Abstract
A major impediment to gene replacement therapy is immune elimination of genetically modified cells. In principle, this can be dealt with by inducing a strong, specific, and enduring tolerance through engraftment of transgene-modified autologous bone marrow (BM). Because usual myeloablation and/or immunosuppression are risk factors in most pathologies, we assessed the potential of monospecific CD4+CD25+ regulatory T cells (Tregs) to engraft minor-mismatched BM without preconditioning. We found that as few as 5 × 104 Tregs directed to the male DBY protein promote the engraftment of foreign male BM into sex-mismatched female hosts, establishing sustained chimerism in all hematopoeitic compartments. We achieved concomitantly strong tolerance to all foreign antigens expressed in the BM, likely occurring through induction of anergy and/or deletion of antidonor T cells. Chimerism was obtained in thymectomized mice too, underlining the major role of peripheral tolerance mechanisms in our system. This allowed us to engraft gene-modified tissues while preserving full immunocompetence to third-party antigens. Our results demonstrate that very few donor-specific Tregs are effective as the sole conditioning to induce mixed molecular chimerism and long-term tolerance to multiple foreign antigens.
Introduction
Gene replacement therapy has raised considerable interest for the treatment of various metabolic diseases and genetic disorders such as muscular dystrophies1 or hemophilia.2 It is, however, very important to avoid deleterious immune responses. Major efforts have been devoted to improving gene delivery procedures, searching for the least immunogenic vectors, for tissue-specific promoters, and for appropriate injection routes and doses.3,4 But these approaches face the risk of creating a status of immune ignorance, in which secondary inflammation and bystander pathogen signals may induce immune rejection of therapeutic gene products.5 Induction of peripheral tolerance using immunosuppressive cotreatments or transient blockade of costimulation pathways, developed initially for transplantation or autoimmunity applications, have been successfully transposed to gene therapy,6,7 but these procedures are accompanied by marked toxicity, increased risks of cardiovascular disease, opportunistic infections, and malignancy in humans. Transgene-specific tolerance approaches are needed to block initial rejection and induce long-term tolerance without affecting overall immune functions in patients. These requirements are met by expressing transgenes of interest in the hematopoietic system prior to gene therapy interventions.
Mixed hematopoietic chimerism generates a robust donor-specific tolerance and represents an attractive method to obviate immune responses in organ transplantation8 or for treatment of autoimmune diseases.9,10 Mixed hematopoietic chimerism was recently used to induce tolerance to specific proteins by transferring genes into autologous hematopoietic stem cells (HSCs) for gene therapy.11,12 However, induction of a stable chimerism has entailed partial myeloablation to create space within the host marrow microenvironment and long-lasting immunosuppression to avoid graft rejection. High doses of bone marrow (BM) cells can overcome the limited accessibility of available niches,13,14 but immunosuppression is still needed to block immune responses directed against minor histocompatibility antigens15,16 and foreign transgenes introduced in the graft.
In place of nonspecific immunosuppression, we tested whether infusion of regulatory T cells directed against minor antigens inhibits immune rejection of transduced HSCs. Of the regulatory T-lymphocyte subsets, naturally arising CD4+CD25+ T cells (Tregs) governed by the forkhead transcription factor Foxp3 represent a unique T-cell lineage dedicated to maintenance of immunologic self-tolerance.17,18 They are particularly appealing for transplantation tolerance15,19 because they can prevent graft versus host disease (GVHD)20,21 and inhibit BM allograft rejection in sublethally irradiated recipients.22 Although their mode of action in vivo is still a matter of debate,23 immune regulation by Tregs appears to require antigen recognition both in vitro and in vivo.24,25 This is consistent with the fact that populations of CD4+CD25+ cells of the appropriate antigen specificity are more powerful in regulating autoimmune responses than Tregs of broad specificity.26,27 Likewise, we showed in a gene therapy setting that Tregs can exert potent immunosuppression against foreign antigen provided they recognize this very same antigen.28
Because tissue engraftment of male origin into female generates well-qualified immune response against UTY, SMCY, and DBY antigens in C57BL/6 (B6) mice,16 we chose this model to test whether male-specific Tregs could promote long-term engraftment of BM progenitors. This model mimics situations in which autologous HSCs transduced with a foreign transgene are rejected due to transgene-specific responses in the host. Furthermore, the presence of several male antigens introduces an appreciable level of complexity, which allowed us to test whether Tregs directed to a single epitope can suppress immune responses induced against all other expressed proteins. We used Tregs directed against the male DBY antigen, obtained from T-cell receptor (TCR) transgenic Marilyn mice,29 and found that they allowed long-term engraftment of appreciable doses of male BM within B6 female hosts. Importantly for therapeutic interventions, B6 mice tolerized by this procedure became permissive for secondary engraftment of autologous tissues modified with the transgene present in the initial BM, independently of the DBY antigen recognized by infused Tregs.
Materials and methods
Mice
Six- to 8-week-old C57BL/6 (CD45.2) and congenic CD45.1 (PtprcaPep3b/BoyJ [CD45.1]) mice were from Charles River (L'abresle, France). Marilyn mice carrying a transgenic TCR specific for a DBY peptide (NAGFNSNRANSSRSS) complexed with IAb (CD45.1, RAG–/–) were a gift from O. Lantz (INSERM U653, Institut Curie, Paris, France).29 For Treg isolation, we used females obtained by crossing C57BL/6 mice (CD45.2) with Marilyn mice (CD45.1+) (CDTA, Orleans, France). Hemizygous transgenic CD45.2 EGFP mice (C57BL/6-TgN [ACTB-EGFP] 1Osb/J; Jackson Laboratory, Bar Harbor, ME) express EGFP cDNA under the control of a chicken β-actin promoter and cytomegalovirus enhancer. We also crossed EGFP mice with CD45.1 mice to generate EGFP × CD45.1 mice. They were both bred in our animal facility. All animal experiments were performed according to institutional guidelines for animal care and use.
Fluorescence-activated cell sorting analysis
All reagents were from BD-Pharmingen (Le Pont de Claix, France). Red blood cells were eliminated by hypotonic shock with PharmLyse buffer. Peripheral blood mononuclear cells (PBMCs) were incubated for 15 minutes at 4°C with 2.4G2 antibody against FcII/III receptors and then stained 30 minutes in PBS–0.01% bovine serum albumin with saturating amounts of combinations of the following monoclonal antibodies (mAbs): FITC-conjugated anti-CD3, anti-CD4, anti-CD11c, anti-B220, anti-CD45.1, PE-conjugated anti-CD8, anti-CD11b, anti-CD25, anti-Gr1, anti-CD45.2, anti-NK1.1, anti-Vβ6, biotin-conjugated anti-CD45.2, and allophycocyanin (APC)–conjugated streptavidin. Dead cells were excluded using 7-actinomycin D (7-AAD) (Sigma Chemicals, St Louis, MO) staining. Flow cytometric analysis was performed on a FACSCalibur using CELLQuest software (BD, Le Pont de Claix, France).
Regulatory T-cell purification
Splenocytes and lymph node cells were incubated with saturating amounts of biotinylated anti-CD25 (7D4) plus streptavidin microbeads (Miltenyi Biotec, Paris, France), followed by magnetic cell separation using LS columns (Miltenyi Biotec) according to the manufacturer's instructions. Cells were further stained 10 minutes on ice with streptavidin-FITC plus 7-AAD, sorted on a MoFlow (DakoCytomation, Freiburg, Germany), and injected into the tail vein in 0.2 mL PBS. More than 70% of the injected cells were CD4+Foxp3+, as detected by intracellular staining.
Bone marrow grafting
Donor bone marrow was flushed with PBS from femurs and tibias. Red blood cells were lysed with ACK buffer and BM cells injected into the tail vein in 0.2 mL PBS 1 times] in the presence or absence of Tregs.
In vivo killing assay
Female and male C57BL/B spleen cells or sex-matched spleen cells loaded or not with 10 μM OVA257 (2 × 107/mL in RPMI 1640) were incubated with 5 μM or 0.5 μM CFSE (Molecular Probes, Cambridge, United Kingdom) at 37°C for 10 minutes. After washing, the cells were mixed, and 2 × 107 cells were injected into the tail vein in 0.2 mL PBS 1 ×. PBMCs were collected from individual mice at regular time points, labeled with PE anti-B220 and 7-AAD, and analyzed for CFSE expression by fluorescence-activated cell sorting (FACS). The percentage of specific lysis of male over female cells (m/f) was calculated on B220+ cells for each time point tx as follows: % specific lysis = ([m/f]t0 – [m/f]tx)/([m/f]t0) × 100. The quantities m and f are measured on standard gates placed on the male CFSE low and female CFSE high histograms peaks.
Antiovalbumin immune responses
Mice were challenged subcutaneously at the base of the tail with 100 μg ovalbumin protein (Sigma Chemicals) or 50 μg OVA257 (SIINFEKL) peptide (Epytop, Nimes, France) emulsified in incomplete Freund adjuvant (IFA) (Difco Laboratories, BD-Difco, Le Pont de Claix, France). IFNγ enzyme-linked immunospot (ELISPOT) assays and enzyme-linked immunosorbent assays (ELISAs) were performed 8 to 10 days later as previously described.28 Briefly, for IFNγ ELISPOT assay, freshly isolated splenocytes (2 × 106 per well and serial dilutions) were cultured in complete medium with or without 10 μM OVA257. For each assay, concanavalin A (ConA) was added (5 μg/mL) as a positive control. After 20 hours, spots were revealed and counted using a Bioreader 2000 (BIO-SYS, Karben, Germany). Spot-forming units (SFUs) are represented after subtraction of background values obtained with unpulsed splenocytes (fewer than 15 spots per well).
Real-time PCR analysis
Total RNA was extracted using the RNAeasy Micro Kit (QIAGEN, Courtaboeuf, France) from 105 sorted cells of each population. Complementary DNA was synthesized from each RNA sample using MuLV reverse transcriptase and random hexamers as primers (Applied Biosystems, Courtaboeuf, France). The real-time polymerase chain reaction (PCR) was performed on an ABI prism 7700 using Absolute QPCR ROX Mix (ABgene, Courtaboeuf, France) in duplicates, and the average threshold cycles (Ct) of the duplicates were used to calculate the Foxp3 mRNA level in each population. All reported mRNA levels are normalized to the mPO mRNA level, where mPO = 1. PCR primers where as follows: Foxp3, 5′-GGCCCTTCTCCAGGACAGA-3′,5′GCTGATCATGGCTGGGTTGT-3′, 5′-ACTTCATGCATCAGTCTCCACTGTGGAT-3′; mPO, 5′-CTCCAAGCAGATGCAGCAGA-3′, 5′-ATAGCCTTGCGCATCATGGT-3′, 5′-CCGTGGTGCTGATGGGCAAGAA-3′.
Skin grafting
Skin grafts of 1 cm in diameter were prepared from backs of EGFP female mice and grafted onto the flanks of the recipients using tissue adhesive (3M Vetbond; 3M, Santé, France) in place of surgical seam. The bandages were removed on day 5. The grafts were monitored every 2 to 3 days until day 20, and every week thereafter, and scored as rejected when less than 10% viable tissue remained.
Results
Antigen-specific in vivo expansion of CD4+CD25+ T cells from Marilyn mice
In the C57BL/6 strain (B6), female mice reject H2-matched male BM grafts generating CD4 T-cell responses against a DBY peptide in the context of I-Ab molecules and helper-dependent CD8 responses against Db-restricted UTY and SMCY epitopes.16 As a source of Tregs directed to a single male antigen, we isolated CD4+CD25+ T cells from female CD45.1/CD45.2 (45.1/45.2) Marilyn mice, which express a transgenic TCR specific for the male I-Ab–restricted DBY epitope NAGFNSNRANSSRSS29 (DBY-Tregs).
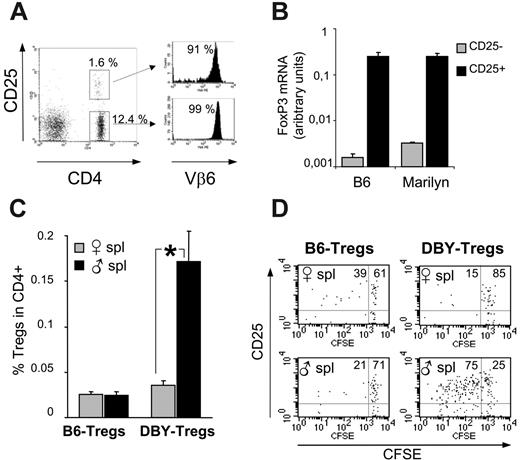
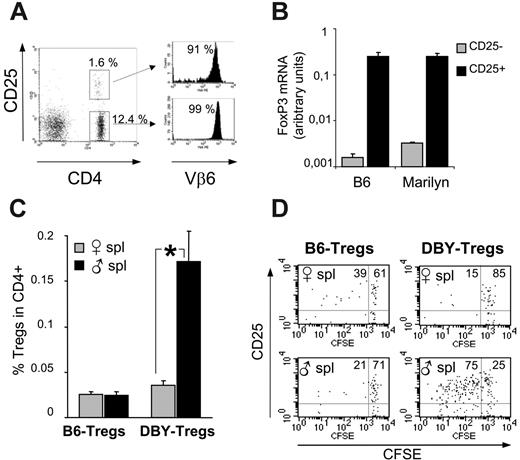
Phenotypically, RAG+/– female Marilyn mice exhibit a distinct population of CD4+CD25+ DBY-Tregs expressing the Vβ6 TCR transgene, amounting to 6% to 11% among total CD4+ T cells (Figure 1A). More than 85% of this CD4+CD25+ population expresses a high level of the recently identified lineage/differentiation Treg marker Foxp3, as revealed by intracellular staining (data not shown). The phenotype of purified Tregs was confirmed by Foxp3 mRNA quantification by real-time PCR. DBY-Tregs expressed Foxp3 levels similar to those observed in B6-Tregs and 80-fold higher than those found in CD25– T cells (Figure 1B). We thereafter characterized the antigen specificity of DBY-Tregs (compared with female B6-Tregs) in vivo by their proliferation capacity in female 45.1 recipients challenged with either male or female 45.1 splenocytes. We found that expansion of DBY-Tregs at day 6 in the spleen occurred only after immunization with male splenocytes (Figure 1C). This antigen-specific proliferation was confirmed by CFSE dilution experiments (Figure 1D). B6-Tregs remained mostly undivided in all conditions (Figure 1C-D), in agreement with previous results from Fisson et al.30 Altogether, DBY-Tregs harbored the phenotype of naturally occurring CD4+CD25+ T cells and were uniquely activated in vivo in the presence of male cells.
Antigen-specific in vivo expansion of CD4+CD25+ T cells from Marilyn mice. (A) FACS analysis of lymph node cells from female Marilyn mice labeled with anti-CD4, Vβ6, and CD25. (B) FoxP3 mRNA levels of purified CD25+ and CD25– cells from female B6 and Marilyn mice were determined by real-time PCR analysis on fresh splenocytes. (C-D) In vivo expansion of DBY-Tregs: B6- or DBY-Tregs labeled with 5 μM CFSE were transferred together with 10 × 106 female or male 45.1 splenocytes into recipient female 45.1 mice; n = 3 mice per group. At day 6, splenocytes were labeled with CD25, CD4, and CD45.2. Dot plots are gated on CD4+CD45.2+ cells. Statistical analysis was performed using the Mann-Whitney t test; *P ≤ .05. Panel A is representative of 3 experiments, and panels B-D are each representative of 2 experiments. Error bars in panels B and C show standard error of the mean.
Antigen-specific in vivo expansion of CD4+CD25+ T cells from Marilyn mice. (A) FACS analysis of lymph node cells from female Marilyn mice labeled with anti-CD4, Vβ6, and CD25. (B) FoxP3 mRNA levels of purified CD25+ and CD25– cells from female B6 and Marilyn mice were determined by real-time PCR analysis on fresh splenocytes. (C-D) In vivo expansion of DBY-Tregs: B6- or DBY-Tregs labeled with 5 μM CFSE were transferred together with 10 × 106 female or male 45.1 splenocytes into recipient female 45.1 mice; n = 3 mice per group. At day 6, splenocytes were labeled with CD25, CD4, and CD45.2. Dot plots are gated on CD4+CD45.2+ cells. Statistical analysis was performed using the Mann-Whitney t test; *P ≤ .05. Panel A is representative of 3 experiments, and panels B-D are each representative of 2 experiments. Error bars in panels B and C show standard error of the mean.
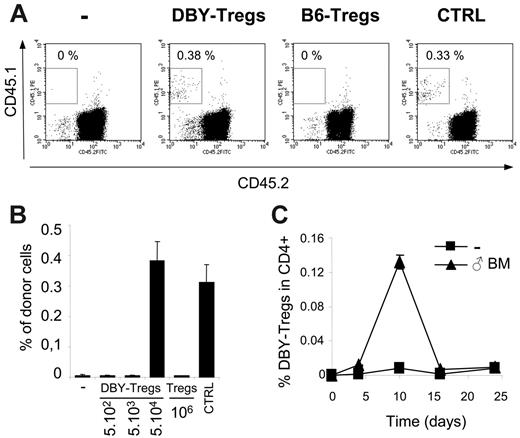
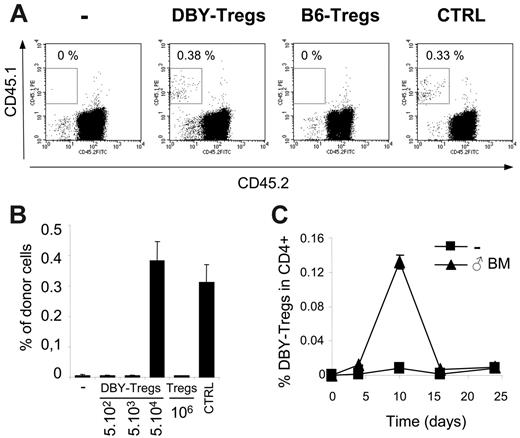
Short-term engraftment of male BM and transient expansion of DBY-Tregs. (A-B) Short-term engraftment (day 28) of congenic male 45.1 BM (5 × 106 cells) transferred into B6 female 45.2 mice either untreated (-) or conditioned with one intravenous injection of a various number of DBY-Tregs or 1 × 106 B6-Tregs. Male B6 mice were engrafted as a positive control (CTRL). Percentages of CD45.1+ donor cells were analyzed in PBMCs. Results represent the mean of 3 to 6 mice per group ± standard error of the mean (SEM). (C) Transient expansion of DBY-Tregs. DBY-Tregs (1 × 105 cells) were transferred into B6 female mice with or without male BM (107 cells). At each time point, mice were killed and their splenocytes stained with CD45.1 and CD4. Graph represents the percentage of CD45.1+ cells in CD4+ 7-AAD– cells. Results represent the mean of 2 mice killed at each time point from groups of 8 mice per condition. Bars represent the 2 values of single-animal results.
Short-term engraftment of male BM and transient expansion of DBY-Tregs. (A-B) Short-term engraftment (day 28) of congenic male 45.1 BM (5 × 106 cells) transferred into B6 female 45.2 mice either untreated (-) or conditioned with one intravenous injection of a various number of DBY-Tregs or 1 × 106 B6-Tregs. Male B6 mice were engrafted as a positive control (CTRL). Percentages of CD45.1+ donor cells were analyzed in PBMCs. Results represent the mean of 3 to 6 mice per group ± standard error of the mean (SEM). (C) Transient expansion of DBY-Tregs. DBY-Tregs (1 × 105 cells) were transferred into B6 female mice with or without male BM (107 cells). At each time point, mice were killed and their splenocytes stained with CD45.1 and CD4. Graph represents the percentage of CD45.1+ cells in CD4+ 7-AAD– cells. Results represent the mean of 2 mice killed at each time point from groups of 8 mice per condition. Bars represent the 2 values of single-animal results.
Induction of mixed hematopoietic chimerism using DBY-Tregs
We then explored the ability of these monospecific Tregs to promote the establishment of mixed chimerism in the absence of preconditioning. We used DBY-Tregs to facilitate direct engraftment of a moderate dose of male BM in B6 female hosts in the absence of myeloablation and immunosuppression. As a control, in the absence of immune responses, injection of 8 × 106 congenic male 45.1 BM cells led to 0.3% to 0.4% of chimerism in 45.2 male mice (Figure 2A). The same graft was completely rejected in 45.2 female mice by day 28. In these female mice, engraftment was already compromised at day 14 and nearly absent at day 21 (data not shown). As expected, we found that infusion into female of 5 × 104 DBY-Tregs concomitantly with male BM suppressed graft rejection and enabled us to achieve nearly 0.4% of chimerism (Figure 2A). We then varied the dose of DBY-Tregs and observed an all-or-none effect on BM engraftment between 5 × 104 and 5 × 103 DBY-Tregs (Figure 2B). Importantly, all doses of DBY-Tregs above threshold promoted maximal engraftment in all female mice, reaching identical level in control male recipients treated with the same amount of BM cells. High doses of 106 nonspecific female B6-Tregs remained ineffective (Figure 2A-B). Altogether these results underline the extreme potency of DBY-specific Tregs to promote mixed hematopoietic chimerism.
Regarding the extremely low number of DBY-Tregs required for male BM engraftment, we wondered whether they proliferate and found a 10- to 15-fold expansion of DBY-Tregs in the spleen at day 10 (Figure 2C). A 3- and 4-fold expansion of DBY-Tregs was found in lymph nodes and blood, respectively (data not shown). This is consistent with their strong proliferative capacity observed against male splenocytes in vivo (Figure 1C). Interestingly, DBY-Tregs returned to lower levels at day 16 without compromising longer-term BM engraftment at day 28 (Figure 2B), suggesting that mechanisms other than active suppression by DBY-Tregs are operating at later time points.
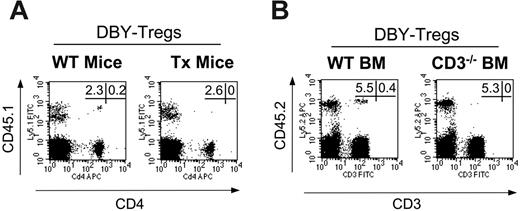
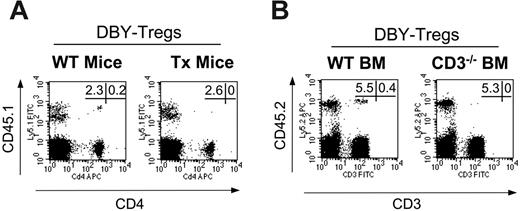
Tolerance to male antigens occurs mainly through peripheral mechanisms. (A) Congenic male 45.1 BM (15 × 106 cells) was transferred into intact (n = 5) or thymectomized female 45.2 B6 mice (n = 5) conditioned with single intravenous injections of 1 × 105 DBY-Tregs. (B) Male 45.2 BM from wild-type mice or from CD3null mice (15 × 106 cells) was transferred into congenic female 45.1 mice (n = 5 for each group) conditioned with single intravenous injections of 1 × 105 DBY-Tregs. In panels A and B, donor chimerism and percentage of T cells, expressed as a percentage of CD45.1+ or CD45.2+ cells, were analyzed in PBMCs at various time points after bone marrow transplantation (BMT). FACS stainings depicted at day 60 are shown. Panels A and B are each representative of 2 experiments.
Tolerance to male antigens occurs mainly through peripheral mechanisms. (A) Congenic male 45.1 BM (15 × 106 cells) was transferred into intact (n = 5) or thymectomized female 45.2 B6 mice (n = 5) conditioned with single intravenous injections of 1 × 105 DBY-Tregs. (B) Male 45.2 BM from wild-type mice or from CD3null mice (15 × 106 cells) was transferred into congenic female 45.1 mice (n = 5 for each group) conditioned with single intravenous injections of 1 × 105 DBY-Tregs. In panels A and B, donor chimerism and percentage of T cells, expressed as a percentage of CD45.1+ or CD45.2+ cells, were analyzed in PBMCs at various time points after bone marrow transplantation (BMT). FACS stainings depicted at day 60 are shown. Panels A and B are each representative of 2 experiments.
Peripheral mechanisms are sufficient to induce mixed chimerism
Transplantation in an allogenic setting often requires conditioning leading to thymic generation of a totally or partially new T-cell repertoire as a main element of tolerance induction. Here in the absence of myeloablation and immunosuppression, we wondered therefore whether tolerance induction initiated by DBY-Tregs may only involve peripheral mechanisms. To examine this, we transferred 45.1 male BM together with DBY-Tregs either into 45.2 wild-type female mice or into 45.2 thymectomized female mice. We found that both groups exhibited comparable chimerism at 2 months in all mice studied (Figure 3A), demonstrating that peripheral mechanisms are sufficient to promote chimerism in mice treated with DBY-Tregs. To determine whether donor bone marrow requires mature donor lymphocytes to induce tolerance, we transplanted CD3–/– 45.2 male BM lacking both CD25– and CD25+ T lymphocytes into 45.1 female mice conditioned by DBY-Tregs. As shown in Figure 3B, the absence of donor T cells had no consequence on BM engraftment in 4 of 5 mice. Of importance, the level of engraftment was of the same magnitude as in the controls.
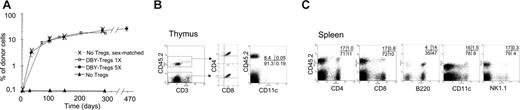
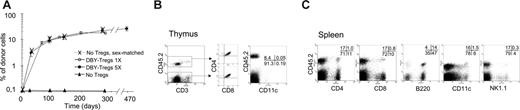
Development of long-term, multilineage mixed chimerism. (A) A total of 15 × 106 male 45.2 BM cells were transferred into congenic female 45.1 mice untreated (▴, n = 3) or conditioned with either 5 weekly intravenous injections of 2 × 105 to 5 × 105 DBY-Tregs (•, n = 5) or a single injection of 1 × 105 DBY-Tregs (○, n = 5). As a positive control, female 45.2 BM cells were transferred into congenic female 45.1 mice (X, n = 3). Donor chimerism expressed as a percentage of CD45.2+ cells was analyzed in PBMCs at various time points after BMT (A). Results represent the mean per group ± SEM. (B-C) Mice chimerized for more than 300 days (5 DBY-Treg injections) were killed, and cells from various organs were analyzed by FACS. (B) Splenocytes were stained with CD45.2-biotin/APC-streptavidin, PE-conjugated anti-CD8, anti-CD4, anti-B220, anti-CD11c, and anti–7-AAD. (C) Thymocytes were stained with CD4, CD3, CD45.2, and CD8 (no gate) or with CD3, CD45.2, and CD11c (FACS gated on CD3– 7-AAD– is shown).
Development of long-term, multilineage mixed chimerism. (A) A total of 15 × 106 male 45.2 BM cells were transferred into congenic female 45.1 mice untreated (▴, n = 3) or conditioned with either 5 weekly intravenous injections of 2 × 105 to 5 × 105 DBY-Tregs (•, n = 5) or a single injection of 1 × 105 DBY-Tregs (○, n = 5). As a positive control, female 45.2 BM cells were transferred into congenic female 45.1 mice (X, n = 3). Donor chimerism expressed as a percentage of CD45.2+ cells was analyzed in PBMCs at various time points after BMT (A). Results represent the mean per group ± SEM. (B-C) Mice chimerized for more than 300 days (5 DBY-Treg injections) were killed, and cells from various organs were analyzed by FACS. (B) Splenocytes were stained with CD45.2-biotin/APC-streptavidin, PE-conjugated anti-CD8, anti-CD4, anti-B220, anti-CD11c, and anti–7-AAD. (C) Thymocytes were stained with CD4, CD3, CD45.2, and CD8 (no gate) or with CD3, CD45.2, and CD11c (FACS gated on CD3– 7-AAD– is shown).
In conjunction with the transient expansion of DBY-Tregs, these results suggest that male antigen-specific effector T cells were altered at the onset of mixed chimerism induction.
Development of long-term, multilineage mixed chimerism
Although DBY-Tregs effectively inhibited antimale immune responses, promoting short-term BM engraftment, their transient expansion could be uniquely associated to short-lived suppression, compromising longer-term engraftment of HSCs and donor-specific tolerance. We thus compared the effect of a single injection of 105 DBY-Tregs corresponding to twice the threshold dose determined in Figure 2B with 5 weekly injections of 2 × 105 to 5 × 105 DBY-Tregs with respect to the establishment of long-term chimerism (Figure 4A). Both conditions led to sustained and high levels of donor chimerism in peripheral blood lymphocytes (PBLs) at 3 to 4 months (9.1 ± 1.8 and 12.3 ± 1.0, respectively), with a gradual increase up to 9 months (21.8 ± 2.6 and 18.0 ± 1.7, respectively) and systematic long-term engraftment at 18 months in all mice. No BM engraftment was achieved with a high dose of 106 unspecific B6-Tregs, even when preactivated overnight with plate-bound anti-CD3 antibody (data not shown). Importantly, the level of chimerism achieved with DBY-Tregs was identical to that achieved in the absence of an immune response with sex-matched control female BM (dotted line, Figure 4A). Hematopoietic compartments (CD3+, B220+, CD11b+, and Gr1+) were chimerized to the same extent in the sex-matched control and DBY-Treg–treated groups (Table 1). However, we always noticed lineage-dependent reconstitution levels favoring B cells over T cells (Table 1 and Figure 4C). Donor cells from various lineages, including dendritic cells and natural killer (NK) cells, were systematically found in the spleen (Figure 4B) and lymph nodes (data not shown). Donor CD11c+ dendritic cells were present in the thymus as well as normally developing thymocytes of donor origin (Figure 4C). These results suggest that the T-cell repertoire generated in the recipient thymus can be educated by hematopoietic cells of recipient and donor origin. We also found that the bone marrow was colonized with donor HSCs as defined by their Lin–Sca+c-Kit+ phenotype or Hoechst exclusion of the side population (data not shown).
Robust tolerance to male antigen and immunocompetence to third-party antigens
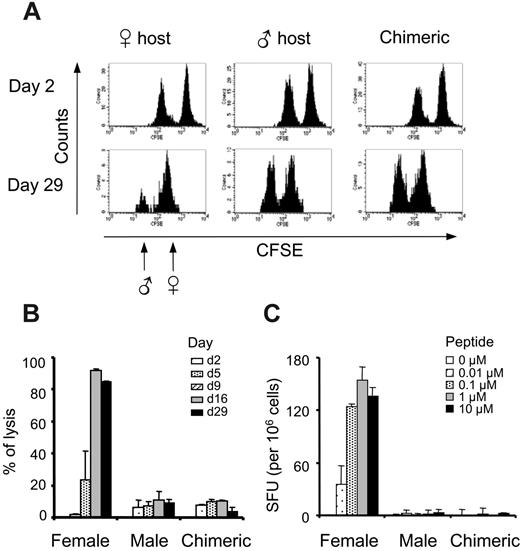
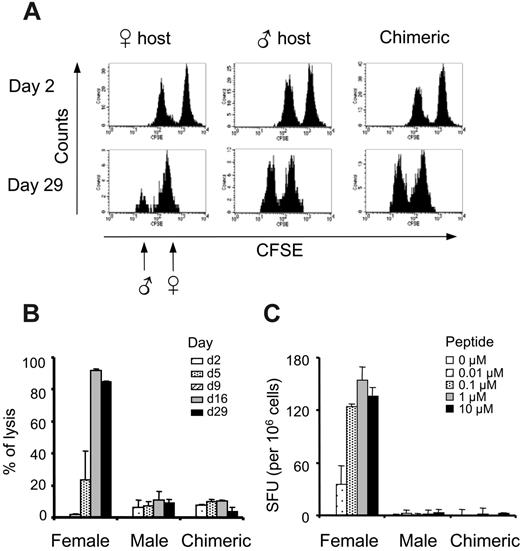
We achieved long-term and multilineage chimerism, and this should in principle ensure a robust tolerance to all antigens present in the graft. To confirm this, we challenged mice exhibiting a minimum of 3% to 5% of chimerism with either male cells or male antigen peptides. We first performed in vivo cytotoxic assays, infusing 5 × 106 male and 5 × 106 female B6 splenocytes labeled with 2 levels of CFSE. Despite CFSE labeling decay and normal loss of infused cells, the same animal can be followed up during 1 month by regular PBL analysis. In this system, naive female mice were primed and reacted selectively against male splenocytes by day 9 after infusion, eliminating almost all male cells by day 16, while no cytoxicity was observed in male hosts (Figure 5A-B). As expected, chimerized mice exhibited no rejection of male targets during the 29 days of the experiment, indicating that long-term peripheral tolerance to male antigen had been reached.
This tolerance could be due to a dominant and sustained regulatory process and/or alteration/deletion of antimale T cells. A sustained suppression by DBY-Tregs was unlikely because they were hardly detectable, being below the detection limit of FACS in the blood and in all primary and secondary lymphoid organs tested several months after BMT (data not shown). To answer to this question, we challenged the mice with a class I–restricted male epitope to avoid a potential immune suppression by undefined Tregs or residual DBY-Tregs. For that, we gave mice injections of the well-characterized UTY epitope in the presence of IFA. No antimale responses were ever detected under these circumstances, as evidenced by the absence of peptide-specific IFNγ responses in ELISPOT assays (Figure 5C).
Strong tolerance to male antigen in chimeric mice. (A-B) The absence of antimale CTL activity in chimeric mice. Male and female splenocytes labeled with 0.5 μM and 5 μM CFSE, respectively, were transferred into female mice, male mice, or chimeric female mice. PBMCs were labeled with PE anti-B220 and 7-AAD at various time points, and percentages of specific lysis of male over female splenocytes were calculated as detailed in “Materials and methods.” Results represent the mean of 2 mice per group assayed at 5 time points. Bars represent the 2 values of single-animal results. (C) The absence of antimale T-cell responses. Female, male, or chimeric mice were challenged subcutaneously with 50 μg UTY peptide emulsified in IFA. Splenocytes were tested on day 10 in a standard IFNγ ELISPOT assay against various doses of the UTY peptide. Results represent the mean of 3 mice per group ± SEM.
Strong tolerance to male antigen in chimeric mice. (A-B) The absence of antimale CTL activity in chimeric mice. Male and female splenocytes labeled with 0.5 μM and 5 μM CFSE, respectively, were transferred into female mice, male mice, or chimeric female mice. PBMCs were labeled with PE anti-B220 and 7-AAD at various time points, and percentages of specific lysis of male over female splenocytes were calculated as detailed in “Materials and methods.” Results represent the mean of 2 mice per group assayed at 5 time points. Bars represent the 2 values of single-animal results. (C) The absence of antimale T-cell responses. Female, male, or chimeric mice were challenged subcutaneously with 50 μg UTY peptide emulsified in IFA. Splenocytes were tested on day 10 in a standard IFNγ ELISPOT assay against various doses of the UTY peptide. Results represent the mean of 3 mice per group ± SEM.
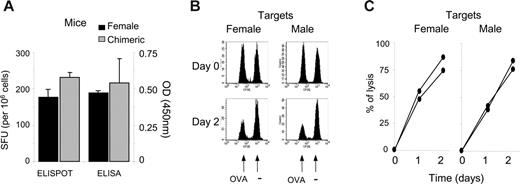
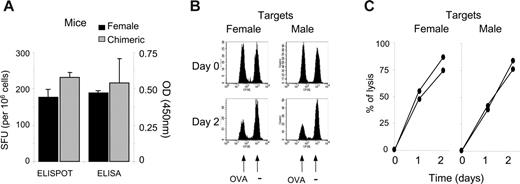
To exclude the possibility of a general state of unresponsiveness in the chimera, we analyzed the immune response to a third-party antigen absent from the initial graft. After OVA immunization, we monitored both the frequency of antigen-specific IFNγ-producing T cells and titers of OVA-specific IgG antibodies. Chimeric and control female mice responded equally to the OVA antigen (Figure 6A). Lastly, we determined whether male antigen-specific tolerance impaired rejection of male splenocytes loaded with OVA257 peptide. Using in vivo cytotoxic assays, we found that peptide-loaded male splenocytes were as susceptible to CTL lysis as peptide-loaded female splenocytes (Figure 6B-C). Importantly, despite strong cytotoxicity against male splenocytes loaded with a third-party antigen, long-term chimerism was unaffected (data not shown).
Altogether, we observed a robust tolerance to all antigens initially present in the graft such as UTY protein but not to third-party antigens introduced afterward. Moreover, this tolerance did not impair normal immune responses against donor target cells.
Applications to secondary engraftment of engineered tissues
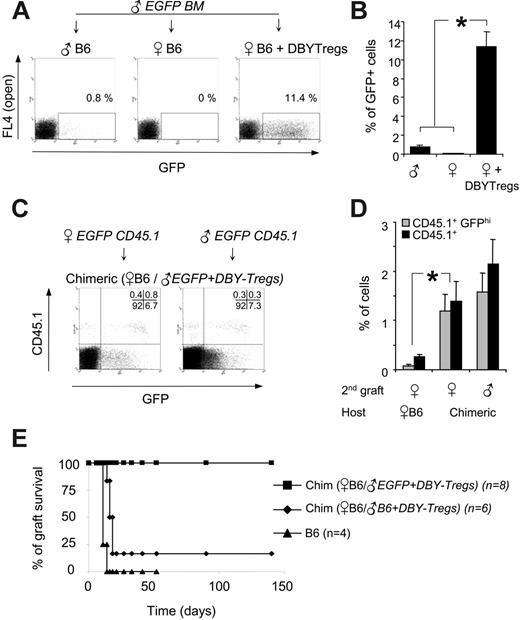
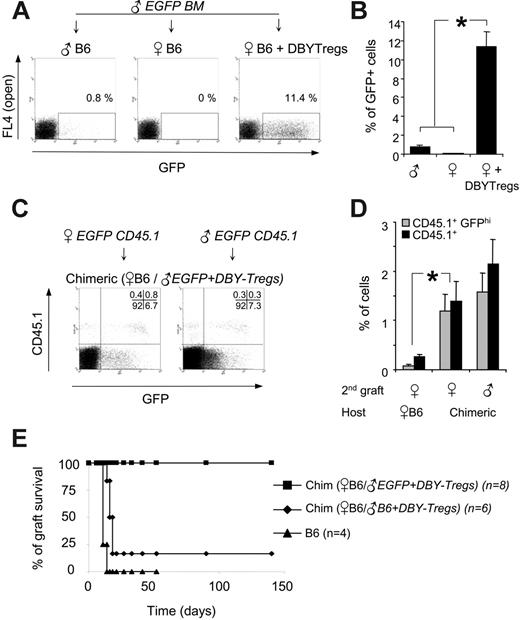
The absence of immune responses to all male antigens observed here in chimeric mice is of importance for the long-term, transgene-specific tolerization required for gene transfer applications. We decided therefore to extend this tolerance to a foreign transgene by creating a mixed molecular chimerism with additional antigens. We chose to introduce the enhanced green fluorescent protein (EGFP) and used male BM from EGFP transgenic mice (C57BL/6-TgN mice). As shown previously with skin grafts experiments,12,31 EGFP and presumably other associated minor antigens precipitated rejection of EGFPhigh male BM cells in unconditioned male hosts (Figure 7A). Rejection of all male EGFP BM cells occurred also in untreated female hosts, probably due to a strong antimale response (Figure 7A). In marked contrast, females conditioned with 105 DBY-Tregs exhibited stable chimerism up to 10% at 5 months, with high expression of the EGFP transgene (Figure 7A-B). This result demonstrates that a single infusion of DBY-Tregs directed to a unique MHC class II DBY epitope is sufficient to induce long-term tolerance to multiple minor antigens present in the graft.
Last, we determined whether the strong tolerance generated to all donor antigens present in the first graft could be exerted in the absence of DBY expression. To test this hypothesis, female 45.2 B6 mice chimerized with 45.2 EGFP male bone marrow and DBY-Tregs for 5 months (Figure 7A-B) received transplants a second time with either male or female EGFP BM cells carrying a traceable CD45.1 marker. We found that CD45.1+ EGFPhigh cells of male or female origin were equally engrafted, showing that tolerance induction applied to all minor antigens even in the absence of DBY coexpression (Figure 7C-D).
Hematopoietic chimerism does not impair immune response to third-party antigens. (A) Chimeric or naive female mice were challenged subcutaneously with 100 μg OVA protein emulsified in IFA. Splenocytes were tested at day 10 in a standard IFNγ ELISPOT assay against the OVA257 epitope. Serum was tested by ELISA for anti-OVA antibody. Results represent the mean of 3 mice per group ± SEM. (B-C) Susceptibility of male cells to immune cytolytic activity. Chimeric mice were challenged subcutaneously with OVA protein in IFA as in panel A and were infused at day 8 with male splenocytes (n = 2) or female splenocytes (n = 2) either pulsed with OVA257 (0.5 μM CFSE) or left unpulsed (5 μM CFSE). PBMCs were analyzed at day 0, 1, and 2. The percentage of specific lysis of pulsed over unpulsed cells was calculated as detailed in “Materials and methods,” similarly to Figure 5 with male over female cells. Panels B and C represent 1 of 2 similar results.
Hematopoietic chimerism does not impair immune response to third-party antigens. (A) Chimeric or naive female mice were challenged subcutaneously with 100 μg OVA protein emulsified in IFA. Splenocytes were tested at day 10 in a standard IFNγ ELISPOT assay against the OVA257 epitope. Serum was tested by ELISA for anti-OVA antibody. Results represent the mean of 3 mice per group ± SEM. (B-C) Susceptibility of male cells to immune cytolytic activity. Chimeric mice were challenged subcutaneously with OVA protein in IFA as in panel A and were infused at day 8 with male splenocytes (n = 2) or female splenocytes (n = 2) either pulsed with OVA257 (0.5 μM CFSE) or left unpulsed (5 μM CFSE). PBMCs were analyzed at day 0, 1, and 2. The percentage of specific lysis of pulsed over unpulsed cells was calculated as detailed in “Materials and methods,” similarly to Figure 5 with male over female cells. Panels B and C represent 1 of 2 similar results.
To extend these results to a stringent organ transplantation setting, we used female EGFP skin grafts as secondary grafts. Because EGFP skin grafts from the tail are slowly rejected (100 days) in only 80% of mice,12 we performed trunk skin graft, which is more sensitive than tail skin graft in detecting minor-antigen differences.32,33 All naive B6 female mice rejected their EGFP skin graft from the trunk promptly between days 12 and 16 (Figure 7E). In sharp contrast, 100% of B6 female mice chimerized with male EGFP BM tolerated their transplants, accepting their skin grafts indefinitely (n = 8). Of importance, control mice chimerized with 45.2 male BM, which were not tolerized to EGFP, did not accept this third-party EGFP allograft (5 of 6), illustrating the strength and the specificity of the tolerance created toward the EGFP-expressing cells independently of the male antigens.
Secondary engraftment of tissues expressing EGFP transgene in the absence of DBY antigen. (A) A total of 7 × 106 male 45.2 BM cells from transgenic EGFP mice were transferred into 45.2 male hosts, 45.2 female hosts, or 45.2 female hosts conditioned with 105 DBY-Tregs. One representative FACS analysis of 3 experiments is shown (day 150). (B) Donor chimerism expressed as a percentage of EGFP-positive cells analyzed in PBMCs 5 months after EGFP BM transfer as in panel A. Results represent the mean of 3 mice per group ± SEM. (C-D) BM from EGFP × CD45.1 male or female mice was transferred into 45.2 EGFP chimeric or naive female mice. Representative FACS analysis (C) and percentage of EGFPhigh and CD45.1+ cells (D) in PBMCs at 5 months are shown. Results represent the mean of 3 mice per group ± SEM. Statistical analysis was performed using the Mann-Whitney t test; *P ≤ .05. (E) Long-term engraftment of EGFP female skin grafts in EGFP male-female chimeric mice (n = 8, ▪). In the controls, 5 of 6 chimeric mice lacking EGFP (♦) and 4 of 4 female B6 mice (▴) rejected the EGFP female skin graft between day 12 and day 16. The results presented are pooled from 2 independent experiments.
Secondary engraftment of tissues expressing EGFP transgene in the absence of DBY antigen. (A) A total of 7 × 106 male 45.2 BM cells from transgenic EGFP mice were transferred into 45.2 male hosts, 45.2 female hosts, or 45.2 female hosts conditioned with 105 DBY-Tregs. One representative FACS analysis of 3 experiments is shown (day 150). (B) Donor chimerism expressed as a percentage of EGFP-positive cells analyzed in PBMCs 5 months after EGFP BM transfer as in panel A. Results represent the mean of 3 mice per group ± SEM. (C-D) BM from EGFP × CD45.1 male or female mice was transferred into 45.2 EGFP chimeric or naive female mice. Representative FACS analysis (C) and percentage of EGFPhigh and CD45.1+ cells (D) in PBMCs at 5 months are shown. Results represent the mean of 3 mice per group ± SEM. Statistical analysis was performed using the Mann-Whitney t test; *P ≤ .05. (E) Long-term engraftment of EGFP female skin grafts in EGFP male-female chimeric mice (n = 8, ▪). In the controls, 5 of 6 chimeric mice lacking EGFP (♦) and 4 of 4 female B6 mice (▴) rejected the EGFP female skin graft between day 12 and day 16. The results presented are pooled from 2 independent experiments.
Discussion
Our goal was to induce antigen-specific tolerance through establishment of a mixed hematopoietic chimerism without myeloablative and immunosuppressive treatments. We explored the potential of Foxp3+CD4+CD25+ Tregs directed to a single donor antigen as the sole conditioning to engraft a moderate dose of allogenic BM. We found that animals chimerized by virtue of antigen-specific Tregs are rendered tolerant to a set of defined donor antigens and exhibited full immunocompetence. Notably, the tolerized male alloantigens are weak immunogens compared with major histocompatibility antigens. They nevertheless induce strong immune responses compared with EGFP-associated minor antigens (Figure 7A); male antigen triggered rapid elimination of all male cells in female hosts while EGFP expression led only to partial rejection in sex-matched recipients.
Several reports indicate that Tregs need to be activated via their TCR to efficiently inhibit immune responses.24,25 Trenado et al have shown that Tregs activated in vitro with host-type antigen-presenting cells (APCs) controlled GVHD while irrelevant Tregs activated with third-party APCs mediated only partial protection from GVHD.20,34 In our setting, Tregs proliferated in an antigen-specific manner, which may explain why as few as 5 × 104 DBY-Tregs were efficient suppressors. Indeed, DBY-Tregs expanded vigorously in vivo only after stimulation with male cells (Figures 1C-D and 2C) and exhibited prominent antigen-specific suppressive functions both in vitro (data not shown) and in vivo (Figure 2A-B). After BMT, DBY-Treg expansion was transient with a peak around day 10 (Figure 2C), consistent with a recent report showing that infused Langerhans islet–specific Tregs accumulate transiently in the corresponding pancreatic draining lymph node.30 In contrast, only a few nonspecific B6-Tregs did proliferate in vivo (Figure 1C-D), in agreement with the cell-cycling capability reported for self-reactive Tregs.30,35 The fact that B6-Tregs were inefficient can be explained by a too low precursor frequency of male-specific Tregs among self-reacting Tregs, as proposed by others groups.26,27 Male-specific Tregs could also be totally absent in B6 female mice, because Treg differentiation results from serial interactions with thymic APCs presenting self-peptides.36,37 Altogether, DBY-Tregs directed against a single MHC class II epitope were extremely potent to promote mixed male-female chimerism.
Several reports showed that Tregs can suppress CD8+ T-cell activities at different stages, such as cell proliferation,38 cytokine secretion,14 or control of cytolytic activity in a TGF-β receptor–dependent manner.14,39 From the small number of DBY-Tregs required here and from the fact that monospecific Tregs were more efficient for the prevention than for the treatment of autoimmune diabetes,26,27 we think that our antigen-specific Tregs are mostly effective during the early phase of the immune response. Because BM engraftment occurred also in thymectomized mice, tolerization of preexisting antimale T cells can effectively operate in the periphery (Figure 3). Early inhibition of the CD8 response was also consistent with the following observations: (1) DBY-Tregs exert an all-or-none effect on BM engraftment instead of a gradual effect (Figure 2A-B); (2) we always reach maximal engraftment compared with a control engraftment without Tregs in male mice (Figures 2 and 4); and (3) DBY-Tregs undergo transient expansion and become undetectable without compromising longer-term BM engraftment.
Mixed hematopoietic chimerism is a state in which multilineage hematopoietic populations of donor and recipient origin coexist.8 This strategy has been explored to achieve organ graft tolerance in animal models, because it requires only mild immunosuppression and myeloablative cotreatment to engraft donor HSCs. In elegant experiments, Iacomini and colleagues40,41 have induced tolerance to nominal antigens by establishing molecular chimerism using engineered autologous HSCs in mice pretreated with a mild irradiation. In our system, antigen-specific Tregs were instrumental in promoting male-female chimerism, nullifying the initial immune response generated against male antigens without the risks associated with preconditioning.
Regarding the efficiency of BM engraftment, we found that DBY-Treg–mediated engraftment led to a high level of chimerism of 0.7% to 1.2% per 106 injected BM cells, which was identical in control congenic recipients after 1 month (Figure 2A-B) or after 1 year (Figure 4A and data not show). Quesenberry and colleagues42 have demonstrated that, in the absence of an immune response, engraftment into normal nonmyeloablated BALB/c hosts can be achieved by infusing a high dose of 2 × 108 BM cells. The percentage of engraftment increased with the dose of BM and number of injections, and the levels of engraftment per million cells infused were similar to ours at 6 months (0.3% per 106 BM cells).43
Progressive engraftment of donor HSCs and the ensuing mixed chimerism generally leads to tolerization of residual preexisting antidonor T cells, probably by peripheral deletion.8,44 In our model, several arguments led us to believe that donor-specific tolerance became independent of DBY-Treg suppressive functions: (1) chimeric mice are unresponsive to male CD8 peptide immunization in the presence of adjuvant, demonstrating that female APCs loaded with class I–restricted male peptide failed to initiate antimale response; and (2) chimeric mice are fully immunocompetent against third-party antigens such as OVA and can exert equal cytotoxic activity against cells of male and female origin, showing that male cells are not protected from immune cytolysis. We also assessed whether long-term tolerance established toward minor antigens such as EGFP required constant expression of male antigens, and we gave chimeric mice transplants with a second graft of female origin expressing EGFP, either BM or skin. Our results demonstrate that tolerance against minor antigens present in the first BM graft was extended to female tissues lacking the antigen recognized initially by DBY-Tregs. We cannot exclude that tolerance to EGFP occurred in these mice due to linked suppression operating through the presence in the host of male cells expressing EGFP and DBY. However, the resistance of chimeric mice to donor-antigen immunization suggests that alteration/deletion of antidonor T-cell mechanisms occurs rather than a sustained DBY-Treg–mediated regulatory process.
In conclusion, we demonstrate that minimal conditioning with a small number of CD4+CD25+ cells directed toward a single DBY epitope promotes BM engraftment and establishes antigen-specific tolerance to multiple minor histocompatibility antigens in H2-matched adults while preserving full immunocompetence. The safe tolerance induction toward defined minor antigens evidenced here bears potential implications for (1) autologous stem cell transplantation to cure severe autoimmune diseases resistant to conventional treatment, (2) solid organ transplantation, and (3) gene and cell therapy whenever the immune response to the transgene or to gene-modified cells is a major hurdle for the treatment. For clinical applications, de novo generation and/or expansion of Treg populations are highly desirable.45,46 Conditions to expand antigen-specific Tregs have been proposed, either with anti-CD3– and anti-CD28–coated beads27 or dendritic cells.26,47 De novo generation of suppressor Tregs was recently achieved in vitro by transduction of the Foxp3 gene into murine polyclonal antigen-specific CD4+ effector T cells.48,49 However, as recently reported, ectopic expression of different FOXP3 isoforms did not generate potent suppressor T cells in humans.50 Other approaches were successful in vivo such as immunization with altered peptide ligand51 or targeting the antigen to tolerogenic dendritic cells using mAb conjugates.52 With the advent of such protocols, antigen-specific Tregs could provide new opportunities to replace myeloablative and life-threatening immunosuppressive treatments for transplantation of gene-modified autologous HSCs.
Prepublished online as Blood First Edition Paper, June 1, 2006; DOI 10.1182/blood-2006-02-011981.
Supported by Association Française contre les Myopathies (AFM), an Action Thématique Incitative du Génopole d'Evry (ATIGE) grant from Génopole Evry, and European Project QLKT3-LT-2001-00427. D.-A.G. designed research; D.-A.G., P.C., M.L., V.M., and L.V.W. performed research; and D.-A.G., O.D., and J.D. analyzed data and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Terry Partridge for critical reading of the manuscript and Olivier Lantz for helpful comments and for Marilyn mice. We also thank Philippe Rameau for cell sorting and Thibaut Marais and Ludovic Arandel for mouse preparation.