Abstract
MRL/lpr mice develop a human lupuslike syndrome and, as in autoimmune lymphoproliferative syndrome (ALPS), massive lymphoproliferation due to inactivation of Fas-mediated apoptosis. Presently, no effective therapy exists for ALPS, and long term, therapies for lupus are hazardous. We show herein that arsenic trioxide (As2O3) is able to achieve quasi-total regression of antibody- and cell-mediated manifestations in MRL/lpr mice. As2O3 activated caspases and eliminated the activated T lymphocytes responsible for lymphoproliferation and skin, lung, and kidney lesions, leading to significantly prolonged survival rates. This treatment also markedly reduced anti-DNA autoantibody, rheumatoid factor, IL-18, IFN-γ, nitric oxide metabolite, TNF-α, Fas ligand, and IL-10 levels and immune-complex deposits in glomeruli. As2O3 restored cellular reduced glutathione levels, thereby limiting the toxic effect of nitric oxide, which is overproduced in MRL/lpr mice. Furthermore, As2O3 protected young animals against developing the syndrome and induced almost total disease disappearance in older affected mice, thereby demonstrating that it is a novel promising therapeutic agent for autoimmune diseases.
Introduction
The pathogenesis of systemic lupus erythematosus (SLE) is multifactorial and polygenic. The apoptosis gene Fas is a candidate contributory gene in both human SLE and murine models of it. In humans, FAS mutations result in a familial autoimmune lymphoproliferative syndrome (ALPS), characterized by nonmalignant accumulation of an unusual population of double-negative (DN) B220+CD4–CD8–α/β+ T lymphocytes and development of autoimmune diseases.1 Overexpression of Th2 cytokine IL-10 is associated with the manifestations of ALPS.2 In mice with the MRL genetic background, the autosomal recessive gene lpr (Tnfrsf6lpr) is responsible for a syndrome characterized by the progressive accumulation of DN T-cell population in peripheral lymphoid tissues. The lpr mutation engenders much less expression of the Fas death receptor, which results in the accumulation, in lymph nodes (LNs) and spleens, of numerous activated DN T lymphocytes that are not normally regulated by the Fas-mediated mechanism controlling apoptosis of mature T cells.3,4 Therefore, MRL/lpr T and B cells massively overexpress Fas ligand (FasL), which renders them able to kill Fas+ cells in vitro5-8 and in vivo.9-11 However, lpr mutation cannot account for the entire autoimmune syndrome of MRL/lpr mice and other genes of pathologic importance in the MRL background have been mapped.12,13
MRL/lpr mice spontaneously develop a lupuslike syndrome that can affect many organs. The murine cutaneous lesions resemble human discoid lupus erythematosus. Mononuclear cells infiltrate joints and lachrymal and salivary glands, resulting in rheumatoid arthritis and Sjögren syndrome, respectively.14 As in patients with SLE, MRL/lpr mice have neurologic manifestations caused by cellular infiltrations into the central nervous system.15 MRL/lpr mice develop hypergammaglobulinemia and high levels of autoantibodies, including anti-DNA antibodies, associated with immune-complex–mediated glomerulonephritis and vasculitis.16,17 Glomerulonephritis, interstitial nephritis, and vasculitis begin by 8 weeks of age, and MRL/lpr females die at about 17 weeks of age and males at 22 weeks.18
Because of the resemblance between the murine and human diseases, MRL/lpr mice have been used extensively to attempt to determine SLE etiology and to evaluate therapies. Indeed, MRL/lpr mice provide an attractive model because their syndrome is spontaneous, predictable, and rapid; it exhibits the characteristic multifaceted tissue destruction and sexual dimorphism, and its severity varies with the individual. Th1 cytokines, including IFN-γ, are present in the tissues of patients severely affected with SLE. IFN-γ production by peripheral-blood cells and in kidneys of patients with severe lupus glomerulonephritis was higher than that of patients with milder renal disease.19 Disease severity in MRL/lpr mice is also linked to Th1 cytokines, IFN-γ and IL-12.20-23 IFN-γ gene21,23 or IFN-γ receptor (IFN-γR) deletion,24,25 and less IgG2a and IgG3 but not IgG1 anti-DNA antibody synthesis dramatically limits glomerulonephritis.26,27 IL-18 is associated with severe inflammatory conditions, for example, autoimmune diseases, allergies, or neurologic disorders,28 and its concentration is elevated in sera from patients with SLE and MRL/lpr mice. IL-18 has pleiotropic immunoregulatory functions: stimulating IFN-γ production, Fas-mediated cytotoxicity, and developmental regulation of Th1.28 Increased nitric oxide (NO) production plays an important role in various inflammatory diseases. Indeed, excessive NO synthesis can be followed by NO interaction with superoxide to form peroxynitrite, which causes severe oxidative damage to lipids, proteins, and DNA. Therefore, the intracellular content of reduced glutathione (GSH) appears to play a key role in dictating cell susceptibility to NO and peroxynitrite. MRL/lpr mice overproduce NO in their disease-affected organs, and this overproduction parallels the development of clinical disease manifestations. The major contribution of NO to disease manifestations is further supported by observations that treatment of MRL/lpr mice with NO synthase inhibitor prevents glomerulonephritis, arthritis, and vasculitis.29 TNF-α, a pleiotropic cytokine with proinflammatory properties, is also increased in the autoimmune MRL/lpr mice.30 Enhanced production of TNF-α has been clearly associated with several autoimmune and inflammatory diseases, such as rheumatoid arthritis31 and inflammatory bowel disease.32 Paradoxically, the Th2 cytokine, IL-10, has also been associated with the lupus disease in MRL/lpr mice.33 Likewise, patients with lupus produce large amounts of IL-10, and its serum level correlates with disease activity.34,35
Arsenic trioxide (As2O3) has shown substantial efficacy in the treatment of patients with newly diagnosed or relapsed acute promyelocytic leukemia (APL).36,37 Side effects are more likely to occur with intravenous than with oral administration of As2O3.38 As2O3 induces remissions in patients with APL in part through degradation of the aberrant promyelocytic leukemia-retinoic acid receptor α (PML-RAR) fusion protein.36,39,40 Although numerous studies have been conducted on the molecular mechanisms accounting for PML-RAR degradation, neither the precise basis for the selectivity of As2O3 for PML-RAR that it targets nor the exact molecular pathway through which As2O3 induces remission in APL patients were understood. As2O3 acts on signaling,41 caspases and apoptosis, cellular redox, and cellular responses to stress.42 Although mostly focused on the APL response to As2O3, investigators using many different experimental systems concluded that As2O3 may be beneficial against hematopoietic malignancies and solid tumors.42 We examined the therapeutic impact of As2O3 on the lymphoproliferative and severe autoimmune disorders manifested in MRL/lpr mice.
Materials and methods
Mice and As2O3 treatment
Wild-type MRL/MpJ (MRL+/+), MRL/MpJ-Tnfrsf6lpr/J mutant (MRL/lpr), and C57BL/6J mice originally from the Jackson Laboratory (Bar Harbor, ME) were maintained in our animal facilities. All experiments were performed in accordance with institutional Animal Research Committee guidelines. A stock solution was prepared by dissolving As2O3 powder (Sigma, St Louis, MO) in 1 M NaOH, then further diluting it in PBS; mice were injected intraperitoneally daily with 2.5, 5, 7.5, 10, or 15 μg/g body weight; controls received a daily intraperitoneal injection of PBS (volume weight-determined).
Phenotyping
Spleen- and LN-cell suspensions were phenotyped by flow cytometry using either FITC-, phycoerythrin-, or biotin-conjugated monoclonal antibody (mAb): rat anti–Thy-1.2, anti-CD4, anti-CD8, anti-B220 (all from PharMingen, San Diego, CA), and rat IgG2a mAb as the isotype control ((Serotec France, Cergy Saint-Christophe, France). Use of mAb to mouse Fcγ receptor (PharMingen) avoided nonspecific antibody binding.
Western blotting
Lymphoid-cell pellets were directly lysed in Laemmli sample buffer and boiled for 5 minutes. Protein samples (30 μg each) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting using rabbit polyclonal anti-FasL (Santa Cruz Biotechnology, Santa Cruz, CA) or antiactin antibodies (Sigma).
Histopathology and immunohistochemistry
Kidney, liver, skin, and lung samples were fixed overnight in GlyoFix (Shandon Lipshaw, Pittsburgh, PA), then dehydrated, and paraffin-embedded; 4-μm thick sections were cut. After hematoxylin, eosin, and saffron staining, sections were examined. For immunohistochemical labeling, kidney samples were frozen in OCT compound, and immune complexes deposited in glomeruli were detected by incubating cryosections with horseradish peroxidase-conjugated goat anti–mouse IgG (1:200, Vector Laboratories, Burlingame, CA). Sections were then counterstained with hemalum. The photomicrographs were obtained using an Axiophot microscope (Carl Zeiss, Oberkochen, Germany) and a PCO sensicam 12 bit cooled imaging camera with SensiControl Utility software (PCO, Klenheim, Germany). Images were processed with Adobe Photoshop (Adobe Systems, San Jose, CA).
ELISA detection of anti-DNA autoantibodies, rheumatoid factor, and cytokines
To detect serum IgG (IgG1, IgG2a, IgG2b, IgG3) anti-DNA autoantibodies, 96-well plates were coated with 10 μg/mL calf thymus DNA (Sigma). For detection of IgM and IgG rheumatoid factor (RF), plates were coated with rabbit IgG (1 μg/mL; Sigma), and for detection of IgG3 RF, plates were coated with mouse IgG2a (1 μg/mL; Sigma).43 After blocking with 1% BSA, serial serum dilutions (starting at 1:400) were added in triplicate for 2 hours at room temperature, then washed; bound IgG anti-DNA was detected with biotin-labeled rat anti–mouse IgG, streptavidin-alkaline phosphatase, and pNPP (Sigma). Bound RF was detected with alkaline phosphatase-labeled goat anti–mouse IgM, IgG (both Sigma), or IgG3 (Caltag Laboratories, Burlingame, CA) and pNPP. OD was determined at 405 nm.
Serum levels of IFN-γ, soluble FasL, IL-18, TNF-α, and IL-10 were assayed using the mouse IFN-γ ELISA Ready-SET-Go (eBioscience, San Diego, CA), mouse Fas ligand immunoassay (Quantikine M kit; R&D Systems, Minneapolis, MN), mouse IL-18 ELISA (R&D Systems), mouse TNF-α immunoassay (R&D systems), and mouse IL-10 immunoassay (R&D Systems), respectively, following the manufacturers' instructions.
Determination of NO production
Serum levels of nitrite were measured using the NO quantification kit (Active Motif, Rixensart, Belgium), according to the manufacturer's instructions.
Apoptosis analysis and GSH detection
Activated caspase levels in spleen and LN cells were measured using the CaspaTag kit (Chemicon, Temecula, CA), according to the manufacturer's instructions. GSH levels were detected in spleen and LN cells using 5 μM CellTracker probe CMFDA, following the manufacturer's instructions (Molecular Probes, Eugene, OR) and flow cytometry.
Statistical analysis
Data are reported as the mean ± SE. Comparisons between experimental and control groups were made by Student t test. Statistical difference was accepted at P below .05.
Results
As2O3 prevents and reduces lymphoproliferation in MRL/lpr mice
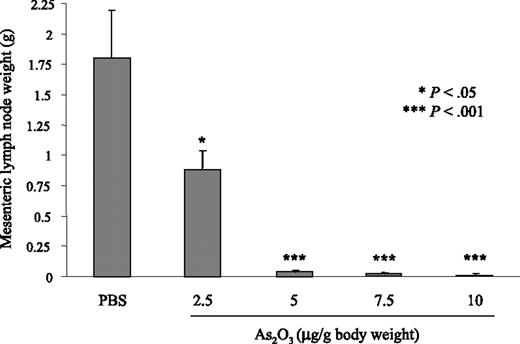
Accumulation of DN B220+CD4–CD8– T lymphocytes in MRL/lpr LN and spleen starts at 2 months and this lymphoproliferation peaks at 4 to 5 months. In experiments starting at 2 months of age, MRL/lpr and MRL+/+ mice were treated for 2 months with As2O3 daily (2.5-10 μg/g body weight) or PBS. With 2.5 μg/g As2O3 the lymphoproliferation was significantly reduced (P < .05) when compared to PBS-treated MRL/lpr mice. However, with 5, 7.5, or 10 μg/g, no lymphoproliferation was seen in the spleens and LNs of treated MRL/lpr mice (Figure 1 and data not shown). Because 5 μg/g/d was the lowest dose of As2O3 efficient to block the lymphoproliferation, this dose was used for subsequent experiments. To assess As2O3 activity on nonlymphoid organs, MRL+/+ and MRL/lpr mice received 5 μg/g As2O3 daily for 2 months and then their spleens, mesenteric LNs, axillary LNs, livers, hearts, lungs, and kidneys were weighed (Table 1). In MRL/lpr mice, mean spleen weight increased 6-fold and their mean axillary and mesenteric LN weights rose 45- and 48-fold, respectively, versus MRL+/+. Mean MRL/lpr liver and lung weights were also higher than MRL+/+ values, at about 1.5- and 1.7-fold, respectively. Under As2O3, the abnormally high MRL/lpr lymphoid organ, liver, and lung weights were sharply reduced, whereas kidney and heart weights remained unaffected (Table 1). In As2O3-treated MRL+/+ mice, spleen- and LN-cell numbers as well as liver, lung, kidney, and heart weights were unchanged (Table 1).
Absence of lymphoproliferation in As2O3-treated MRL/lpr mice. Mesenteric LN weights from 2-month-old MRL/lpr mice that had been treated daily for 2 months with 2.5, 5, 7.5, or 10 μg/g As2O3 or PBS. Results are from 6 individual mice per group. Error bars indicate SE.
Absence of lymphoproliferation in As2O3-treated MRL/lpr mice. Mesenteric LN weights from 2-month-old MRL/lpr mice that had been treated daily for 2 months with 2.5, 5, 7.5, or 10 μg/g As2O3 or PBS. Results are from 6 individual mice per group. Error bars indicate SE.
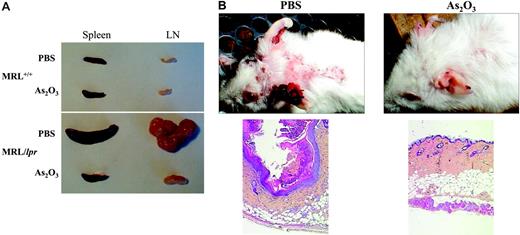
To determine whether As2O3 could also be curative, 4-month-old MRL/lpr mice, with severe lymphoproliferation, were given daily As2O3 (5 μg/g) injections for 2 months; lymphoproliferation in their spleens and LNs regressed under therapy (Figure 2A). Importantly, As2O3 had no effect on lymphoid organs from MRL+/+ mice (Figure 2A). Flow cytometry quantification of spleen-cell subpopulations from MRL/lpr mice treated with PBS or As2O3 using mAb specific to CD3, CD4, CD8, CD19, and B220 cell-surface antigens yielded (mean ± SE), for 4-month-old PBS-treated MRL/lpr mice, 7.2 ± 0.7 × 108 splenocytes (n = 8), among which 77% ± 1.5% and 13% ± 0.5% were CD3+ T and CD19+ B lymphocytes, respectively. DN represented 75% ± 7% of the CD3+ T cells, whereas CD4+ and CD8+ subsets accounted for 13% ± 2% and 12% ± 1.5%, respectively. The number of splenocytes in As2O3-treated MRL/lpr mice was reduced about 15-fold to 0.49 ± 0.6 × 108 (n = 10), among which 51% ± 2% and 49% ± 3% were CD3+ T and CD19+ B lymphocytes, respectively. Abnormal DN T lymphocytes represented 23% ± 1.5% of CD3+ T lymphocytes, with 50% ± 4% and 25% ± 2% normal CD4+ and CD8+ subpopulations, respectively. Similarly, mesenteric LN-cell counts were 10-fold lower. These data demonstrate the ability of As2O3 to specifically eliminate abnormal DN T lymphocytes. To completely exclude that As2O3 acts as a cytotoxic agent by causing generalized immunosuppression, we determined bone marrow, thymus, blood, spleen, and LN-cell numbers in autoimmune MRL/lpr and nonautoimmune C57BL/6J mice treated with As2O3 (5 μg/g/d) for 2 months. In As2O3-treated C57BL/6J mice, bone marrow, thymus, red blood, spleen, and LN-cell numbers were unchanged. White blood cell numbers from these mice were slightly, but not significantly, decreased (Table 2). In As2O3-treated MRL/lpr mice, the number of white blood cells is normalized and the lymphoproliferation in their spleens and LNs regressed, whereas bone marrow, thymus, and red blood cell numbers remained unchanged (Table 2). Taken together our results suggest that As2O3 has a selective effect on DN T lymphocytes, which accumulate in autoimmune MRL/lpr mice.
As2O3 suppresses skin lesions in MRL/lpr mice
Human lupus exhibits several clinical phenotypes. Discoid lupus affects the skin, causing rashes and lesions, usually on the face and upper torso, and the cutaneous lesions of MRL/lpr mice resemble it. MRL/lpr mice develop necrotic skin lesions on their ears, hair loss, and scab formation, typically on the upper back (Figure 2B). Light microscopy examination of the skin lesions revealed hyperkeratosis, acanthosis, liquefaction, vasodilatation, and mononuclear-cell infiltrates in the dermis (Figure 2B left panel). Unexpectedly, injecting As2O3 (5 μg/g/d, for 1-2 months) into MRL/lpr mice led to the quasi-total disappearance of cervical cutaneous lesions and hair grew back (Figure 2B). The quasi-total regression of skin lesions in As2O3-treated mice was confirmed by light microscopy examination of skin sections (Figure 2B right panel). Thus, in arsenic-treated animals, dermal lymphoid infiltration was dramatically attenuated, and hyperkeratosis, acanthosis, hypergranulosis, liquefaction, and dermal vasodilatation disappeared.
As2O3 returns FasL levels to normal in MRL/lpr mice
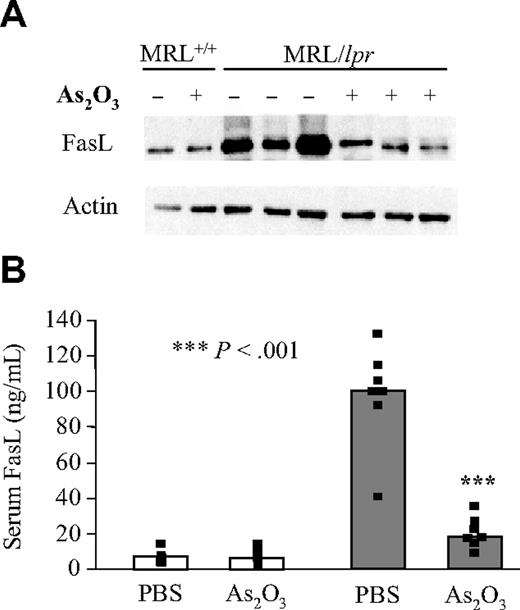
FasL is synthesized by lymphoid cells in membrane-associated and soluble forms.44,45 Both forms were studied in MRL+/+ and MRL/lpr mice treated with PBS or As2O3. Cell-surface–anchored FasL was analyzed by Western blotting on lymphoid-cell lysates, whereas soluble FasL in mouse sera was quantified by ELISA. As expected, in Fas-deficient MRL/lpr, but not MRL+/+ mice, both FasL forms were overexpressed (Figure 3A-B). As2O3 treatment of MRL/lpr mice sharply decreased soluble and membrane-associated FasL levels to approach those found in MRL+/+ mice (Figure 3A-B). Administering As2O3 to MRL+/+ mice did not affect FasL levels, suggesting that FasL-overexpressing T lymphocytes are the main target of this agent.
Absence of lymphoproliferation and regression of skin lesions in As2O3-treated MRL/lpr mice. Two-month-old mice had been treated daily for 2 months with PBS or As2O3 (5 μg/g). (A) Spleens and mesenteric LNs from 4-month-old MRL/lpr and MRL+/+ mice. (B) Skin lesions on the back and ears from 4-month-old MRL/lpr mice. Light microscopy histology of these skin lesions (bottom panels, original magnification ×50, with 5×/0.15 NA objective).
Absence of lymphoproliferation and regression of skin lesions in As2O3-treated MRL/lpr mice. Two-month-old mice had been treated daily for 2 months with PBS or As2O3 (5 μg/g). (A) Spleens and mesenteric LNs from 4-month-old MRL/lpr and MRL+/+ mice. (B) Skin lesions on the back and ears from 4-month-old MRL/lpr mice. Light microscopy histology of these skin lesions (bottom panels, original magnification ×50, with 5×/0.15 NA objective).
IFN-γ, IL-18, TNF-α, and IL-10 syntheses decline in As2O3-treated MRL/lpr mice
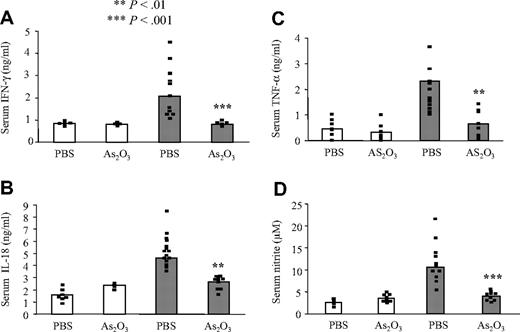
Because IFN-γ, IL-18, and TNF-α are major mediators of several autoimmune and inflammatory diseases,23,31,32,46 we compared their concentrations in the sera of MRL+/+ and MRL/lpr mice treated with PBS or As2O3. In MRL+/+ mice, IFN-γ, IL-18, and TNF-α syntheses were not affected by As2O3 treatment (Figure 4A-C). As expected, PBS-treated MRL/lpr mice had very high serum IFN-γ, IL-18, and TNF-α levels, but As2O3 treatment maintained normal cytokine concentrations, comparable to those of MRL+/+ mice (Figure 4A-C).
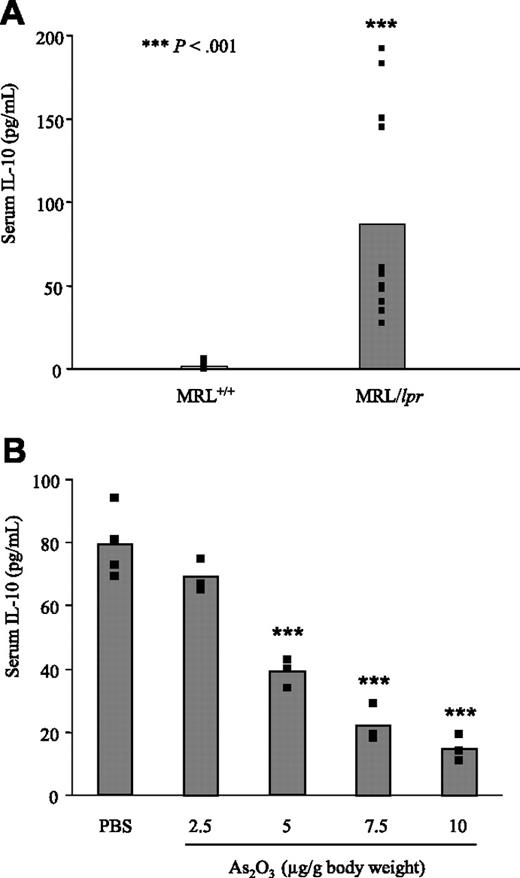
Like patients with ALPS2 and human SLE patients,35 untreated MRL/lpr mice produce large amounts of IL-10 when compared to MRL+/+ mice (Figure 5A). As expected, PBS-treated MRL/lpr mice had very high serum IL-10 levels, but As2O3 treatment significantly reduced IL-10 concentrations, in a dose-dependent manner (Figure 5B). However, even at the highest dose of As2O3 tested (10 μg/g), serum from MRL/lpr mice contained significantly higher concentrations of IL-10 than those from MRL+/+ mice. The DN T cells are primary producers of IL-10 in ALPS patients2 ; we therefore quantified these cells in MRL/lpr mice treated with PBS or As2O3 by flow cytometry using mAbs specific to CD3, CD4, CD8, CD19, and B220 molecules. In LNs from PBS-treated MRL/lpr mice, DN T cells represented 66.8% ± 4.35% of CD3+ T cells. In MRL/lpr mice treated with 2.5, 5, 7.5, or 10 μg/g As2O3, DN T cells represented 48% ± 5.40%, 19.33% ± 3.80%, 15% ± 2.34%, or 8.51% ± 2.75% of CD3+ T lymphocytes, respectively. Importantly, the reduction of serum IL-10 paralleled the decrease in DN T-cell numbers.
Concentrations of nitrites decline in As2O3-treated MRL/lpr mice
Measuring NO synthase (NOS) activity by monitoring the accumulation of nitrite, a stable oxidative end product of NO, is a standard assay for NOS activity. MRL/lpr mice overproduce NO as autoimmune disease progresses.29 Therefore, serum from MRL/lpr mice contained significantly higher concentrations of nitrite than those from MRL+/+ mice (Figure 4D). Although As2O3 did not modify nitrite levels in serum from MRL+/+ mice, it sharply decreased nitrite levels in serum from MRL/lpr mice to approach those found in MRL+/+ mice (Figure 4D).
FasL levels return to normal in As2O3-treated MRL/lpr mice. Level of cell-surface–anchored form of FasL from 4-month-old MRL+/+ (white bars) and MRL/lpr (gray bars) mice that had been treated daily with PBS (–) or As2O3 (5 μg/g) (+) for 2 months were analyzed by Western blotting of lymphoid-cell lysates from 8 individual mice using anti-FasL mAb. The blot was stripped and reprobed with antiactin mAb. (B) ELISAs were used to determine their serum FasL concentrations. Black squares represent data from individual animals and bars correspond to the mean for 6 to 12 mice per group.
FasL levels return to normal in As2O3-treated MRL/lpr mice. Level of cell-surface–anchored form of FasL from 4-month-old MRL+/+ (white bars) and MRL/lpr (gray bars) mice that had been treated daily with PBS (–) or As2O3 (5 μg/g) (+) for 2 months were analyzed by Western blotting of lymphoid-cell lysates from 8 individual mice using anti-FasL mAb. The blot was stripped and reprobed with antiactin mAb. (B) ELISAs were used to determine their serum FasL concentrations. Black squares represent data from individual animals and bars correspond to the mean for 6 to 12 mice per group.
As2O3 prevents inflammatory infiltrates in lungs and kidneys and inhibits immune-complex deposition in kidneys of MRL/lpr mice
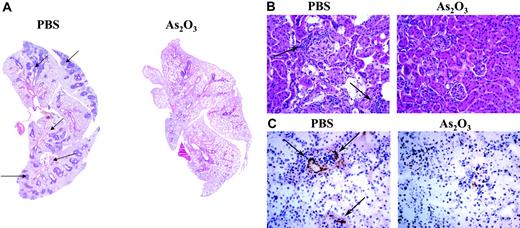
SLE, usually more severe than discoid lupus, can attack any organ. Pleurisy is a common pulmonary manifestation of SLE and, more rarely, patients develop acute lupus pneumonitis. Similarly, the MRL/lpr disease is characterized by massive accumulation of leukocytes in the lungs,47 with widespread perivascular and peribronchiolar mononuclear-cell infiltrates (Figure 6A). In contrast, in As2O3-treated MRL/lpr mice, the pulmonary architecture was normal, with mononuclear cells rarely seen around vessels and never around the airways (Figure 6A).
Lupus nephritis or glomerulonephritis is another severe manifestation of human SLE. The kidney disease developing in MRL/lpr mice is fatal and caused by lymphoid cells infiltrating into glomeruli, interstitium, and perivascular compartments (Figure 6B). In contrast, MRL/lpr mice treated with As2O3 for 2 months had normal kidney structure, with a few mononuclear cells around glomeruli (Figure 6B). As in humans, MRL/lpr lupus nephritis is caused by the deposition of immune complexes (Figure 6C) in kidney parenchyma. Treating 2-month-old MRL/lpr mice with As2O3 for 2 months completely prevented immune-complex deposits in their glomeruli (Figure 6C).
IFN-γ, IL-18, nitrites, and TNF-α levels return to normal in As2O3-treated MRL/lpr mice. Four-month-old MRL+/+ (white bars) and MRL/lpr (gray bars) mice had been treated daily with PBS or As2O3 (5 μg/g) for 2 months. ELISAs were used to determine their serum IFN-γ (A), IL-18 (B), and TNF-α (C) concentrations, and Griess reagents to measure their serum nitrite concentrations (D). Black squares represent data from individual animals and bars correspond to the mean for 6 to 9 mice per group.
IFN-γ, IL-18, nitrites, and TNF-α levels return to normal in As2O3-treated MRL/lpr mice. Four-month-old MRL+/+ (white bars) and MRL/lpr (gray bars) mice had been treated daily with PBS or As2O3 (5 μg/g) for 2 months. ELISAs were used to determine their serum IFN-γ (A), IL-18 (B), and TNF-α (C) concentrations, and Griess reagents to measure their serum nitrite concentrations (D). Black squares represent data from individual animals and bars correspond to the mean for 6 to 9 mice per group.
Reduction of serum IL-10 in As2O3-treated MRL/lpr mice. Serum IL-10 levels were quantified by ELISA in the sera of (A) untreated 4-month-old MRL+/+ and MRL/lpr (B) 2-month-old MRL/lpr mice that had been treated daily for 2 months with 2.5, 5, 7.5, or 10 μg/g As2O3 or PBS. Black squares represent data from individual animals and bars correspond to the mean for 3 to 4 mice per group.
Reduction of serum IL-10 in As2O3-treated MRL/lpr mice. Serum IL-10 levels were quantified by ELISA in the sera of (A) untreated 4-month-old MRL+/+ and MRL/lpr (B) 2-month-old MRL/lpr mice that had been treated daily for 2 months with 2.5, 5, 7.5, or 10 μg/g As2O3 or PBS. Black squares represent data from individual animals and bars correspond to the mean for 3 to 4 mice per group.
As2O3 inhibits autoantibody and RF production in MRL/lpr mice
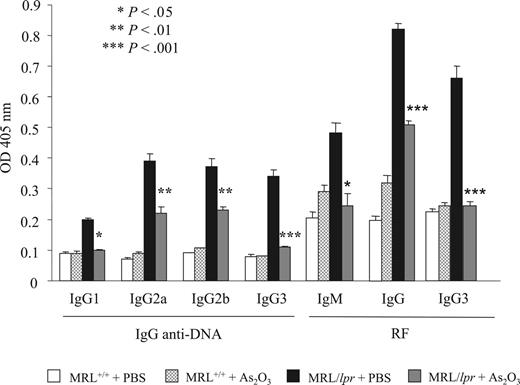
Anti-DNA antibodies and RF, commonly found in patients with SLE, are thought to play important pathogenic roles in lupus nephritis.17,48,49 IgG1, IgG2a, IgG2b, and DNA-reactive IgG3, and IgM, IgG, and IgG3 RF were quantified by ELISA in sera from MRL+/+ and MRL/lpr mice treated with PBS or As2O3. IgG1 and IgG3 anti-DNA and IgG and IgG3 RF levels were markedly lower in As2O3-treated mice, and IgG2a and IgG2b anti-DNA and IgM RF were moderately lower (Figure 7).
As2O3-induces regression of pulmonary and renal inflammatory infiltrates and inhibits immune-complex deposition in kidneys of MRL/lpr mice. Histologic examination of mononuclear-cell infiltrates (arrows) in (A) lung (original magnification ×50, with 5×/0.15 NA objective) and (B) kidney (original magnification ×200, with 20×/0.50 NA objective) sections from a 4-month-old MRL/lpr mouse that had been treated daily for 2 months with As2O3 (5 μg/g) or PBS. (C) Glomerular IgG deposits in frozen kidney sections labeled with HRP-conjugated goat anti–mouse IgG (original magnification ×200, with 20×/0.50 NA objective).
As2O3-induces regression of pulmonary and renal inflammatory infiltrates and inhibits immune-complex deposition in kidneys of MRL/lpr mice. Histologic examination of mononuclear-cell infiltrates (arrows) in (A) lung (original magnification ×50, with 5×/0.15 NA objective) and (B) kidney (original magnification ×200, with 20×/0.50 NA objective) sections from a 4-month-old MRL/lpr mouse that had been treated daily for 2 months with As2O3 (5 μg/g) or PBS. (C) Glomerular IgG deposits in frozen kidney sections labeled with HRP-conjugated goat anti–mouse IgG (original magnification ×200, with 20×/0.50 NA objective).
As2O3 significantly improves survival of MRL/lpr mice
As2O3 significantly prolonged survival (P < .001) of male and female MRL/lpr mice with established disease (Figure 8 and data not shown). Indeed, 18 weeks after starting treatment, all As2O3-treated female mice were alive, whereas all PBS-treated MRL/lpr mice had died. After 45 weeks of treatment, As2O3-treated MRL/lpr mice were still alive with no sign of lymphoproliferation. Finally, 75 weeks after starting treatment, all As2O3-treated MRL/lpr had died. Likewise, most of the MRL+/+ mice died by 77 weeks. Therefore, the survival curves of As2O3-treated MRL/lpr are similar to those of MRL+/+ mice.
As2O3 inhibits anti-DNA autoantibody and RF production in MRL/lpr mice. IgG1, IgG2a, IgG2b, and lgG3 anti-DNA autoantibodies, and IgM, IgG and IgG3 RF levels were quantified by ELISA in the sera (dilution, 1:1600) of 4-month-old MRL+/+ and MRL/lpr mice that had been treated daily for 2 months with As2O3 (5 μg/g) or PBS. Results are from 10 individual mice per group. Error bars indicate SE.
As2O3 inhibits anti-DNA autoantibody and RF production in MRL/lpr mice. IgG1, IgG2a, IgG2b, and lgG3 anti-DNA autoantibodies, and IgM, IgG and IgG3 RF levels were quantified by ELISA in the sera (dilution, 1:1600) of 4-month-old MRL+/+ and MRL/lpr mice that had been treated daily for 2 months with As2O3 (5 μg/g) or PBS. Results are from 10 individual mice per group. Error bars indicate SE.
As2O3 activates caspases and normalizes glutathione levels in MRL/lpr mice
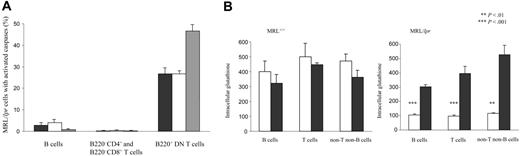
Because As2O3 was shown to induce apoptosis of various cell types,42 we determined the levels of activated caspase-2, -8, and -9 in lymphocyte populations from mice treated with As2O3 for 1 up to 90 days. Surprisingly, as early as day 4 of treatment, about 50% of MRL/lpr B220+ DN T lymphocytes contained activated caspases, as opposed to only a few MRL/lpr B cells, and MRL/lpr B220–CD4+ and B220–CD8+ T cells (Figure 9A). No activated caspases were detected in B lymphocytes or the B220–CD4+ and B220–CD8+ T-cell subpopulations from MRL+/+ mice treated with As2O3 for 4 days or longer (data not shown).
Because NO is overproduced in the diseased organs of MRL/lpr mice,29 we measured levels of GSH, a critical antioxidant in cellular defenses against free radical-induced damage. Notably, GSH concentrations were 4 to 5 times lower in MRL/lpr spleen and LN-cell populations than in those from MRL+/+ mice (Figure 9B; data not shown). Although MRL+/+ GSH levels were not significantly modified by As2O3, intracellular GSH levels in MRL/lpr spleen and LN-cell populations had increased significantly (P < .001) 3- to 5-fold as of day 4 of treatment to reach the levels found in MRL+/+ mice (Figure 9B and data not shown).
As2O3 dramatically prolongs survival of MRL/lpr mice. Starting at 2 months of age, MRL/lpr mice (15/group) were treated daily with As2O3 (5 μg/g) (solid line) or PBS (dotted line) and monitored to establish mortality rates.
As2O3 dramatically prolongs survival of MRL/lpr mice. Starting at 2 months of age, MRL/lpr mice (15/group) were treated daily with As2O3 (5 μg/g) (solid line) or PBS (dotted line) and monitored to establish mortality rates.
As2O3 activates caspases and restores cellular levels of GSH in MRL/lpr mice. (A) Activated caspase-2 (black bars), -8 (white bars), and -9 (gray bars) were detected by flow cytometry in the spleen-cell subpopulations of 4-month-old MRL/lpr mice (4/group) that had been treated daily for 4 days with As2O3 (5 μg/g). (B) Intracellular levels of GSH were detected by flow cytometry in the spleen-cell populations of 4-month-old MRL+/+ and MRL/lpr mice (5/group) that had been treated daily for 4 days with As2O3 (5 μg/g; ▪) or PBS (□). Error bars indicate SE.
As2O3 activates caspases and restores cellular levels of GSH in MRL/lpr mice. (A) Activated caspase-2 (black bars), -8 (white bars), and -9 (gray bars) were detected by flow cytometry in the spleen-cell subpopulations of 4-month-old MRL/lpr mice (4/group) that had been treated daily for 4 days with As2O3 (5 μg/g). (B) Intracellular levels of GSH were detected by flow cytometry in the spleen-cell populations of 4-month-old MRL+/+ and MRL/lpr mice (5/group) that had been treated daily for 4 days with As2O3 (5 μg/g; ▪) or PBS (□). Error bars indicate SE.
Discussion
Our data presented herein demonstrate that As2O3 is a novel therapeutic agent to delete autoreactive lymphocytes and block the progression of autoimmune diseases. Arsenic therapy strongly limited or even abrogated, depending on the mouse, the following autoimmune manifestations of MRL/lpr disease: cytokine and autoantibody production, lymphoid hyperplasia, skin lesions, and mononuclear-cell infiltration into lungs and kidneys, immune-complex deposition in glomeruli, and early mortality. Remarkably, As2O3 administration achieved the quasi-total disappearance of the disease, even when it was initiated at an advanced stage, and its prevention when initiated at the predisease stage. This model of disease reversal makes As2O3 application in humans with lupus or other autoimmune diseases highly promising.
In MRL/lpr mice, DN T-lymphocyte accumulation in LNs and spleen starts at 2 months and peaks at 4 to 5 months. In MRL/lpr mice treated with As2O3 more than 50% of DN T lymphocytes contained activated caspases-2, -8, and -9, and the number of DN T cells was sharply diminished, suggesting that these cells were eliminated by apoptosis. In contrast, in As2O3-treated MRL+/+ mice only a few T cells contained activated caspases, and the number of T cells was unchanged. Therefore, we showed herein that As2O3 specifically activated caspases in the abnormal FasL-overexpressing DN T cells, thereby maintaining normal FasL levels.
Under As2O3, IgG1, IgG2a, IgG2b, and IgG3 anti-DNA autoantibodies as well as IgM, IgG, and IgG3 RF concentrations were much lower. Pathogenic autoantibodies in lupus mice generally belong to the IgG2a, IgG2b, and IgG3 subclasses, which are predominantly regulated by IFN-γ.26,50 IFN-γ, which is produced primarily by Th1 lymphocytes and natural killer (NK) cells, is a pleiotropic cytokine that modulates immune function, cell proliferation, apoptosis, and numerous other cellular responses. In the immune system, IFN-γ modulates macrophage-effector functions, influences isotype switching, and potentiates immunoglobulin secretion by B cells.51 IFN-γ synthesis by peripheral-blood cells and in the kidneys of patients with severe lupus glomerulonephritis was elevated, compared to individuals with milder renal disease.19 Although, this enhanced production was recently ascribed a beneficial role in murine lupus,52 the authors of several studies accorded it a major pathogenic role. In MRL/lpr mice, the IFN-γ concentration gradually rises over a prolonged period. Moreover, IFN-γ administration exacerbates the disease both in humans and mice, whereas MRL/lpr mice, with defective IFN-γ or IFN-γR expression, develop less severe forms.20-25,53-55 Indeed, IFN-γ is responsible of an overproduction of NO in diseased organs, and this overproduction parallels the development of clinical disease manifestations.29 Pertinently, we showed herein that antioxidant GSH levels were much lower in MRL/lpr than MRL+/+ spleen and LN cells. This GSH depletion most likely amplifies the toxic effect of NO and peroxynitrite on tissues. Interestingly, As2O3 treatment rapidly (4 days) restored intracellular GSH levels in MRL/lpr mice, thus limiting the deleterious effects of NO or peroxynitrite. Finally, we showed that the quasi-total disease regression observed in MRL/lpr mice treated with As2O3 corresponded to normal IFN-γ secretion, and consequently NO production, comparable to that found in MRL+/+ mice. We hypothesize that Th1 lymphocytes and NK cells are the targets of As2O3 because IFN-γ and FasL, molecules predominantly expressed by these cells, were maintained at near-normal levels by As2O3 therapy. Because activated caspase-2, -8, and -9 are not detected in CD4+ T cells from As2O3-treated MRL/lpr mice, these cells might have been eliminated by a caspase-independent mechanism. Our hypothesis is in agreement with a recent study showing that As2O3 triggers a caspase-independent necrotic-cell death.56
IL-18 also participates in the progression of this autoimmune syndrome. This multifunctional cytokine is expressed by various cell types, including antigen-presenting cells (macrophages and dendritic cells [DCs]) and cells not primarily involved in immune responses, for example, keratinocytes, articular chondrocytes, synovial fibroblasts, and osteoblasts, and also within the adrenal cortex and pituitary gland.28 IL-18 induces IFN-γ synthesis by T lymphocytes and NK cells. Enhanced IL-18 synthesis at the site of inflammation has been described in numerous inflammatory disorders,28 but the factors influencing its production are still poorly understood. In human SLE, elevated levels of circulating IL-18 paralleled disease activity.57 MRL/lpr lymphoid cells are hypersensitive to IL-18 and express high levels of it.58 Pertinently, IL-18 levels, which were abnormally high in MRL/lpr mice, returned to normal MRL+/+ levels in As2O3-treated MRL/lpr mice. This observation suggests that, in addition to eliminating DN T lymphocytes, activated Th1 and NK cells, As2O3 can also eradicate activated macrophages and DCs. As2O3-mediated regulation of IL-18 is probably involved in the maintenance of normal IFN-γ levels and minimal renal damage. Our results are in agreement with a study showing that lower IL-18 activity protects against autoimmune diseases.46 Because IL-18 had been shown to up-regulate FasL expression on NK cells,28 less IL-18 also contributes to maintaining normal levels of membrane-bound and soluble forms of FasL in As2O3-treated MRL/lpr mice. Finally, TNF-α concentrations, which were abnormally high in MRL/lpr mice, returned to normal MRL+/+ levels in MRL/lpr mice treated with As2O3. The major sources of this proinflammatory cytokine are the cells of the monocyte/macrophage lineage, with T lymphocytes, neutrophils, mast cells, and endothelium also contributing under different circumstances. This observation is in agreement with our hypothesis that As2O3 eliminates activated macrophages.
Overexpression of Th2 cytokine, IL-10, is associated with the manifestations of ALPS2 and has a disease-promoting effect in humans and mice with lupus.33,35 IL-10 can be secreted by many cell types, with multiple biologic effects.59 For example, IL-10 can inhibit inflammatory responses as well as promote B-cell proliferation and antibody production. IL-10 contributed to the abnormal production of immunoglobulin and of autoantibodies in SLE.34 Administration of anti-IL-10 antibodies delayed onset of autoimmunity in NZB/W F1 mice.60 Moreover, anti-IL-10 mAb administration to SLE patients with active disease led to a reduction in disease activity.61 However, the precise role of IL-10 in the pathogenesis of lupus remains uncertain.62 IL-10 levels, which were abnormally high in MRL/lpr mice, decreased significantly in As2O3-treated MRL/lpr mice. Importantly, the reduction of serum IL-10 paralleled the decrease in DN T-cell numbers in peripheral lymphoid tissues. These observations suggest that like DN T cells from ALPS patients,2 the DN T cells are primary producers of IL-10 in MRL/lpr mice. As2O3-mediated regulation of IL-10 probably reduced serum immunoglobulins and autoantibodies, and renal immune-complex deposition.
The prototypical autoimmune disease, SLE is currently treated with corticosteroids and cytotoxic or immunosuppressive drugs. These therapies prolong survival but are associated with severe side effects, particularly infections. The therapeutic intraperitoneal route of arsenic in our mice model of autoimmune disease is different from intravenous or oral administration used in patients with APL. It would be interesting to compare the 3 routes of AS2O3 administration in MRL/lpr mice.38 As2O3 significantly prolonged survival of MRL/lpr mice by preventing young mice from developing the syndrome and quasi-totally reversing established disease in older animals, thereby demonstrating its potential as a novel therapeutic agent for autoimmune diseases.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 22, 2006; DOI 10.1182/blood-2006-04-020610.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We would like to thank Dr Jurgen Brosius (University of Muenster, Germany) for his critical reading of the manuscript and Magalis Berger and Elisabeth Connault for technical assistance.
This work was supported by the Centre National de la Recherche Scientifique and grants from Ligue Nationale Contre le Cancer and Association for Research on Rheumatoid Arthritis (ARP).