Abstract
This multicenter, open-label, randomized phase 2 study evaluated 2 dose regimens of lenalidomide for relapsed, refractory myeloma. Seventy patients were randomized to receive either 30 mg once-daily or 15 mg twice-daily oral lenalidomide for 21 days of every 28-day cycle. Patients with progressive or stable disease after 2 cycles received dexamethasone. Analysis of the first 70 patients showed increased grade 3/4 myelo-suppression in patients receiving 15 mg twice daily (41% versus 13%, P = .03). An additional 32 patients received 30 mg once daily. Responses were evaluated according to European Group for Blood and Marrow Transplantation (EBMT) criteria. Overall response rate (complete, partial, or minor) to lenalidomide alone was 25% (24% for once-daily and 29% for twice-daily lenalidomide). Median overall survival in 30-mg once-daily and twice-daily groups was 28 and 27 months, respectively. Median progression-free survival was 7.7 months on once-daily versus 3.9 months on twice-daily lenalidomide (P = .2). Dexamethasone was added in 68 patients and 29% responded. Time to first occurrence of clinically significant grade 3/4 myelosuppression was shorter in the twice-daily group (1.8 vs 5.5 months, P = .05). Significant peripheral neuropathy and deep vein thrombosis each occurred in only 3%. Lenalidomide is active and well tolerated in relapsed, refractory myeloma, with the 30-mg once-daily regimen providing the basis for future studies as monotherapy and with dexamethasone.
Introduction
Multiple myeloma, the second most common hematologic malignancy in the United States,1 remains incurable despite conventional chemotherapy2,3 or high-dose therapy.4,5 Recently, novel drug classes targeting both myeloma and its microenvironment have shown clinical activity.6 Bortezomib, a proteasome inhibitor, improves time to progression and survival rates in patients with relapsed and refractory myeloma.7-9 Thalidomide achieves 25% response in patients with refractory myeloma,10,11 and combinations of thalidomide with dexamethasone result in 40% to 55% responses in patients resistant to either agent alone.10,12 The combination has also been shown to be superior to high-dose dexamethasone for initial treatment.13-15 Somnolence, constipation, and neuropathy with dose escalation or prolonged administration are dose limiting,16 and additional toxicities occur when combined with dexamethasone, including deep vein thrombosis (DVT).13-15
Lenalidomide (CC-5013, Revlimid) is a potent thalidomide analog with a different toxicity profile from the parent molecule. It induces apoptosis of myeloma cells; overcomes cytokine and bone marrow stromal cell-mediated drug resistance; has antiangiogenic effects; enhances dexamethasone cytotoxicity; and stimulates host antimyeloma T-cell and natural killer (NK)-cell immunity.17-21
Two phase 1 dose escalation trials of lenalidomide in advanced myeloma defined 25 mg per day as the maximal tolerated dose.22,23 Of importance, no significant somnolence, constipation, or neuropathy was observed. Responses occurred in the majority of patients, including patients who had received prior thalidomide. Pharmacokinetic studies revealed rapid absorption, monophasic elimination, as well as low to moderate intersubject variability for area under the curve and maximum concentration.22
These preclinical and phase 1 studies provided the framework for this phase 2 clinical trial evaluating oral lenalidomide, 30 mg once daily versus 15 mg twice daily for 21 of 28 days to treat patients with relapsed or relapsed and refractory multiple myeloma. Dexamethasone was added in patients with progressive disease or in those patients who did not respond (ie, achieved stable disease) with lenalidomide alone.
Patients and methods
Patients
Patients were at least 18 years old with relapsed or relapsed and refractory myeloma. Measurable disease was a monoclonal immunoglobulin concentration on serum electrophoresis of at least 10 g/L (1 g per deciliter) for IgG or 5 g/L (0.5 g per deciliter) for IgA, or urinary excretion of at least 200 mg monoclonal light chain per 24 hours; patients with nonsecretory or oligosecretory myeloma had other measurable disease. All patients had relapsed after prior chemotherapy, with the majority having progressed during or within 60 days after salvage treatment.
Eligibility criteria included Eastern Cooperative Oncology Group (ECOG; Zubrod) performance status of 0 to 2; serum concentration of aspartate aminotransferase or alanine aminotransferase no higher than 3 times upper limit of normal; serum total bilirubin concentration no greater than twice upper limit of normal; a serum creatinine level no more than 176.8 μM (2 mg/dL); platelet count of at least 50 × 109/L (50 000 per μL); and absolute neutrophil count of at least 1 × 109/L (1000/μL). Prior thalidomide was permitted, but patients had to be off treatment for at least 21 days. Exclusion criteria included clinically relevant infection, serious comorbidity, or prior malignancy. Patients agreed to use contraception, and a confirmed negative pregnancy test before enrollment was required for women. All patients gave written informed consent before entering the study, which was obtained in accordance with the Declaration of Helsinki, under the auspices of protocols approved by the institutional review board of each participating center.
Study design and treatment
Patients were randomized to receive either 30 mg once-daily or 15 mg twice-daily oral lenalidomide for 21 of every 28 days. Patients were assessed before treatment, every 2 weeks for 4 weeks, and then monthly while on therapy. Patients with progressive or stable disease after 2 cycles continued lenalidomide treatment and received dexamethasone 40 mg/d orally for 4 days every 14 days. Treatment was discontinued in patients who progressed on lenalidomide plus dexamethasone; was withheld for grade 3 or worse nonhematologic toxicity, or grade 4 hematologic toxicity; and then resumed at lower dose level when toxicity was grade 2 or better.
The investigators and representatives from Celgene designed this study. Participating institutions received grant support from the sponsor; there were no limits on the content of the article, and all authors agreed with the publication of the results.
Assessment of efficacy
The primary end point was best overall response (complete response [CR], partial response [PR], or minor response [MR]) to each regimen of single-agent lenalidomide. Secondary end points were duration of response with lenalidomide, response to lenalidomide in combination with dexamethasone, safety, progression-free survival (PFS), and overall survival. Response or progression was assessed at each cycle according to the criteria of the European Group for Blood and Marrow Transplantation (EBMT) and confirmed by an independent review committee (IRC). Additional secondary end points included the following: pharmacokinetics (PK); biomarker expression (blood and cellular angiogenic factors, cytokines, and adhesion molecules); and assessment of CD4+, CD8+, and CD56+ lymphocyte counts.
Progression-free survival was time from initial administration of lenalidomide to progression or time last known alive. Overall survival was time from its initial administration to death or time last known alive. Duration of response was time from achievement of a response on lenalidomide to disease progression. To evaluate duration of response to lenalidomide alone, patients who received dexamethasone prior to progressive disease were censored when dexamethasone was added. Progression-free survival was evaluated both with and without censoring for the addition of dexamethasone.
Assessment of safety and other secondary end points
Adverse events, graded according to National Cancer Institute Common Toxicity Criteria (version 2.0; Bethesda, MD), were assessed at each visit until 30 days after last dose of lenalidomide. Abnormal laboratory values were considered adverse events if the abnormality resulted in discontinuation from the study, required treatment modification, or was judged by the investigator to be of significant clinical impact. Toxicity is also reported including all laboratory abnormalities.
Corollary studies and pharmacokinetics
To evaluate lenalidomide's mechanism of action and to identify markers of response, serum levels of soluble intercellular adhesion molecule 1 (sICAM-1), interleukin-2 (IL-2), interleukin-12 (IL-12), and interferon-gamma (IFN-γ) were measured in 35 patients (21 on once-daily and 14 on twice-daily lenalidomide) before treatment and at initiation of cycle 2 of lenalidomide therapy by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). Percent CD4+, CD8+, and CD56+ lymphocyte counts was measured at baseline and serially with each cycle of lenalidomide therapy.
Plasma (PK) concentrations were determined in 39 patients during the first and second cycles of lenalidomide in both dose groups, and when dexamethasone was added due to PD or SD on lenalidomide alone. This was scored as Cycle X and the next cycle was scored as Cycle X + 1.
Statistical analyses and sample size
The trial enrolled 70 patients (cohort 1) randomized to 2 doses of lenalidomide in a one-stage pick-the-winner design,24 with at least 84% power of selecting more effective dose (response rate ≥ 40%), and low probability (< 5%) of selecting less effective dose (response rate ≤ 10%). This design was selected because it allows for concurrent evaluation of 2 doses of lenalidomide and ensures through randomization similar patient populations on both arms. It was not selected to formally compare the 2 arms, as this analysis would require a larger number of patients. Analysis of the first cohort of 70 patients showed increased rate of grade 3/4 myelosuppression (neutropenia or thrombocytopenia) with 41% reported in the 15-mg twice-daily arm versus 13% in the 30-mg once-daily arm (P = .03). Therefore, an additional 32 patients (cohort 2) were treated with once-daily lenalidomide. With these additional patients, the maximum width of the 95% confidence interval is 25% (n = 67).
The 95% confidence intervals were reported for the response rate to lenalidomide alone, improvement in response after addition of dexamethasone, and toxicity. Time-to-event outcomes were estimated using Kaplan-Meier method and compared using the log-rank test. Cytokine levels between baseline and initiation of cycle 2 of treatment and the change between responders and nonresponders were compared using the Wilcoxon signed rank tests and Wilcoxon rank-sum tests, respectively. All P values were 2-sided.
Results
Patients and treatment
From May 2002 to July 2003, 102 patients with relapsed or relapsed and refractory myeloma enrolled at 4 centers. Seventy patients were enrolled to cohort 1, with 35 patients randomized to receive 15 mg twice-daily and another 35 patients randomized to 30 mg once-daily lenalidomide. An interim analysis of cohort 1 included the toxicity data of the first 57 patients (27 in twice daily and 30 in once daily) and showed increased grade 3/4 myelosuppression (neutropenia or thrombocytopenia) in the twice-daily group versus the once-daily group (41% versus 13%, P = .03). Subsequently, a second cohort of 32 patients was enrolled and assigned to receive 30-mg once-daily lenalidomide. Overall, 67 and 35 patients received the once-daily and twice-daily regimen, respectively (Table 1). The 2 arms were well matched for age and sex. Ninety-three percent of patients were white, 5% African-American, and 2% of Asian American or of Pacific Island heritage.
At diagnosis, 52% of patients had Durie-Salmon stage III disease. Fifty-three (52%) patients had relapsed and refractory myeloma at study entry. IgG isotype was most common, and 60% of patients had Bence-Jones proteinuria (Table 1). Lytic bone disease occurred in 74% of patients. The median percentage of bone marrow plasma cells was 31%; cytogenetics were abnormal in 31 patients (38%), with chromosome 13 deletion in 19 (23%) cases. Median serum albumin level for all patients was 39 g/L (3.9 g/dL); hemoglobin level, 116 g/L (11.6 g/dL); LDH level, 433 U/L (range, 72-1305 U/L); platelet count, 207 × 109/L (207 000/μL); and white blood cell count, 4.6 × 109/L (Table 1).
The median number of prior lines of therapy was 4 (range, 1-13), and 56% of patients had received more than 3 lines of prior therapy. Moreover, 61%, 76%, and 18% of patients had received prior high-dose chemotherapy followed by stem cell transplantation, thalidomide, and bortezomib, respectively.
Response
The overall rate of response (CR, PR, MR) to lenalidomide was 25% (Table 2). In the once-daily cohort, the response rate was 24% (95% CI, 14%-36%) with 6% CR and 12% PR, while 29 patients (43%) achieved stable disease (SD). Among the 16 patients who achieved at least MR to lenalidomide alone, 9 (56%) were refractory to their last therapy. The median duration of response to lenalidomide, with censoring at the time of dexamethasone addition, was 19 months (range, 2-22 months).
In the twice-daily dose group, the response rate was 29% (95% CI: 15%-46%), with no CR and 14% PR. Fourteen patients (40%) had stable disease (Table 2). The median duration of response, with censoring at time of dexamethasone addition, was 23 months (range, 2-25 months).
Sixty-eight patients (67%; 27 on the twice-daily arm and 41 on the once-daily arm) of 102 patients had dexamethasone added. In the once-daily group, 12 patients (29%) achieved a response to the combination, with 1 (2%) CR, 8 (20%) PR, 3 (7%) MR, and 13 (32%) SD. In the twice-daily group, no patients achieved CR, with 6 (22%) PR, 2 (7%) MR, and 1 (4%) SD. Overall, 20 (29%) of 68 patients responded to the addition of dexamethasone with 14 (21%) SD.
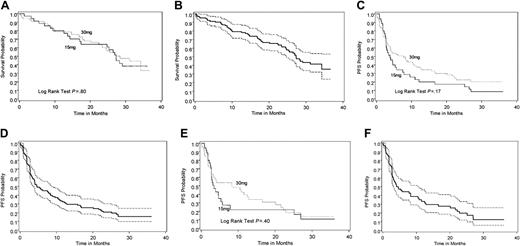
Overall survival and progression-free survival of lenalidomide-treated patients. (A) Overall survival for the 15-mg twice-daily and 30-mg once-daily dose groups. (B) Overall survival combined for both doses with 95% confidence interval. (C) Progression-free survival to lenalidomide (without censoring for addition of dexamethasone) for the 15-mg twice-daily and 30-mg once-daily dose groups. (D) Progression-free survival to lenalidomide (without censoring for addition of dexamethasone) for both doses combined (with 95% confidence interval). (E) Overall progression-free survival (with censoring for addition of dexamethasone) for 15 mg twice-daily and 30 mg once-daily dose groups. (F) Overall progression-free survival (with censoring for addition of dexamethasone) for both doses combined with 95% confidence interval. Dotted lines in panels B, D, and F represent the upper and lower boundaries of the 95% CI for each Kaplan-Meier curve.
Overall survival and progression-free survival of lenalidomide-treated patients. (A) Overall survival for the 15-mg twice-daily and 30-mg once-daily dose groups. (B) Overall survival combined for both doses with 95% confidence interval. (C) Progression-free survival to lenalidomide (without censoring for addition of dexamethasone) for the 15-mg twice-daily and 30-mg once-daily dose groups. (D) Progression-free survival to lenalidomide (without censoring for addition of dexamethasone) for both doses combined (with 95% confidence interval). (E) Overall progression-free survival (with censoring for addition of dexamethasone) for 15 mg twice-daily and 30 mg once-daily dose groups. (F) Overall progression-free survival (with censoring for addition of dexamethasone) for both doses combined with 95% confidence interval. Dotted lines in panels B, D, and F represent the upper and lower boundaries of the 95% CI for each Kaplan-Meier curve.
No significant differences in response rate (CR + PR + MR) to single-agent lenalidomide were detected between patients who had received prior thalidomide (in the once-daily group, 18% for those who had received prior thalidomide versus 39% for those who did not, P = .11; in the twice-daily group, 25% for those who received versus 43% for those who did not. P = .38) or bortezomib (for the once-daily group, 9% for those who received prior bortezomib versus 27% for those who did not, P = .28; for the twice-daily group, 29% for those who received versus 29% for those who did not, P = .99). Similarly, no significant differences were detected in response rate (CR + PR + MR) to single-agent lenalidomide and dexamethasone between patients who received prior thalidomide (in the once-daily group, 23% response rate for those who received prior thalidomide versus 50% for those who did not, P = .12; in the twice-daily group, 30% for those who received versus 25% for those who did not, P = .99) or bortezomib (20% for those who received versus 31% for those who did not, P = .99; 15 mg: 20% for those who received versus 32% for those who did not, P = .99).
Progression-free and overall survival
The median overall survival among patients in the twice-daily and once-daily arms was 27 and 28 months, respectively (Figure 1A), and 27 months for both arms combined (Figure 1B). The median follow-up time among patients who were alive at the time of this analysis was 31 months, with 20 and 34 patients having died on the twice-daily and once-daily arms, respectively. The median PFS was 3.9 months (95% CI, 2.8-7.5) for the twice-daily arm and 7.7 months (95% CI, 3.8-11.5) for the once-daily arm (Figure 1C), and 4.6 months for both arms combined (Figure 1D). The median PFS for lenalidomide, censoring for addition of dexamethasone, was 3.5 months (95% CI, 2.8-5.4) for the twice-daily arm and 8.3 months (95% CI, 3.2-12.8) for the once-daily arm, (Figure 1E) and 4.1 months for both arms combined (Figure 1F). The median PFS for lenalidomide, considering the addition of dexamethasone as an event, was 2.8 months (95% CI, 2.5-3.9) for the twice-daily arm and 3.0 months (95% CI, 2.7-4.7) for the once-daily arm, and 3.0 months for both arms combined. No significant difference was detected between arms in overall survival (P = .8), PFS (P = .17), or PFS for lenalidomide either with (P = .7) or without (P = .4) the addition of dexamethasone (Figure 1A-F).
Toxicity
The most common grade 3 or higher adverse events during therapy were neutropenia and thrombocytopenia (Table 3). Grade 4 neutropenia occurred in 4 (11%) of 35 patients treated twice daily versus 8 (12%) of 67 patients treated once daily. Grade 4 thrombocytopenia occurred in 6 (17%) of patients on twice-daily treatment versus 11 (16%) of 67 patients on once-daily lenalidomide. Among 102 patients, no significant differences were detected, at the conclusion of the study (both cohorts 1 and 2), in the proportion of grade 3/4 myelosuppression (69% for once daily vs 80 for twice daily, P = .3). However, the time to the first occurrence of clinically significant grade 3/4 myelosuppression was shorter in the twice-daily group (1.8 vs 5.5 months, P = .05). Including all laboratory abnormalities, the time to first occurrence of grade 3/4 myelosuppression showed the same trend (1.7 vs 3.6 months, P = .09). The median number of cycles was 6.0 (range, 1-36) for twice daily and 9 (range, 1-39) for once daily (P = .6). Among patients who developed grade 3/4 hematologic toxicity, a total of 3 cases of febrile neutropenia was observed: 1 case in the 15-mg twice-daily group (of a total of 35 patients) and 2 cases in the 30-mg once-daily group (of a total of 67 patients). No significant bleeding was reported in association with grade 3/4 thrombocytopenia.
The proportion of patients who experienced grade 3 or 4 myelosuppression was not significantly different by number of prior therapies (grades 3/4 myelosuppression: 63% (n = 22) for 1-2 prior therapies, 61% (n = 23) for 3 prior therapies, and 81% (n = 57) for > 3 prior therapies; P = .11). However, the proportion of patients who experienced grade 3 or 4 myelosuppression was significantly higher for patients who had prior high-dose chemotherapy and stem cell transplantation (SCT) (82% vs 58%, P = .01).
The most common other grade 3 or greater toxicities included leukopenia (34% versus 37%), anemia (14% versus 16%), and fatigue (9% versus 7%). Of importance, the incidence of treatmentemergent peripheral neuropathy was 8 (23%) of 35 in the twice-daily arm versus 7 (10%) of 67 for the once-daily arm. Similarly, constipation occurred in 11 (31%) versus 17 (25%) patients of the twice- and once-daily arms, respectively. With regard to diarrhea, 2 cases of grade 1 toxicity were observed in the 15-mg twice-daily group, while 9 cases of grade 1, 1 case of grade 2, and 1 case of grade 3 diarrhea were observed in the 30-mg once-daily group.
In terms of thromboembolic events, 2 cases of DVT were seen in the 15-mg twice-daily group and 1 case in the 30-mg once-daily group. In both groups, DVTs occurred only when dexamethasone was added. No pulmonary emboli were reported. No thromboprophylaxis was administered in patients enrolled in this trial.
Surrogate markers and pharmacokinetics
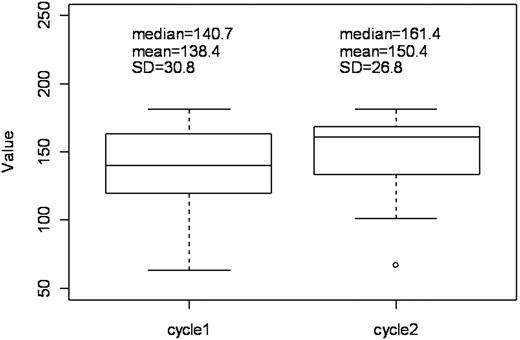
ELISAs were performed in 35 evaluable patients (21 and 14 patients with once- and twice-daily treatment, respectively) to measure the serum levels of sICAM-1, IL-2, IL-12, and IFN-γ at baseline and cycle 2. A significant increase was observed in median sICAM-1 levels (median = 7.8, mean = 11.4, SE = 3.2 ng/mL) from baseline (median = 140.7, mean = 138.4, SE = 5.2 ng/mL) (P = .001, Wilcoxon signed rank test) (Figure 2). This increase was observed with both once- and twice-daily treatment (P = .021 and P = .041, respectively). No significant differences between responders and nonresponders were detected in sICAM-1 levels at baseline or at cycle 2, or between baseline versus cycle 2, or between the 2 treatment arms for serum levels of IL-2, IL-12, and IFN-γ. Similarly, no differences in progression-free survival or survival were detected.
In the same subset of patients (n = 35), lenalidomide administration was associated with significant increase in the mean (± SE) percentage of CD4+ T cells at cycle 2 versus baseline (36.59 ± 2.11 vs 32.66 ± 2.16; median change of 4%; mean change of 4.44%; SE, 1.25%; 95% CI, 1.9-7.0; P = .001, Wilcoxon signed rank test). Significant increase in mean (± SE) CD4/CD8 ratio at cycle 2 versus baseline (1.70 ± 0.22 vs 1.43 ± 0.17; median change of 0.18; mean change of 0.34; SE, 0.1; 95% CI, 0.135-0.538; P = .005, Wilcoxon signed rank test) was also observed. In contrast, no significant differences were observed with respect to percentages of CD8+ or CD56+ NK cells.
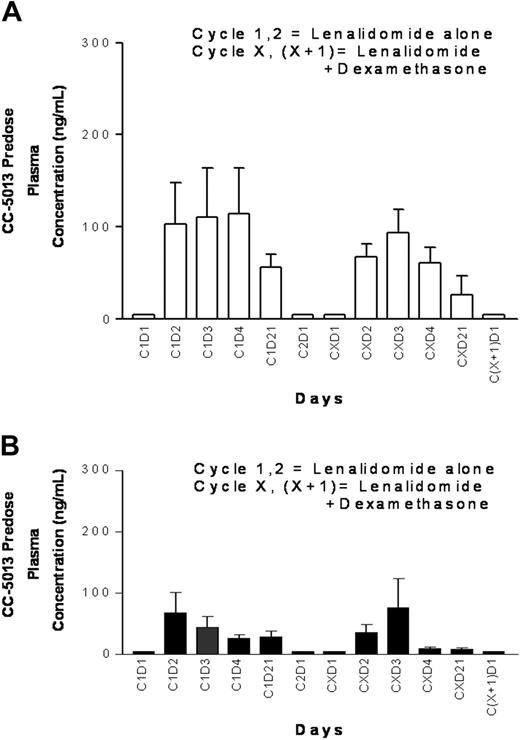
The mean minimum (Cmin) plasma lenalidomide concentrations on days 1, 2, 3, 4, and 21 during the first and second 21-day cycles of lenalidomide alone and with the addition of dexamethasone are shown for the once-daily and twice-daily cohorts (n = 39) (Figure 3). The average Cmin plasma levels were less in the twice-daily compared with daily dosing regimens. No obvious effect on lenalidomide plasma concentrations was seen with addition of dexamethasone in either once- or twice-daily treatment.
Discussion
Lenalidomide, a member of the IMiD class of immunomodulatory drugs, confers a broad spectrum of effects against myeloma cells, including direct induction of apoptosis; abrogation of protection of bone marrow microenvironment on tumor cells; inhibition of angiogenesis; and stimulation of host antitumor immunity.6,17,19-21,25,26 Preclinical studies also suggest that caspase-8-mediated activity of lenalidomide can be enhanced by agents such as dexamethasone19,21 and bortezomib.21 Two phase 1 clinical trials of lenalidomide showed a favorable side effect profile, defined its maximum tolerated dose, and demonstrated its ability to overcome drug resistance in patients with advanced myeloma.22,23 However, these trials did not determine an optimal schedule for dosing and, prior to this study, no clinical experience had prospectively tested the laboratory observation that corticosteroids could improve the antimyeloma effect of lenalidomide.
In this study, which evaluated 2 treatment regimens in patients with heavily pretreated, relapsed, refractory myeloma, the overall response rate (CR, PR, MR), assessed by EBMT criteria and confirmed by an IRC, was 25%, with a further 42% of patients achieving SD.
With addition of dexamethasone in patients in whom lenalidomide either failed to achieve a response or who subsequently progressed, 29% did respond and 21% achieved SD. These clinical observations support that caspase-8-mediated effects of thalidomide and lenalidomide can potentiate caspase-9-mediated antimyeloma effects of dexamethasone.19,21
Change in serum sICAM-1 levels during single-agent lenalidomide treatment. Baseline (beginning of first cycle) versus beginning of second cycle.
Change in serum sICAM-1 levels during single-agent lenalidomide treatment. Baseline (beginning of first cycle) versus beginning of second cycle.
Pharmacokinetics of lenalidomide treatment. (A) Minimum lenalidomide plasma levels for the 30-mg once-daily dose group. (B) Minimum lenalidomide plasma levels in the 15-mg twice-daily dose group. Lenalidomide (CC-5013): mean ± SE.
Pharmacokinetics of lenalidomide treatment. (A) Minimum lenalidomide plasma levels for the 30-mg once-daily dose group. (B) Minimum lenalidomide plasma levels in the 15-mg twice-daily dose group. Lenalidomide (CC-5013): mean ± SE.
Responses were durable, with median duration of response to lenalidomide alone of 20 months for both dose groups combined (95% CI, 8-25 months), and are favorable when compared with results seen with bortezomib monotherapy.7-9 Median progression-free survival was 7.7 months on the 30-mg once-daily arm, and median overall survival was 28 months,7-9 which is similar to that achieved with bortezomib-based treatment. No significant differences were observed in response rates to single-agent lenalidomide (or to its combination with dexamethasone) between patients who had received prior thalidomide or bortezomib versus those who had not, although the number of patients who had received prior bortezomib at the time of this study was relatively small. However, there was a trend suggesting that those patients with prior thalidomide exposure achieved lower response rates compared with those who were IMiD naive. This observation may have implications for the sequencing of therapy, and further studies are needed to address this question.
In the once-daily arm, the most common hematologic grade 3/4 adverse events were neutropenia (61%) and thrombocytopenia (31%). It was noteworthy that the proportion of patients who experienced grade 3 or 4 myelosuppression was significantly higher for patients who had prior SCT (82% vs 58%, P = .01). Nonhematologic toxicity included development of grade 3 neuropathy and fatigue in 3% and 7% patients, respectively. There was a low incidence of DVT, which occurred only when dexamethasone was added. Of importance, once-daily dosing proved preferable to twice-daily dosing in terms of clinically significant neutropenia and thrombocytopenia, which was otherwise manageable with dose reduction and granulocyte colony-stimulating factor (GCSF) support.
Corollary studies showed significant increases in average levels of sICAM-1 with lenalidomide treatment. The precise source of this sICAM-1 increase remains to be determined. Increase of sICAM-1 levels has been observed in clinical and preclinical studies after thalidomide treatment,16,27,28 as well as in various conditions with endothelial/vascular damage and immune stimulation (reviewed in Zeldis et al29 and Witkowska and Borawska30 ), or after tumor cell targeting via NF-kB inhibition, which occurs in myeloma cells exposed to lenalidomide in vitro.19,21 Ongoing studies are addressing whether sICAM-1 or related molecules can serve as biomarkers of response to lenalidomide, or could predict for toxicity.
The increase in mean percentage of CD4+ cells is consistent with the reported immunomodulatory effects of lenalidomide and other thalidomide analogs,17 and was observed after only one cycle of lenalidomide treatment. It is conceivable that more prolonged exposure to lenalidomide may lead to more pronounced increases in mean percent of CD4+ cells and perhaps other immunologic parameters: this hypothesis is being tested in follow-up studies of lenalidomide-treated patients. These current data, along with the accumulating evidence on the immunomodulatory role of lenalidomide, thalidomide, and other members of this family of compounds, support the notion that lenalidomide could be used to stimulate responses of myeloma patients to cell-based immunotherapy.17,25
The PK profile observed in this study demonstrated lower Cmin levels for the twice-daily dosing regimen compared with the once-daily regimen. This implies that the increased neutropenia and thrombocytopenia noted in the twice-daily dose group cannot be explained by minimal concentration effect, and further studies are needed to better understand this aspect of lenalidomide toxicity. Of importance, there was no obvious effect on the Cmin of lenalidomide with addition of dexamethasone for either once-daily or twice-daily dosing, suggesting that the observed increased incidence of thrombosis with the addition of dexamethasone cannot be attributed to an impact on serum lenalidomide levels.
In summary, the encouraging response rate, PFS, and overall survival observed after lenalidomide treatment in this study, either as a single agent or combined with dexamethasone, support the role of lenalidomide as an important new therapy for advanced myeloma.22,23 Of importance, efficacy was observed in heavily pretreated patients, including those who had received prior thalidomide. This once-daily regimen and the increased response rates observed with added dexamethasone have provided the framework for 2 placebo-controlled, phase 3 studies in patients with relapsed myeloma, comparing the combination of high-dose dexamethasone with lenalidomide to high-dose dexamethasone alone.31 Our study also provided the basis for a large, multicenter phase 2 study of lenalidomide alone in patients with relapsed and refractory myeloma, combination studies with high-dose dexamethasone for patients with newly diagnosed disease, and for studies of combinations with other agents, including bortezomib.32-34 This reflects the evolution of lenalidomide in the treatment of myeloma as a paradigm for the development of novel therapy, used either as a single agent or in combination, to improve patient outcome in this otherwise incurable malignancy.35
Prepublished online as Blood First Edition Paper, July 17, 2006; DOI 10.1182/blood-2006-04-015909.
Supported by National Institutes of Health (NIH) grants RO-1 CA50947, PO-1 78373, and Specialized Program of Research Excellence (SPORE) P50 CA100707 (K.C.A.); a Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.); the Multiple Myeloma Research Foundation (K.C.A.); the Myeloma Research Fund; and the Fund to Cure Myeloma (K.C.A.).
P.G.R., S.J., N.C.M., and K.C.A. have participated in Advisory Boards for Celgene Corp. S.V.R. and K.C.A. have received research support from Celgene Corp. R.L.S. and D.D. have received honoraria from Celgene Corp for lectures. M.O., K.W., J.Z., and R.K. are employees of Celgene Corp.
P.G.R., E.B., and C.S.M. contributed equally to this work.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
The authors gratefully acknowledge the assistance of Amy Potenza in the preparation of the article; the research nurses, nurse practitioners, and research coordinators for their invaluable help with the study; and especially the patients and their families for their participation.