Comment on Dumitriu et al, page 1198
Sox6 cooperates with EPO in a cell-autonomous manner to enhance definitive erythropoiesis, a possible new target for therapy of anemias and erythrocythemias.
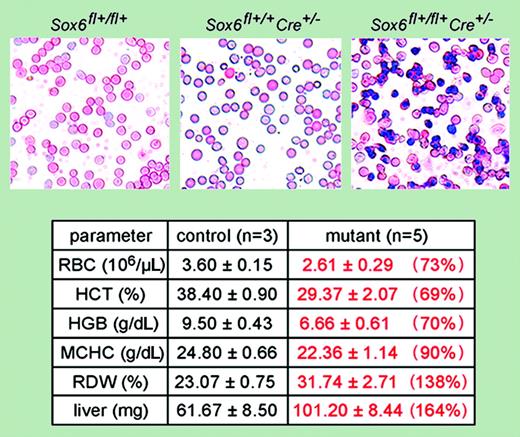
In the current issue of Blood, Dumitriu and colleagues describe important functions for the Sry-related high-mobility-group (HMG) box DNA-binding domain group 6 (Sox6) gene family member in red blood cell development and maturation. This gene family comprises 20 members that play important roles in development of various tissues. Sox6 is highly expressed in neurons, chondrocytes, and muscle, and in chondrogenesis Sox6 functions downstream of Bmp signaling.1 Sox6 also mediates expression of Wnt-1–, N-cadherin–, and E-cadherin–mediated signaling during aggregation and neuronal differentiation of embryonic carcinoma cells.2 Of interest, the expression pattern of Sox6 parallels hematopoietic development both temporally and spatially, with high expression in E12.5 fetal liver and high level expression in adult mouse bone marrow hematopoietic stem cells.3 Despite this strong developmental relationship, the role of Sox6 in hematology is just now being uncovered. Mice with a chromosome 7 inversion (p100H) that disrupts the p gene and Sox6 have constitutive expression of embryonic globin at E14.5, and accordingly Sox6 can directly function to regulate ϵy-globin gene expression.4 These findings, combined with an increased prevalence of nucleated red blood cells in Sox6–/– mice, suggested a possible role for Sox6 in erythropoiesis. However, it was not possible to rule out cell-extrinsic phenotypes that could impact upon definitive erythropoiesis by altering the microenvironment.FIG1
Dumitriu and colleagues have used a new conditional knock-out mouse model for Sox6 to study definitive erythropoiesis in the fetal liver. These studies confirm previous findings with Sox6–/– mice and now demonstrate that EPO receptor–mediated deletion of Sox6 in committed erythroid progenitors is additive with EPO for maturation, proliferation, and survival. This figure demonstrates the increase in nucleated red blood cells, anemia, and increase in erythropoietic tissue volume associated with conditional deletion. Of importance, this approach indicates that Sox6 functions in a cell-intrinsic manner to regulate erythroid development and suggests that Sox6 may be an important new therapeutic target. See the complete figure and table in the article beginning on page 1198.
Dumitriu and colleagues have used a new conditional knock-out mouse model for Sox6 to study definitive erythropoiesis in the fetal liver. These studies confirm previous findings with Sox6–/– mice and now demonstrate that EPO receptor–mediated deletion of Sox6 in committed erythroid progenitors is additive with EPO for maturation, proliferation, and survival. This figure demonstrates the increase in nucleated red blood cells, anemia, and increase in erythropoietic tissue volume associated with conditional deletion. Of importance, this approach indicates that Sox6 functions in a cell-intrinsic manner to regulate erythroid development and suggests that Sox6 may be an important new therapeutic target. See the complete figure and table in the article beginning on page 1198.
To address the limitations of studies using p100H or Sox6–/– mice, Dumitriu and colleagues have now generated mice containing a floxed Sox6 allele, permitting conditional deletion studies to be performed while avoiding perinatal lethality.5 This work is important because it describes a new animal model that may be useful for further study of Sox6 function in hematopoiesis. The authors have used this approach to delete Sox6 in cells after they are committed to the erythroid lineage and are expressing the EPO receptor. In this issue of Blood, the authors show that like Sox6–/– fetuses, conditional deletion of Sox6 using EPOR-GFPCre mice leads to cell-autonomous increases in nucleated red blood cells, anemia, red blood cell anisocytosis, and an enlarged liver. Of interest, Sox6–/– mice had a compensation for the anemia due to a 10-fold increase in EPO level. This is somewhat complicated by the mixed genetic background that might have favored the increase in erythropoietic tissue volume. Backcross of these mice to the C57BL/6 strain might lead to more severe defects. Nevertheless, since these defects were additive with those of EPO signaling in favoring the early expansion, maturation, survival, and proliferation of red blood cells, the study raises interesting questions about whether Sox6 is EPO regulated and what downstream targets are important for Sox6 function. In this model, unlike other erythroid-restricted transcription factors, Sox6 could also function independent of lineage commitment and was required for promoting proerythroblast and erythroblast proliferation and survival. The additional finding that F-actin assembly or stability in erythrocytes is dependent on Sox6 expression suggests that Sox6 may have similar functions in leukocytes. The identification of this important role for Sox6 may lead to new targets for controlling erythrocythemias and for treating hemoglobinopathies. ▪