Comment on Liu et al, page 1123
B-lymphocyte reconstitution following hematopoietic stem cell transplantation is frequently delayed and functionally compromised. In this issue of Blood, Liu and colleagues describe a potentially novel mechanism implicating recipient pro-B cells in negatively impacting donor B-cell engraftment.
Production of B lymphocytes that express an antibody repertoire with antimicrobial protective effects is a lifelong process that originates in 8-week fetal liver and continues in individuals enjoying their eighth decade of life.1 However, reconstitution of B-cell function in individuals undergoing allogeneic hematopoietic stem cell transplantation (HSCT) is frequently delayed and/or functionally compromised. Recapitulation of normal age-matched B-cell development following HSCT does not occur. How can this be explained?
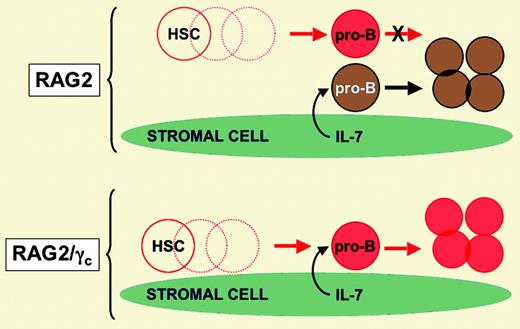
In this issue of Blood, Liu and colleagues used a murine model of HSCT to test the intriguing hypothesis that residual host pro-B cells (early marrow B-lineage cells that do not yet express functional immunoglobulin [Ig] gene rearrangements) somehow interfere with engraftment of donor B-lineage cells. Using a nonmyeloablative protocol, the basic approach involved transfer of C57BL/6 (CD45.1) donor stem cells into CD45.2 congenic recipients that harbor targeted disruptions in genes that encode proteins essential for immune system development. The targeted genes included the recombinase activating gene (RAG2), critical for immunoglobulin and T-cell receptor rearrangement, and the common gamma chain (γc) subunit of the interleukin (IL) receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21. RAG2-deficient mice have no functional T or B cells, but do have pro-B cells and mature natural killer (NK) cells. RAG2/γc double-deficient mice have essentially no T, B, or NK cells. When the 2 strains were used as recipients in the HSCT model, donor B-cell engraftment was almost completely restorative in the marrow, lymph node, spleen, and peritoneal cavity of RAG2/γc double-deficient recipients. In contrast, B-cell engraftment was 100- to 200-fold less efficient in RAG2-deficient recipients that harbored host pro-B cells (see the figure). The possibility that host NK cells present in RAG2-deficient mice were disrupting B-cell engraftment was largely excluded by demonstrating that RAG2/IL-2Rβ double-deficient mice (which have pro-B cells but no mature NK cells due to a failure of IL-15 and IL-2 signaling) also exhibited severe deficiencies in donor B-cell engraftment.FIG1
Model proposing that availability of IL-7 (or some other stromal cell–derived factor/nutrient) limits B-cell development in the study by Liu and colleagues. Red cells are of donor origin. Brown cells are of recipient origin. Dashed circles are lymphohematopoietic intermediates that are not shown for simplicity. Illustration courtesy of Rebecca Asch.
Model proposing that availability of IL-7 (or some other stromal cell–derived factor/nutrient) limits B-cell development in the study by Liu and colleagues. Red cells are of donor origin. Brown cells are of recipient origin. Dashed circles are lymphohematopoietic intermediates that are not shown for simplicity. Illustration courtesy of Rebecca Asch.
This interesting report prompts several questions. First, what is the mechanism whereby the presence of recipient pro-B cells compromises donor B-cell engraftment in the murine model? One possibility is that limiting amounts of IL-7 might exist in a “B lymphopoietic niche,” adequate to support proliferation of host pro-B cells but inadequate to support expansion of donor pro-B cells as well. In a similar vein, and as discussed elsewhere,2 an important nutrient such as arginine could be rate limiting. Donor pro-B cells may also have reduced expression of the IL-7 receptor making them unable to compete, reminiscent of the situation with reduced IL-7 receptor expression on T lymphocytes.3 In the event that the murine study holds relevance for human allogeneic HSCT, what strategy could be used to promote donor B-cell engraftment? It is noteworthy that severe combined immunodeficiency (SCID) patients with residual pro-B cells who undergo HSCT in the absence of myeloablation often retain host B-lineage cells many years after transplantation.4 Depletion of residual host B-lineage cells before transplantation with, for example, anti-CD19 antibodies (potentially conjugated to drugs or radionuclides) might facilitate donor B-cell engraftment. Administration of IL-7 after transplantation might also overcome the putative competitive disadvantage of donor B-lineage cells and facilitate their engraftment, given evidence that IL-7 can transduce a replicative signal to human pro-B cells.5 ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal