|---|---|---|
| IgG levels for infants off IVIG† | 13 | 1 | 1 |
| IgA levels | 15 | 2 | 4 |
| IgM levels | 17 | 4 | 0 |
| IgE levels‡ | 15 | 3 | 2 |
All values listed were obtained at least 1 year from transplantation and most are within 1 year of the present time. Eighteen infants were off IVIG. Of the 3 on IVIG, two subjects have not been taken off IVIG and have not been tested for antibody formation by the local physician. The patient with ectodermal dysplasia remains on IVIG because this patient does not have T-cell proliferative responses to tetanus toxoid.
The years after transplantation are used instead of years of life for the Ig levels. Thus, normal values in mg/dL are as follows: IgA, 15 to 108 for 2 to 3 years, 21 to 144 for 4 to 5 years, 22 to 141 for 6 years, 38 to 240 for 7 years, 32 to 248 for 8 to 10 years, and 40 to 277 for 11 to 12 years; IgM is 43 to 202 for 2 to 3 years, 31 to 163 for 4 to 5 years, 43 to 177 for 6 years, 37 to 151 for 7 years, 42 to 185 for 8 to 10 years, and 42 to 174 for 11 to 12 years. The normal values for IgE in IU/mL are as follows: 0-126 for 2 years, 3-135 for 3 years, 1-219 for 4 years, 2-158 for 5-7 years, 2-524 for 7-10 years, and 4-437 for 10-15 years. Note: “low” is below these levels and “high” is above these levels.
IgG levels are not included for 6 patients. This includes the 3 patients on IVIG. Three others have not had IgG levels obtained since stopping IVIG.
Not done after year 1 in one patient.
Specific antibodies in children more than 2 years after transplantation
| . | n . |
|---|---|
| Tetanus antibodies* | |
| Normal | 12 |
| Low | 1 |
| Pneumococcal antibodies† | |
| Response to 3 or more serotypes | 9 |
| Responses to 1-2 serotypes | 4 |
| No significant responses | 0 |
| Isohemagglutinins‡ | |
| Normal for age | 4 |
| Low | 9 |
| Negative | 4 |
| . | n . |
|---|---|
| Tetanus antibodies* | |
| Normal | 12 |
| Low | 1 |
| Pneumococcal antibodies† | |
| Response to 3 or more serotypes | 9 |
| Responses to 1-2 serotypes | 4 |
| No significant responses | 0 |
| Isohemagglutinins‡ | |
| Normal for age | 4 |
| Low | 9 |
| Negative | 4 |
All values listed were obtained at least 1 year from transplantation and most are within 1 year of the present time.
Tetanus antibodies have not been tested in 8 subjects.
Pneumococcal titers to multiple serotypes (unconjugated) were assessed in 13 subjects. One additional subject (not included in the table) also made antibodies in response to a conjugated pneumococcal vaccine.
Isohemagglutinins have not been tested in 4 subjects.
Fourteen subjects immunized after stopping IVIG have developed antigen-specific antibodies. Thirteen have normal responses to pneumococcal carbohydrate antigens in unconjugated vaccines. A fourteenth subject was immunized with a conjugated pneumococcal vaccine and made a specific response. Isohemagglutinins have developed in some subjects. After 2 years, most subjects stop IVIG therapy. The persistence of protective titers is a topic of current investigation in our laboratory.
Infections
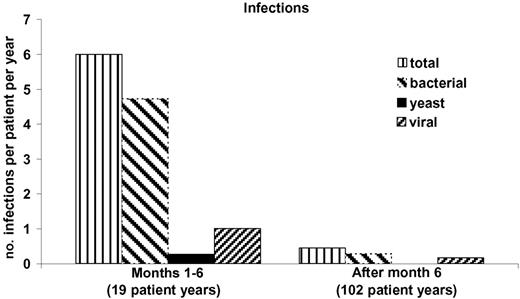
Figure 4 shows the number of infections per subject normalized per year for the initial 6 months after transplantation when T cells have not yet developed versus the infections after the first 6 months. All subjects are included, although some died in the first 6 months. A marked fall in infection frequency is seen even if only the surviving subjects are analyzed (data not shown). The most common infections in the first 6 months were bacterial line infections representing 40% (44 of 113) of the total infections recorded. None of these infections was related to the transplantation procedure. The other infections in the first 6 months included 20 bacterial lung infections, 19 bacterial urine infections, 19 viral infections, 6 episodes of diarrhea associated with Clostridium difficile, and 5 yeast infections. The viral infections that were clinically the most problematic in the first 6 months after transplantation were RSV (4 cases with 2 deaths), CMV (3 cases with 2 deaths), and parainfluenza virus (2 cases).
Infections after thymus transplantation. The number of infections per patient per year is presented for the first 6 months after transplantation before normal T-cell function has developed and for the remainder of follow-up time. All 44 patients who received a transplant are included in the data for the initial 6 months, resulting in 19 patient-years of follow-up. The follow-up time from 6 months after transplantation until the end of November 2006 totaled 82 patient-years of follow-up. A decrease in rate of infections is seen.
Infections after thymus transplantation. The number of infections per patient per year is presented for the first 6 months after transplantation before normal T-cell function has developed and for the remainder of follow-up time. All 44 patients who received a transplant are included in the data for the initial 6 months, resulting in 19 patient-years of follow-up. The follow-up time from 6 months after transplantation until the end of November 2006 totaled 82 patient-years of follow-up. A decrease in rate of infections is seen.
After 6 months, there were relatively few line infections, likely because most central lines were removed after immune function developed. After 6 months, viral infections had similar courses to viral infections in healthy children. All preexisting viral infections cleared after circulating naive T cells developed. Nine subjects had 15 documented viral infections. Eight of the 9 subjects have antigen-specific T-cell responses (and B-cell antigen-specific responses documented for those who are not on IVIG). The ninth is the subject with ectodermal dysplasia. She never developed antigen-specific T-cell responses and has had serious post-transplantation viral infections. We acknowledge a significant underreporting of viral infections in the follow-up period. This could be due to lack of surveillance cultures and lack of reporting by the pediatrician. Taken as a whole, it appears that the flattening of the survival curve (Figure 1) after 6 months is secondary to survival after viral infections which normally have a high mortality rate prior to the development of T cells.
Other adverse events
There have been few adverse events related to thymus transplantation. Two subjects developed erythema at the site of a suture after the surgery. These subjects were treated with antibiotics and suture removal. Three subjects developed respiratory abnormalities within 3 months of transplantation prior to development of naive T cells8,28 ; another subject developed elevated levels of eosinophils. These abnormalities resolved with steroid therapy.8
Five subjects developed rashes in the first 2 months after transplantation, 4 of which were biopsied. The most common diagnosis was spongiotic dermatitis. This was usually treated with low-potency steroid creams. Three rashes resolved after a week or 2; others resolved after naive T cells could be detected in the blood at 3 to 5 months after transplantation. Of note, GVHD secondary to donor thymic cells has not been seen.
The most significant post-transplantation adverse event has been autoimmune disease that was seen in 8 subjects, 2 of whom had atypical complete DiGeorge anomaly. Two subjects developed brief episodes of thrombocytopenia.8,9 One (atypical) subject developed nephrotic syndrome which responded to steroid therapy.9 One (atypical) subject developed severe enteritis which developed at 5 months after transplantation. Biopsies showed T-cell infiltration of the entire gut with destruction of colonic architecture. The subject is currently undergoing immunosuppressive treatment. Five infants (one of whom had nephrotic syndrome earlier) developed Hashimoto thyroiditis in the first 2 years after transplantation. The 5 subjects had elevated levels of thyroid-stimulating hormone (TSH) and low thyroxine levels. They are on treatment with thyroxine. Four of these subjects were tested for antithyroperoxidase and antithyroglobulin antibodies and were positive. One of these subjects also developed alopecia totalis that has been resistant to therapy. An additional subject (not counted in the total above) may be developing Graves disease. He had low TSH and slightly elevated free T4 levels, although the latter has normalized. This subject is positive for antithyroperoxidase and anti-TSH receptor antibodies. He is not on therapy. It is interesting that 6 additional subjects (of the 54 enrolled) developed thyroid disease prior to transplantation. Ten additional subjects have been screened for antithyroid antibodies after transplantation. In 7 cases, they were normal. Two of the other 3 had elevated antibodies but normal thyroxine levels, although one has a slightly elevated TSH level.
Discussion
The 54 subjects with complete DiGeorge anomaly were heterogeneous with respect to the organs affected, the severity of the abnormalities, and the genetic and syndromic associations. Approximately half were hemizygous for chromosome 22q11. The group that was normal at 22q11 included infants with CHARGE association and infants of diabetic mothers. Thirty-one percent of the 54 subjects developed the atypical phenotype of complete DiGeorge anomaly. The low percentage of subjects with conotruncal congenital heart disease was not anticipated. Likely, the higher percentage of conotruncal congenital heart disease reported in partial DiGeorge anomaly2,4,37–39 is secondary to ascertainment bias in that many centers screen all infants with truncus arteriosus and interrupted aortic arch for DiGeorge anomaly.
The surgical transplantation of thymus tissue was a safe procedure. There were no adverse events associated with the surgical procedure except for 2 minor stitch abscesses that developed at the site of the incision and resolved with antibiotic therapy. Thymus tissue–derived infections with cytomegalovirus and EBV33,34,40 have not been observed, presumably because of screening of the thymus donors.
Only the phase 2 clinical trial has the end point of survival. In that trial 8 of 8 subjects are alive with 7 more than 1 year and the eighth at 51 weeks after transplantation. This survival supports the primary hypothesis that thymus transplantation is efficacious in extending life in these patients.
