While likely being neither a necessary nor a sufficient factor, Germeshausen and colleagues demonstrate that 78% of patients with severe congenital neutropenia who undergo a malignant transformation to leukemia harbor mutations in the G-CSF receptor gene, CSF3R.
Treatment with pharmacologic doses of recombinant granulocyte colony-stimulating factor (G-CSF) has dramatically changed the clinical course of patients with severe congenital neutropenia. With more than 95% of patients responding to G-CSF, infection rates have fallen, mortality has been reduced, and quality of life has increased. The increased life expectancy in severe congenital neutropenia has unearthed a new problem, an approximate 15% risk for transformation to myelodysplasia or leukemia.1 Initial fears that the G-CSF treatment itself caused the problem have been dispelled. It is now becoming more clear that as patients with severe congenital neutropenia (CN) live longer, somatic mutations are acquired in their hematopoietic cells that are associated with the progression to a malignant leukemia phenotype. The neutrophil elastase gene, ELA2, has been demonstrated to be the causative defect in cyclic neutropenia,2 presumably by mutated ELA2 causing impaired proliferation and accelerated apoptosis in myeloid progenitor cells. Similarly, heterozygous ELA2 mutations have been found in 60% of patients with CN, indicating that it is likely a cooperating molecular event in CN pathogenesis.
The earliest reports of another somatic event, CSF3R mutations in patients with CN that had transformed to leukemia, began to appear in the literature more than a decade ago.3,4 Prior to the report by Germeshausen and colleagues in this issue of Blood, 8 different mutations had been described in the cytoplasmic carboxyterminal–negative regulatory domain. The present report importantly describes 10 new mutations. This report of an extensive cohort of patients with a lengthy longitudinal analysis contains data on 148 patients with CN, 23 of whom developed secondary monosomy 7, myelodysplasia, acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), or chronic myelomonocytic leukemia (CMML). Of the cohort of 148 patients with CN, 61 (41%) were found to harbor CSF3R mutations, including 5 of 30 patients with CN who never received G-CSF therapy, adding further evidence that G-CSF therapy does not cause these mutations. Since only 23 in the cohort developed subsequent transformation, and not 61, this proves that CSF3R mutations are not sufficient for malignant transformation. Most (18 [78%]) of the 23 patients with CN who had secondary transformation had CSF3R mutations in their hematopoietic cells, also proving that this is not a necessary condition for transformation.
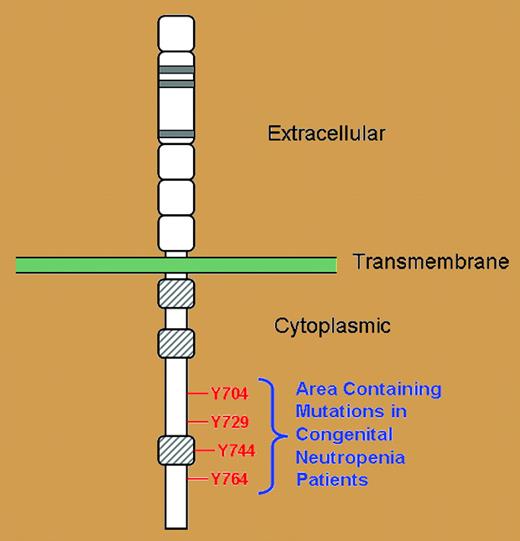
Important sequence analysis in this study demonstrated that all of the CSF3R mutations in all patients led to loss of at least 1 intracytoplasmic tyrosine residue (Y764), but in many cases, 2, 3, or sometimes all 4 critical tyrosine residues on the G-CSF receptor were lost in the nonsense mutations (see figure). These 4 tyrosine residues are vital docking sites for intracytoplasmic SH2-containing signaling molecules necessary for the transmission ofregulatory signals for the proliferation, differentiation, and survival properties of myeloid progenitor cells.5,6 Without these critical tyrosine residues serving as docking sites, these intracytoplasmic SH2-containing signaling molecules are left to precariously drift in the cytoplasmic sea, unable to exert their normal regulatory control.
Schematic representation of the G-CSF receptor with extracellular, transmembrane, and cytoplasmic regions as indicated. Gray-hatched boxes represent conserved cytoplasmic subdomains. Four cytoplasmic tyrosine residues indicated in red.
Schematic representation of the G-CSF receptor with extracellular, transmembrane, and cytoplasmic regions as indicated. Gray-hatched boxes represent conserved cytoplasmic subdomains. Four cytoplasmic tyrosine residues indicated in red.
Therefore, while CSF3R mutations are neither necessary nor sufficient for secondary transformation, they are, at the very least, a highly predictive marker that patients with CN who have CSF3R mutations need to be kept under close observation. But the more likely scenario is that CSF3R mutations in fact contribute at some stage, along with other cooperating molecular events that lead to leukemogenesis.
The author declares no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal