Abstract
Mesenchymal stromal cells (MSCs) have enormous potential for the regeneration of bone, cartilage, and other tissues derived from primitive mesoderm. Despite extensive research, there is still no single marker that reliably identifies MSCs within the bone marrow. Using immunocytochemistry and flow cytometry, we demonstrate here that the neural ganglioside GD2 is expressed by MSCs either newly isolated from bone marrow or expanded in tissue culture; this finding was supported by reverse transcriptase–polymerase chain reaction (RT-PCR) analysis showing expression of the mRNA for GD2 synthase, an essential enzyme for GD2 biosynthesis. GD2 was also expressed on MSCs isolated from adipose tissue, but not on foreskin fibroblasts. Importantly, MSCs were the only cells within normal marrow that expressed this marker. Thus, GD2 appears to be the first reported single surface marker that uniquely distinguishes MSCs from other marrow elements. GD2 may prove valuable to study MSC biology and for the preparation of MSCs for clinical applications.
Introduction
Human mesenchymal stromal cells (MSCs) are multipotent progenitors that can differentiate to bone, fat, cartilage, and other mesenchymal tissues.1,2 Termed mesenchymal stem cells by some investigators, MSCs can be isolated from a variety of tissues, but those from bone marrow are the most widely studied and best characterized. Interest in MSCs for diverse applications has grown rapidly over the last decade3–6 ; however, a single surface marker that would uniquely identify this population of cells within the bone marrow remains elusive.2
The first monoclonal antibodies used to characterize MSCs were SH2 and SH3,7 which later were shown to recognize epitopes on CD1058 and CD73,9 respectively. While these antigens remain the cornerstone of human MSC identification,2 they are also expressed on hematopoietic and endothelial cells. GD2 is a disialoganglioside found mainly in the nervous system. Reports of neural antigen expression on MSCs10,11 led us to consider that these cells might express GD2, which could be a useful marker of MSCs, as its expression should not extend to hematopoietic cells or other normal marrow elements. Here, we demonstrate the expression of GD2 by MSCs but not other cells within the bone marrow, suggesting that this antigen might serve as a single definitive marker of marrow-derived MSCs.
Materials and methods
Isolation of human MSCs
Bone marrow MSCs (from multiple donors) were isolated according to a protocol approved by the institutional review board of St Jude Children's Research Hospital, Memphis, TN, as previously described.12 Adipose-derived MSCs were obtained from tissue generously provided by Dr Jeffrey Gimble according to a standard protocol described elsewhere.13
Immunocytochemistry
We performed immunocytochemical studies on a formalin-fixed cell culture expanded on a sterile chamber slide, using a murine monoclonal antibody against GD2 (clone 14.G2A; BD Biosciences, San Jose, CA). The reaction was visualized with a biotinylated goat antimouse secondary antibody with ABC substrate and Nova Red as the chromogen (Vector Labs, Burlingame, CA). All slides were lightly counterstained with hematoxylin. Stained slides were examined on an Olympus BX41 (Olympus America, Melville, NY) with a 4×/0.13 NA dry objective, and photomicrographs were acquired using the attached SPOT RT color camera and SPOT RT v 3.2 software (Diagnostic Instruments, Sterling Heights, MI). Images were cropped and labeled using Photoshop 7.0 and Illustrator 10.0 (Adobe Systems, San Jose, CA).
Reverse transcription PCR
RNA was extracted with Trizol Reagent according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Primers for GD2 synthase were forward 5′-CCAACTCAACAGGCAACTAC-3′, reverse 5′-GATCATAACGGAGGAAGGTC-3′ (230-bp product), and forward 5′-GACAAGCCAGAGCGCGTTA-3′, reverse 5′-TACTTGAGACACGGCCAGGTT-3′ (99-bp product); primers for β2-microglobulin (333-bp product) were forward 5′-CTCGCGCTACTCTCTCTTTCTTGG-3′, reverse 5′-GCTTACATGTCTCGATCCACTTAA-3′. Polymerase chain reaction (PCR) conditions were denaturation at 95°C for 12 minutes, then 35 cycles at 95°C for 1 minute, 59°C for 1 minute, 72°C for 1 minute, and then 72°C for 10 minutes.
MSC differentiation
Culture-expanded MSCs were differentiated in vitro to osteoblasts,14 adipocytes,15 and chondroblasts,16 as described. The differentiated osteoblasts were stained with Alizarin Red S,17 the adipocytes with Oil Red O,18 and the chondroblasts with Alcian Blue,19 according to published protocols. Stained differentiated cells were imaged on a Nikon Eclipse TE300 inverted microscope (Nikon, Melville, NY) with a 40×/0.6 NA objective and an attached Nikon D70 camera (Nikon). Images were cropped and labeled using Photoshop 7.0 and Illustrator 10.0 (Adobe Systems).
Flow cytometry
All analyses were performed on a BD LSR II flow cytometer (BD Biosciences, San Jose, CA), with antibodies from BD Biosciences, except that the monoclonal antibody against human fibroblasts, clone D7-FIB, was from Serotec (Raleigh, NC). The data were analyzed with Cell Quest Pro Software Version 5.2.1 (BD Biosciences).
Immunoselection of MSCs
GD2-expressing MSCs were isolated from freshly harvested bone marrow mononuclear cells using our murine monoclonal antibody against GD2 and an allophycocyanin-conjugated donkey antimouse secondary antibody with the corresponding magnetic beads on an AutoMACS device (Miltenyi Biotech, Bergisch Gladbach, Germany), according to the manufacturer's instructions.
Results and discussion
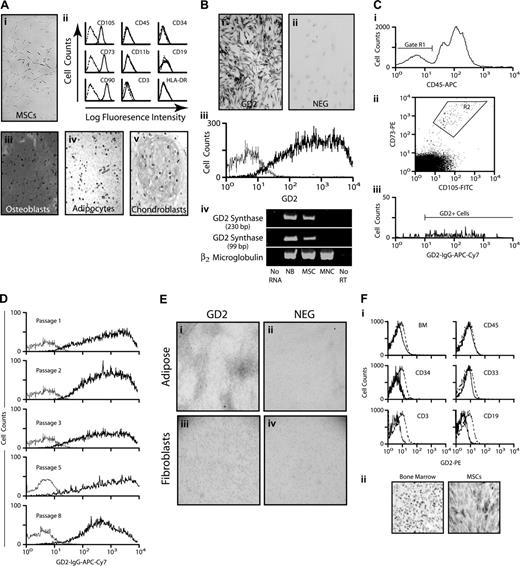
Human MSCs isolated from bone marrow showed the characteristic features of spindle shape and plastic adherence (Figure 1Ai). Flow cytometric analysis demonstrated the expression of distinguishing MSC antigens (CD105, CD73, and CD90), and the absence of hematopoietic and endothelial antigens (CD45, CD34, CD19, CD3, CD11b, and HLA DR; Figure 1Aii). The culture-expanded cells were capable of in vitro differentiation to osteoblasts as demonstrated by Alizarin Red staining; adipocytes, as demonstrated by Oil Red O staining; and chondroblasts as demonstrated by Alcian Blue staining (Figure 1Aiii-v). Thus, the isolated cells met the essential criteria used to define MSCs.2
Immunocytochemical staining of the marrow-derived MSCs revealed striking expression of the neural ganglioside GD2 (Figure 1Bi). Comparable staining was found on all MSCs, indicating a pancellular expression of GD2 among these bone marrow elements. Staining of MSCs without the primary antibody yielded negative results, demonstrating the lack of nonspecific staining (Figure 1Bii). To assess GD2 expression by an alternative method, we analyzed culture-expanded cells by flow cytometry, finding high levels of GD2 surface expression (Figure 1Biii). Reverse-transcription PCR analysis using 2 different primer pairs, with a neuroblastoma cell line and blood mononuclear cells serving as positive and negative controls, respectively, showed that MSCs expressed the mRNA for GD2 synthase (Figure 1Biv), an essential enzyme for GD2 biosynthesis. These results provide crucial supportive evidence of genuine ganglioside expression on MSCs.
GD2 expression on marrow MSCs. (Ai) Photomicrograph of undifferentiated MSCs showing the characteristic spindle shape and adherent properties of the cells. Original magnification, ×40. (ii) Flow cytometry histograms demonstrating the typical expression pattern of surface antigens (—) isotype and control (- - -), as indicated. (iii-v) Immunocytochemical staining demonstrating the differentiation of MSCs into osteoblasts (Alizarin Red stain), adipocytes (Oil Red O stain), and chondroblasts (Alcian Blue stain). (B) Immunocytochemical staining of culture-expanded MSCs for GD2 (i) and staining without the primary anti-GD2 antibody as a negative control (ii). (iii) Flow cytometry histogram showing GD2 expression (bold line) by MSCs and the isotype control (thin line). (iv) Reverse-transcription PCR for GD2 synthase. Results using primers generating a 230-bp product are shown at the top, primers generating a 99-bp product are in the middle, and β2-microglobulin as a control for the quality and quantity of RNA is at the bottom. No RNA indicates a complete reaction omitting the RNA sample; NB, RNA from neuroblastoma cells (positive control), MSC, culture-expanded after marrow derivation; MNCs, RNA from blood mononuclear cells (negative control); No RT, reaction with MSC RNA, but omitting reverse transcriptase. (Ci) Flow cytometry histogram of bone marrow cells for CD45 expression. The R1 gate indicates CD45− cells. (ii) Analysis of the CD45− cells from the R1 gate for CD105 and CD73 expression. The R2 gate indicates the double-positive cells. (iii) Analysis of the CD45−CD105+ CD73+ cells from the R2 gate for GD2 expression. These cells, MSCs from freshly harvested bone marrow, were never in tissue culture. (D) Flow cytometry histograms of GD2 expression on MSCs after serial passage in tissue culture. The experimental and control curves are as indicated in panel B. (E) Immunocytochemical staining of adipose-derived MSCs (i) and a negative control (ii) in which the primary anti-GD2 antibody was omitted. The specimens were lightly counterstained with hematoxylin. Original magnification, ×4. Immunocytochemical staining of foreskin fibroblasts (iii) and a negative control (iv) as for the adipose-derived MSCs. Original magnification, ×4. (Fi) Flow cytometric histograms showing the lack of GD2 expression on unfractionated bone marrow cells (upper left, BM), and on marrow cells expressing CD45 (leukocytes), CD34 (hematopoietic progenitors), CD33 (myeloid cells), CD3 (T-lymphocytes), or CD19 (B-lymphocytes). anti-GD2 antibody (—), isotype control (- - -). (ii) Anti-GD2 immunohistochemical staining of a bone marrow biopsy specimen (left) and MSCs (right, positive control). Both specimens were counterstained with hematoxylin.
GD2 expression on marrow MSCs. (Ai) Photomicrograph of undifferentiated MSCs showing the characteristic spindle shape and adherent properties of the cells. Original magnification, ×40. (ii) Flow cytometry histograms demonstrating the typical expression pattern of surface antigens (—) isotype and control (- - -), as indicated. (iii-v) Immunocytochemical staining demonstrating the differentiation of MSCs into osteoblasts (Alizarin Red stain), adipocytes (Oil Red O stain), and chondroblasts (Alcian Blue stain). (B) Immunocytochemical staining of culture-expanded MSCs for GD2 (i) and staining without the primary anti-GD2 antibody as a negative control (ii). (iii) Flow cytometry histogram showing GD2 expression (bold line) by MSCs and the isotype control (thin line). (iv) Reverse-transcription PCR for GD2 synthase. Results using primers generating a 230-bp product are shown at the top, primers generating a 99-bp product are in the middle, and β2-microglobulin as a control for the quality and quantity of RNA is at the bottom. No RNA indicates a complete reaction omitting the RNA sample; NB, RNA from neuroblastoma cells (positive control), MSC, culture-expanded after marrow derivation; MNCs, RNA from blood mononuclear cells (negative control); No RT, reaction with MSC RNA, but omitting reverse transcriptase. (Ci) Flow cytometry histogram of bone marrow cells for CD45 expression. The R1 gate indicates CD45− cells. (ii) Analysis of the CD45− cells from the R1 gate for CD105 and CD73 expression. The R2 gate indicates the double-positive cells. (iii) Analysis of the CD45−CD105+ CD73+ cells from the R2 gate for GD2 expression. These cells, MSCs from freshly harvested bone marrow, were never in tissue culture. (D) Flow cytometry histograms of GD2 expression on MSCs after serial passage in tissue culture. The experimental and control curves are as indicated in panel B. (E) Immunocytochemical staining of adipose-derived MSCs (i) and a negative control (ii) in which the primary anti-GD2 antibody was omitted. The specimens were lightly counterstained with hematoxylin. Original magnification, ×4. Immunocytochemical staining of foreskin fibroblasts (iii) and a negative control (iv) as for the adipose-derived MSCs. Original magnification, ×4. (Fi) Flow cytometric histograms showing the lack of GD2 expression on unfractionated bone marrow cells (upper left, BM), and on marrow cells expressing CD45 (leukocytes), CD34 (hematopoietic progenitors), CD33 (myeloid cells), CD3 (T-lymphocytes), or CD19 (B-lymphocytes). anti-GD2 antibody (—), isotype control (- - -). (ii) Anti-GD2 immunohistochemical staining of a bone marrow biopsy specimen (left) and MSCs (right, positive control). Both specimens were counterstained with hematoxylin.
All cells analyzed in the preceding experiments had been expanded for 2 passages in culture. Since GD2 can play a role in cellular adhesion20 we considered that plastic adherence of MSCs in vitro might induce or alter the expression of GD2 in a manner different from the normal regulation of the ganglioside. To address this possibility, we analyzed newly harvested bone marrow mononuclear cells (MNCs) by 4-color flow cytometry, gating first on marrow MNCs that lacked CD45 expression (Figure 1Ci, gate R1). These cells (R1) were then analyzed for CD105 and CD73 expression (Figure 1Cii). About 95% of the CD45−CD105+ CD73+ cells (gate R2) expressed GD2 (Figure 1Ciii). Further, newly harvested marrow MNCs that expressed either CD271 or D7Fib, both of which have been suggested to represent MSCs,21,22 were found to coexpress GD2 (data not shown). The intensity of GD2 expression exceeded that of either CD271, which is lost during culture, or D7Fib, which is also expressed on skin fibroblasts. Notably, the entire population of expanded MSCs continued to express GD2 at similar levels through 8 culture passages (Figure 1D).
To determine if GD2 expression is unique to marrow-derived MSCs, we analyzed such cells from adipose tissue. As shown in Figure 1E, the adipose-derived cells also expressed GD2 at approximately the same level as marrow-derived MSCs. Foreskin fibroblasts, by contrast, lacked GD2 expression (Figure 1E).
Finally, flow cytometric analysis of unfractionated marrow cells, leukocytes (CD45), hematopoietic progenitors (CD34), myeloid cells (CD33), T-lymphocytes (CD3), and B-lymphocytes (CD19) demonstrated the absence of GD2 expression (Figure 1F). Our RNA analysis revealed the lack of GD2 synthase expression in circulating blood cells (Figure 1B). Most importantly, immunohistochemical staining of normal bone marrow biopsy specimens (Figure 1F) did not detect other GD2-expressing cells. Collectively, these data indicate the lack of hematopoietic GD2 expression, consistent with prior reports.23,24
Cells isolated from freshly harvested bone marrow by selection for GD2 expression using immunomagnetic beads demonstrated typical MSC morphology and plastic adherence (Figure 2). The culture-expanded cells differentiated to osteoblasts, adipocytes, and chondroblasts (Figure 2), as expected for MSCs.
Ex vivo expansion and trilineage differentiation of GD2-selected MSCs. Photomicrograph of undifferentiated, ex vivo–expanded cells (upper panel) after isolation by GD2 selection. The characteristic spindle shape morphology and plastic adherence of MSCs is shown. Immunocytochemical staining (lower panels) demonstrates the differentiation of the GD2-selected MSCs into osteoblasts (Alizarin Red stain), adipocytes (Oil Red O stain), and chondroblasts (Alcian Blue stain).
Ex vivo expansion and trilineage differentiation of GD2-selected MSCs. Photomicrograph of undifferentiated, ex vivo–expanded cells (upper panel) after isolation by GD2 selection. The characteristic spindle shape morphology and plastic adherence of MSCs is shown. Immunocytochemical staining (lower panels) demonstrates the differentiation of the GD2-selected MSCs into osteoblasts (Alizarin Red stain), adipocytes (Oil Red O stain), and chondroblasts (Alcian Blue stain).
GD2 is the first candidate marker of MSCs to be consistently expressed at a high level on all cells of this population, whether freshly isolated or ex vivo expanded; hence, GD2 selection may offer an improved approach for MSC identification and isolation. Immune recognition of the GD2-expressing MSCs in the marrow microenvironment may underlie the hematopoietic suppression observed when anti-GD2 antibodies are used as immunotherapy for neuroblastoma.
A critical question in MSC biology is whether this cell population possesses a relatively uniform differentiation capability, and responds to specific signals to generate diverse mesenchymal lineages, or is comprised of biologically distinct subsets of progenitors committed to differentiate in particular pathways. The availability of a marker antigen such as GD2 would be useful in resolving this issue, much in the way that CD34 has helped to discriminate among subsets of hematopoietic progenitors.
Authorship
Contribution: C.M. designed, performed, and analyzed research, and assisted with preparation of the manuscript; T.J.H. designed and analyzed research; R.M. provided important analytical tools and performed research; M.D. provided important analytical tools and analyzed research; E.M.H. supervised the entire project, designed and analyzed research, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edwin M. Horwitz, Division of Bone Marrow Transplantation, St Jude Children's Research Hospital, 332 N Lauderdale, Mail Stop 321, Memphis, TN 38105; e-mail: edwin.horwitz@stjude.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge Drs Richard Ashmun, Ann Marie Hamilton-Easton, and James Houston for the flow cytometric analyses, and John Gilbert for editorial review. This work was supported in part by grants NHLBI R01 HL077 643 and NCI T32 CA070 089, Cancer Center Support (CORE) grant NCI P30 CA 21 765, and the American Lebanese Syrian Associated Charities (ALSAC).