Thymus transplantation in subjects with typical and atypical complete DiGeorge anomaly resulted in functional immune reconstitution characterized by diverse T-cell repertoires, the presence of more CD4 than CD8 T cells, and good proliferative T-cell function. B-cell function, as assessed by the ability to respond to primary immunizations, has developed in all tested after 2 years except in the subject with associated ectodermal dysplasia. Immune outcomes were similar for the typical and atypical groups whether immunosuppression was used in the peritransplantation period. CD8 T-cell function and B-cell memory are new areas of study in this research.
The development of in vitro immune function was temporally associated with the subjects' ability to fight infections after thymus transplantation. Once naive T cells developed, subjects previously infected with RSV, parainfluenza virus, adenovirus, or rotavirus cleared their infections. Subsequent infections with varicella, RSV, parainfluenza, adenovirus, rotavirus, HHV6, and influenza have also cleared. We have not given live vaccines to subjects after thymus transplantation. We plan to further study B-cell responses, in particular to bacteriophage immunization, before considering use of the live measles, mumps, and rubella vaccine.
Autoimmune disease has been seen in some subjects after transplantation. Transient autoimmune thrombocytopenia was seen in 2 subjects. This has been reported in patients with partial DiGeorge anomaly who do not receive transplants.41–43 Nephrotic syndrome and enteritis were seen in one subject each. Six subjects developed thyroid disease before, and 5 subjects developed thyroid disease after transplantation. Thyroid disease is associated with partial DiGeorge anomaly44–48 at rates from less than 1% to as high as 7%. Autoimmune hyperthyroidism (Graves disease) has been described in partial DiGeorge anomaly.45,47 Hashimoto thyroiditis has also been reported in partial DiGeorge anomaly.46 Antithyroglobulin and antithyroperoxidase antibodies have been found in both conditions.45–48 We continue to monitor the subjects closely for evidence of thyroid disease.
The outcome of subjects regarding survival, adverse events, and immune function was similar whether immunosuppression was used. Use of immunosuppression was based on the number and function of T cells present prior to transplantation. Only the most recent 8 atypical subjects receiving immunosuppression have been on post-transplantation cyclosporine, tacrolimus, and/or steroids. By 1 year, all developed naive T cells, proliferative responses to mitogens and antigens, and a normal TCRBV repertoire. The use of cyclosporine before and after transplantation has not only prevented graft rejection in subjects with atypical complete DiGeorge anomaly, but it has also improved the clinical condition of the infants who had severe rashes and failure to thrive associated with the oligoclonal T-cell proliferations.
This group of 54 infants is the largest series of infants with complete DiGeorge anomaly ever reported. We report 44 consecutive subjects treated with thymus transplantation with follow-up as long as 13 years. There were no transplantation-related deaths and 75% survive. It is gratifying that the initial promising immune laboratory results after thymus transplantation published in 199928 have translated into long-term good clinical functioning of the children for up to 13 years (median follow-up, 3 years 10 months) from transplantation. It is important to reemphasize that only half of patients with complete DiGeorge anomaly are 22q11 hemizygous. Accurate and prompt diagnosis becomes more important as thymus transplantation continues to be developed as a promising, life-saving therapy.
Authorship
Contribution: M.L.M. designed research, performed research, contributed new reagents or tools, collected data, analyzed data, and wrote the paper; B.H.D. designed research, performed research, collected data, analyzed data, and contributed to writing the paper; M.J.A. performed research and analyzed data; E.A.M. and S.E.G. performed research and collected data; I.K.C. performed research and analyzed data; L.P.H. analyzed data; T.B.K. contributed new tools and analyzed data; M.H. contributed new analytical tools. M.S. performed research, collected data, and analyzed data; J.L., M.A.S., H.E.R., and J.C.H. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: M. Louise Markert, Box 3068, DUMC, Durham, NC 27710; e-mail: marke001@mc.duke.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Page Anderson for identifying which subjects had conotruncal heart defects. We thank Drs J.M. Cook and S. Langdon of the Duke Comprehensive Cancer Center flow cytometry and sequencing facilities; Drs J. Jaggers and A. Lodge for acquiring thymus tissue; Drs Rebecca Buckley, Wesley Burks, Laurie Lee, Joseph Roberts, and Larry Williams in caring for the subjects; members of the protocol data and safety monitoring board (Drs R. Bollinger, R. H. Buckley, J. Dawson, B. F. Haynes, D. Howell, J. Kurtzberg, E. W. St Clair, P. Szabolcs) for their suggestions; Drs G. D. Sempowski and L. Cowell for reviewing the manuscript; and Dr Yi-Ju Li for statistical assistance. We thank the referring physicians for sending blood samples and data to us.
This work was supported by the National Institutes of Health (grants R01-AI47040, R01-AI 54843, R21 AI 60967, P30-AI51445 [Duke Center for Translational Research]), NCRR (clinical research grant M03-RR30), and FDA (grant FD-R-002606).
M.L.M., L.P.H., and T.B.K. are members of the Duke Comprehensive Cancer Center.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal