Abstract
Point mutations in the gene for the granulocyte colony-stimulating factor (G-CSF) receptor CSF3R have been implicated in the progression of severe congenital neutropenia (CN) to leukemia. In this study we present data on a total of 218 patients with chronic neutropenia, including 148 patients with CN (23/148 with secondary malignancies). We detected CSF3R nonsense mutations at 17 different nucleotide positions (thereof 10 new mutations) which lead to a loss of 1 to all 4 tyrosine residues in the intracellular domain of the receptor. Of 23 patients with CN with signs of malignant transformation, 18 (78%) were shown to harbor a CSF3R mutation, indicating that these mutations, although not a necessary condition, are highly predictive for malignant transformation even if detected in a low percentage of transcripts. In serial analyses of 50 patients with CSF3R mutations we were able to follow the clonal dynamics of mutated cells. We could demonstrate that even a highly clonal hematopoiesis did not inevitably show a rapid progression to leukemia. Our results strongly suggest that acquisition of a CSF3R mutation is an early event in leukemogenesis that has to be accompanied by cooperating molecular events, which remain to be defined.
Introduction
Severe congenital neutropenia (CN) is a rare disease characterized by an absolute neutrophil count (ANC) of less than 200 cells/mm3 from birth on, and a predisposition to recurrent and life-threatening infections.1-3
About 95% of all patients respond to pharmacologic doses of recombinant granulocyte colony-stimulating factor (G-CSF) with rising neutrophil counts.4-6 In consequence, infection rates fall, mortality is reduced, and quality of life increases. The diverse effects of G-CSF are mediated through the interaction with its cell-surface receptor G-CSFR, a single chain receptor containing 813 amino acids. The membrane proximal cytoplasmic region of the receptor has been shown to transduce proliferative and survival signals, whereas the distal region transduces maturation signals and suppresses the receptor's proliferative signals.7-9
CN was first described by Kostmann in 1956 as an autosomal recessive disease.10 Since that time sporadic as well as autosomal dominant cases have also been described.3 A correlation between G-CSFR signaling and the pathophysiology of CN could not be established. Several studies in patients with CN have indicated normal production of endogenous G-CSF,11-13 normal G-CSFR expression and affinity,14 and absence of serum inhibitors of myeloid development.15-17
The higher life expectancy under G-CSF treatment revealed the increased risk of patients with CN for developing hematopoietic malignancies. According to the data of the Severe Chronic Neutropenia International Registry there is a cumulative incidence of 21% for a malignant transformation in CN after 10 years on G-CSF.18 However, this does not seem to be a direct effect of G-CSF treatment: malignant conversion is not seen with prolonged use of recombinant G-CSF in patients with CyN or with idiopathic neutropenia,3 and cases of malignant transformation in CN have also been reported in patients not treated with G-CSF.19-21
Mutations in a region of the CSF3R gene coding for the intracytoplasmic domain of the G-CSFR have been discovered in patients with CN and were initially suggested to be the cause of CN.22,23 In the following years it became obvious that these mutations were acquired somatic mutations, which are associated with progression to leukemia in CN.23-25
Until now, 8 different mutations in the region of the CSF3R gene coding for the cytoplasmic domain of the receptor have been described (Table S1, available at the Blood website; see the Supplemental Table link at the top of the online article). Except for one missense mutation reported for one patient with CN/acute myeloid leukemia (AML),26 all introduce a stop codon predicted to lead to the truncation of the intracellular part of the G-CSFR with a loss of the carboxyterminal-negative regulatory domain.23 Expressing these mutations in myeloid cell lines led to enhanced proliferation, inhibition of maturation, resistance to apoptosis, and increased cell survival.27,28 Knock-in mice bearing receptor mutants equivalent to those found in CN showed a hyperproliferative response to exogenous G-CSF27,29 but, despite prolonged G-CSF administration, not one developed leukemia.
From the in vitro and in vivo data and from the fact that somatic CSF3R mutations are frequently detected prior to morphologic evidence of transformation, we have hypothesized that they represent an important step in the progression of CN to myelodysplastic syndrome (MDS)/leukemia.25,30
We report on a long-term study designed to determine the incidence and the course of the occurrence of mutations in the CSF3R gene and their significance for malignant transformation in patients with CN.
Patients, materials, and methods
Patients
The study included 218 patients with severe chronic neutropenia who were screened for mutations in the region of the CSF3R gene coding for the intracytoplasmic domain of the G-CSFR. Of these patients, there were 148 with CN, 8 with Shwachman-Diamond syndrome, 2 with myelokathexis, 4 with glycogen storage disease type 1b, 4 with hyper-IgM syndrome, 2 with Barth syndrome, 18 with cyclic neutropenia, 29 with idiopathic neutropenia, and 3 with autoimmune neutropenia. As control groups we tested 62 patients with severe aplastic anemia (SAA) and 34 patients with primary leukemia. Approval was obtained from the ethics committee of the Hannover Medical School. Informed consent was provided according to the Declaration of Helsinki.
The diagnosis of CN was based on an ANC of less than 200/μL in peripheral blood, maturation arrest at the promyelocyte/myelocyte level in the bone marrow, recurrent severe bacterial infections during the first year of life, and absence of antineutrophil antibodies. For 126 of 148 patients with CN we had information about G-CSF therapy. Most of these patients were regularly treated with G-CSF (117/126).
Molecular analyses of the CSF3R mRNA and genomic DNA
RNA was extracted from the neutrophils and mononuclear cells of peripheral blood and marrow. Total RNA was isolated using the guanidinium thiocyanate method and treated with DNAse. In some rare cases viable cells were not available and genomic DNA was prepared for mutation analysis. Fragments including the mutation-sensitive region 2342-2541 were polymerase chain reaction (PCR)–amplified from cDNA after reverse transcription of mRNA or from genomic DNA and sequenced after subcloning. Detailed protocols and primer sequences are available on request. This procedure has been performed at least twice for the investigation of 2 independently prepared RNAs or genomic DNAs. In total we analyzed between 17 and 45 Escherichia coli clones generated from mRNA and DNA from each patient per time. The purified plasmids have been sequenced using semiautomated sequencing.
Results
Occurrence of CSF3R mutations
Only in the group of patients with CN we detected CSF3R mutations (61/148, 41%). Even in 5 of 30 patients with CN tested without prior G-CSF therapy we detected CSF3R mutations. No mutations were found in any other patient group tested (0/70 severe chronic neutropenia except CN, 0/62 SAA, 0/34 primary leukemia). We detected nonsense mutations in a region of the CSF3R gene spanning nucleotides 2300-2600, which are predicted to lead to a truncation of the carboxyterminal region of the G-CSFR.
The incidence of CSF3R mutations in the group of patients with CN without any signs of malignant transformation up to the time of analysis was 34% (43/125). In contrast, 78% (18/23) of the patients with CN/monosomy 7 and/or MDS/leukemia harbor a CSF3R mutation. The correlation between the acquisition of CSF3R mutations and malignant transformation was highly significant using Pearson chi square test (Table 1)
In 5 of 23 patients with CN who developed MDS/leukemia, no CSF3R mutations could be detected, suggesting that the occurrence of CSF3R mutations is not a necessary prerequisite for leukemogenesis in CN. However, the risk of secondary malignancies was much higher in the group of patients with CSF3R mutations compared with patients without mutations (odds ratio: 6.9; 95% confidence interval: 2.4-19.8).
Diversity of CSF3R mutations
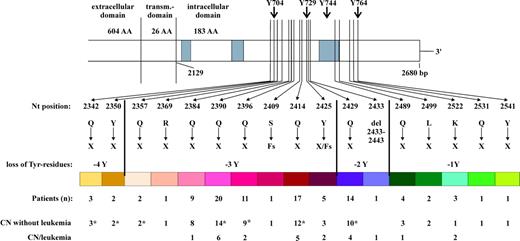
In patients with CN we detected CSF3R mutations at 17 different nucleotide positions (Figure 1). In addition to point mutations we detected 3 frame shift mutations. All mutations are predicted to lead to the loss of a C-terminal fragment of variable length including 1 to 4 of the intracellular 4 tyrosine residues. The mutations detected most frequently in patients with CN were located at nucleotides 2384, 2390, 2414, and 2429, whereas the nonsense mutations at nucleotides 2350, 2369, 2409, 2531, and 2541 and the deletion of nucleotides 2433-2443 were detectable only in a single patient, respectively.
CSF3R mutations in patients with CN. *Including patients who underwent HSCT.
We investigated the specific association of certain mutations with leukemogenesis. Most patients with MDS/leukemia secondary to CN harbored nonsense mutations at nucleotides 2384, 2390, 2396, 2414, 2425, and 2429. There were some rare mutations that were found exclusively in patients who have not yet developed a malignancy (Figure 1). However, since the numbers are small we cannot make a statement about a relative risk for malignant transformation for single rare mutations.
Occurrence of multiple CSF3R mutations
Some patients tested displayed only a single nonsense mutation whereas other patients were found to have nonsense mutations at 2 or more nucleotide positions. We compared the risk of developing leukemia between patients with 1 or multiple mutations in the CSF3R gene. We found no difference in the proportion of patients having single or multiple mutations between the groups of patients with and without malignant transformation (18/43 or 37% with multiple mutations in the group of patients without MDS/leukemia vs 6/18 or 33% in the group of patients with MDS/leukemia). A single CSF3R nonsense mutation seems to have the same predictive value for leukemic progression as multiple mutations. We found no correlation between G-CSF therapy and the number of different mutations in patients. Multiple mutations from single patients were always found segregated in cloned PCR products, indicating that they were not present in the same allele.
Course of occurrence and rate of CSF3R mutations
For 50 of 61 patients with CN with CSF3R mutations analyses could be performed more than once (2-10 times; Table 2) In nearly one third of these patients we detected a single mutation once in one single clone (representing 3%-5% of CSF3R mRNA). At other times (before and after) no mutations were detected. One patient out of this group developed MDS 6 months after detection of the mutation 2396 in a single clone. In 5 patients we found CSF3R mutations in one single clone at different times. We never detected any mutations in the analyzed region of the CSF3R gene in a large number of healthy donors or patients suffering from diseases other than CN using the same methodology.
We could not define an age of increased susceptibility for the acquisition of CSF3R mutations. Although most patients (47/61, 77%) acquired mutations at the age of 0 to 15 years (median: 9 years), the risk for the remaining patients seemed not to decrease with increasing age; a noticeable number of patients developed mutations at an age older than 15 years (8/61: 15-20 years; 6/61: > 20 years). We found no differences between the patient groups with or without development of MDS/leukemia with respect to the age of first detection of a CSF3R mutation. Even the analysis of duration of G-CSF treatment before detection of a CSF3R mutation did not reveal a time span of increased risk (median: 4 years).
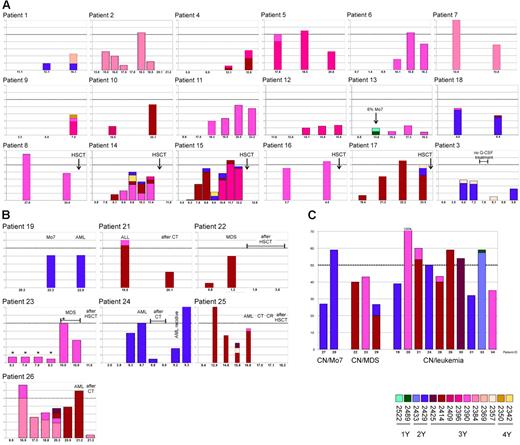
Detailed analysis of 26 patients with mutations in multiple clones at different times revealed a high variability in the time courses of mutation frequencies (Figure 2A-B). In most cases a low percentage of mutated clones was present in first analyses, followed by a phase of continuous increase of mutation frequency. This phase could last for several years and could be intermitted by a transient decline. We also observed drastic changes of the frequency within a few months as well as stable mutation frequencies over several years.
Course and rate of CSF3R mutations in patients with CN. Plotted is the percentage of mutated clones at different times (age in years) for patients with CN. The different mutations are encoded by different colors, which correspond to Figure 1. (A) Patients who have not developed a malignancy so far (including patients who underwent HSCT). (B) Patients with secondary malignancies (*Data from Bernard et al31 and Ancliff et al32 ). (C) Rate of mutated clones at time of malignant transformation.
Course and rate of CSF3R mutations in patients with CN. Plotted is the percentage of mutated clones at different times (age in years) for patients with CN. The different mutations are encoded by different colors, which correspond to Figure 1. (A) Patients who have not developed a malignancy so far (including patients who underwent HSCT). (B) Patients with secondary malignancies (*Data from Bernard et al31 and Ancliff et al32 ). (C) Rate of mutated clones at time of malignant transformation.
Some patients developed different mutations during the course of the disease. The detection of different mutations in one patient at different times is indicative for an independent acquisition of mutations by different cells. In almost all patients with multiple mutations we found a predominant expression of one mutation at a time. A new mutation was able to displace another previously predominant mutation (eg, mutations 2414 and 2396 in patient no. 15). The recurrence of apparently lost mutations argues against a complete disappearance but for maintenance at a level below the detection limit (eg, mutation 2384 in patient no. 26, mutation 2414 in patient no. 15).
We found high proportions of mutated clones at onset of MDS or acute leukemia (Figure 2C). For most of the patients (9/15) the frequency of mutated clones was near 50%, which is consistent with an involvement of nearly 100% of the analyzed cells. In patient no. 20, who has been reported recently,21 we found an exclusive expression of mutated mRNA.
Treatment of MDS/leukemia with chemotherapy led to a strong reduction or even elimination of mutated clones (Figure 2B), indicating that the leukemic cells bear the mutation. In most cases there was no complete disappearance of the mutation after chemotherapy. This may be partly due to an incomplete depletion of leukemic cells but rather more to a low proportion of nonmalignant cells bearing a CSF3R mutation. As expected, we did not find any CSF3R mutations in patients after hematopoietic stem cell transplantation (Figure 2A-B).
The influence of G-CSF on the propagation of cells bearing a CSF3R mutation is an important issue in the discussion about potential risks of G-CSF treatment. For patient no. 3 we had the opportunity to perform analyses before, during, and after a discontinuation of the G-CSF therapy for 2.5 months (Figure 2A). During the time of G-CSF treatment this patient acquired the mutations 2429 and 2357. After discontinuation of G-CSF treatment we did not detect one of the previously found mutations. Both mutations could be detected again in later analyses after continuation of G-CSF treatment.
Discussion
CN and CSF3R mutations
Patients with CN are at increased risk for the development of MDS/leukemia. Recent studies suggest that the occurrence of acquired mutations in the CSF3R gene is correlated with the development of secondary leukemia in CN.24,30,33
In vitro studies demonstrating that truncated G-CSFR molecules transduce strong proliferative but defective maturation signals could provide a mechanistic basis for the involvement of CSF3R mutations in leukemogenesis.7-9 However, there is no easy causal relationship: (1) the acquisition of CSF3R mutations does not inevitably lead to leukemia within a defined time span; (2) a transient presence of CSFR3 mutations has been observed in some patients31 ; (3) cases of leukemia in CN without occurrence of CSF3R mutations have been reported.31,34 From these facts some authors hypothesized that CSF3R mutations do not possess a primary role in leukemogenesis.31
In this report we present data of a long-term survey of a large cohort of patients with CN that allows making more precise statements about the incidence of CSF3R mutations and the correlation with leukemogenesis in CN.
We could confirm that the acquisition of CSF3R mutations is highly specific for patients with CN. These mutations were not found in patients with primary AML, nor in the preleukemic diseases SDS or AA, nor in other subgroups of chronic neutropenia, where long-term treatment with recombinant G-CSF is part of the regular therapy. Besides the already-published 7 nonsense mutations at nucleotide positions 2314 to 2429 (Table S1) we found 10 new mutations extending the region of the CSF3R gene susceptible for the acquisition of mutations.
Localization of CSF3R mutations
All mutations found in this study are predicted to lead to the loss of at least 1 intracytoplasmic tyrosine residue (Y764), the great majority of mutations lead to the loss of 2 or 3 tyrosine residues (Y764, Y744, and Y729), some rare mutations lead to the loss of all 4 tyrosine residues. These conserved tyrosine residues are important docking sites for Src homology 2 (SH2)–containing signaling molecules and are implicated in the control of differentiation of myeloid progenitors.35-38 Early studies dissecting the different roles of intracytoplasmic domains in G-CSFR function demonstrated that the N-terminal region is sufficient to transduce a proliferation signal, whereas the C-terminal region plays an essential role for differentiation.7,9,39 The situation has become more complex due to a number of subsequent studies carried out to assign the control of proliferation, survival, and differentiation pathways to distinct regions of the G-CSFR.29,35-38,40-48 Based on these studies one could expect some heterogeneity in consequence of different CSF3R mutations, which lead to a loss of variable parts of the intracytoplasmic domain and to activation of different signaling pathways. However, there were no obvious differences in the course of the disease and in the risk for developing a secondary leukemia between patients with different mutations. Only in the group of patients with mutations at nucleotides 2342 and 2350 with a loss of all tyrosine residues was there no development of a malignancy until now. However, 3 of 5 patients in this group have been treated with hematopoietic stem cell transplantation (HSCT) referring to a severe course of the disease.49 Moreover, Cassinat et al33 recently described a case of CN and secondary acute lymphoblastic leukemia (ALL) with nonsense mutation 2342 leading to a loss of all intracytoplasmic tyrosines.
It was remarkable that the CSF3R mutations that lead to a loss of 1 or 4 tyrosines (in contrast to the predominant mutations leading to the loss of 2 or 3 tyrosines) were always detected only in a small percentage of clones. None of these mutations were detected predominantly in malignancies secondary to CN (Figure 2B-C). This argues for a strong proliferative advantage only of cells with mutations leading to the loss of 2 or 3 tyrosines (marked with shades of red and violet in Figures 1 and 2).
Frequency of CSF3R mutations and correlation to malignant transformation
The strong correlation between the occurrence of CSF3R mutations and leukemic development together with a mechanism suggested by in vitro studies lead to the concept of CSF3R mutations as an early step in malignant development in CN. The percentage of 40% of CSF3R mutations in patients with CN reported here is higher than that in earlier studies.24,43,50 These early reports documented a high correlation between the occurrence of CSF3R mutations and leukemogenesis. In contrast, a report by Ancliff et al32 revealed a frequency of only 7% in a cohort of 29 patients with CN and a bad correlation to MDS/AML development. Most of the published data on CSF3R mutations in CN cannot be compared with our data since they are biased by the restriction on the analysis of patients with CN with malignancies. For example, in a study of the French neutropenia register including 101 patients, the authors investigated only 4 patients who developed MDS/leukemia and found 3 patients with CSF3R mutations.33
There are a number of possible explanations for the apparent discrepancy between the different reports. Most of the studies (1) used the method of direct sequencing of cDNA or restriction analysis, which could be inappropriate to detect low levels of heterozygously mutated cells and (2) only screened for the 5 previously described mutations. Also, one could assume a higher cumulative effect in this study compared with others since we overlook a large cohort of patients over a period of several years including patients older than 20 years. This is also reflected in the higher percentage of patients transformed to malignancy (16%).
From our investigations on the clonal succession of various CSF3R mutations we can conclude that the acquisition of a CSF3R mutation is an event that does occur prior to malignant transformation. It is not a secondary event giving a growth advantage to an already-transformed cell clone, as suggested by Bernard et al.31 The highly elevated risk for leukemic progression in patients who have acquired a CSF3R mutation argues for a significant contribution of these mutations in leukemogenesis. However, it is unlikely that a CSF3R mutation is a sufficient condition for malignant transformation because (1) there is a great variability in the time course from the detection of mutations and malignant transformation in patients, and (2) some patients harbor CSF3R mutations over years without malignant transformation.
Other molecular events that are necessary for a malignant transformation of cells already bearing a CSF3R mutation have not yet been defined in CN. Mutations in the RAS genes have been discussed as late events in leukemogenesis in CN.21,51,52 Recent results of our group have shown that RAS mutations are less common during malignant transformation in CN than suggested by earlier studies and are rarely preceded by CSF3R mutations.53
Is there a clinical relevance of low level detection of CSF3R mutations?
One possible explanation for the sporadic finding of low level mutations is that mutated cells are stably persistent at a low level and are detected randomly. Another explanation could be that the acquisition of nonsense mutations in the CSF3R gene in hematopoietic progenitors is a more common event. Those cell clones could be eliminated due to either apoptosis or normal clonal succession.54,55 The latter hypothesis is supported by the fact that some patients display different mutations at different times and that mutations which are found in early analyses of patients are not inevitably those which are detected later in leukemia. One could postulate a yet-unknown cellular alteration that increases the susceptibility of the CSF3R gene for mutations as the primary event in leukemogenesis in CN. Anyway, both scenarios support the hypothesis of increased susceptibility of the CSF3R gene for mutations as a predictive marker for malignant transformation.
The early detection of CSF3R mutations as a molecular marker of future malignant transformation demands a sensitive detection method. Even patients with a stable low-level persistence of a mutated CSF3R mRNA over a long period of time31 are at risk of developing a malignancy during the further course of the disease.32 Our results further emphasize the relevance of even the sporadic finding of a single mutated clone. The detection of a mutation even in a single clone is a strong predictor of later leukemic development and therefore should result in a more intensive diagnostic monitoring.
Clonal evolution of hematopoietic cells bearing a CSF3R mutation
The method used in the present study also allows a relative quantification of mutated transcripts in the myeloid cells. In some cases the levels of mutated mRNA were very low at the time of initial detection, rose up to levels around 50%, and the patients developed leukemia or underwent HSCT. At the time of malignant transformation the frequency was about 50% in most cases, suggesting that all leukemic cells were heterozygously mutated. Accordingly, the level of mutated mRNA showed a sharp decline after initiation of chemotherapy.
Intriguingly, in some patients the percentage of mutated clones reached high levels (arguing for a highly clonal hematopoiesis) without subsequent development of leukemia. The occurrence of different CSF3R mutations in the same patient and the fact that this is no more common in patients who progress to leukemia further support the idea that CSF3R mutations contribute to leukemia but are not a sufficient condition. It also argues that cells with these mutations are under strong clonal selection.
In 2 patients (patient no. 15 and no. 26) we observed the occurrence and expansion of a single mutation up to a high level, which in succession was displaced by another mutation. At present we cannot decide whether the growth advantage of the second clone is due to the different CSF3R mutation or to other secondary alterations. The fact that an equal mutation can show either a low-level expression over several years or a rapid expansion over a short period of time argues against a sole influence of the CSF3R mutation. We cannot exclude the possibility that the mutations that are unstable occur in stem cells without other cooperating lesions and therefore do not persist.
CSF3R mutations and treatment
As a consequence of our findings we recommend annual investigations of the CSF3R gene for all patients with CN in addition to the usually performed morphologic and cytogenetic evaluations of bone marrow.1 In case of detection of a CSF3R mutation the patients should be kept under close observation to follow the course of the detected mutation. The option of HSCT should be discussed with the family to start HLA typing. If no HLA identical family donor is available the search for a matched unrelated donor should also be considered, since the outcome of HSCT after MDS or frank leukemia secondary to CN is poor.49
The role of G-CSF treatment for the development of leukemia in CN has been discussed for a long time.4,56 In fact, some studies revealed an increased risk for malignant transformation in patients receiving high G-CSF doses.18,57 A G-CSF–dependent propagation of cells bearing CSF3R mutations could be demonstrated in patient no. 3, in whom G-CSF therapy has been discontinued for several months. Other groups have reported similar courses: Bernard et al31 reported a case in which a CSF3R mutation could no longer be detected after discontinuation of G-CSF treatment. Jeha et al58 could demonstrate a direct relation between G-CSF administration and blast count in a patient with leukemia secondary to CN. On the other hand, the spontaneous disappearance of CSF3R mutations in patients could also be observed in patients under G-CSF therapy (eg, patient no. 2) and could be due to normal clonal succession in hematopoiesis.54,55 Moreover, reports on malignant transformation of patients with CN without cytokine treatment19-21 could argue for the fact that even the normal or increased levels of endogenous G-CSF11-13 are sufficient to drive the propagation of preleukemic cells. Nevertheless, discontinuation of G-CSF therapy has to be taken into consideration in case of highly clonal hematopoiesis with CSF3R mutations since it may reduce the selective advantage for the mutant clone.
A direct effect of G-CSF treatment on the genesis of CSF3R mutations also has to be discussed: increased mitosis rates of G-CSFR–expressing progenitors and the accessibility of the CSF3R gene for transcription could make the CSF3R gene more vulnerable for damage and, in consequence, for mutations. Further investigations are necessary to clarify this issue.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
We are indebted to the patients and families who participated in this study and the referring physicians, in particular Cornelia Zeidler and the local liaison physicians of the Severe Chronic Neutropenia International Registry for their cooperation. We thank Eva Brinkmann, Marly Dalton, Sabine Jakobs, Sabine Lang, Christina Reimer, and Sarina Scharbatke for technical assistance as well as Heinz Geerlings and Bernhard Vaske for help with the statistical analyses. This work was supported by grants of the Deutsche Krebshilfe (M.G.) and of the Federal Ministry of Education and Research (German Network on Congenital Bone Marrow Failure Syndromes; M.B. and M.G.).