Abstract
The immunoglobulin free light chain (FLC) is the precursor protein of amyloid in primary systemic amyloidosis (AL). Historically, the ability to monitor the amyloid protein precursor protein has been crude. We evaluated the utility of the FLC assay in a retrospective analysis of patients with AL undergoing peripheral blood stem cell transplantation (PBSCT). Ninety-three such patients had serial FLC measurements performed. The prognostic effects of the initial concentration and the extent of reduction of monoclonal FLC on survival were studied. There was a significantly higher risk of death in patients with higher baseline FLC (hazard ratio 2.6, P < .04). Baseline FLC correlated with serum cardiac troponin levels, and higher FLC levels were associated with more organs involved by amyloid, suggesting that high FLC levels may be associated with more advanced disease. The percent FLC reduction did not predict for survival, but the absolute level of FLC achieved after therapy did. Normalization of FLC level after PBSCT predicted for both organ response and complete hematologic response. Achievement of FLC response was a better predictor of survival than achievement of complete hematologic response or normalization of the FLC ratio. FLC measurements both before and after PBSCT are important predictors of patient outcome.
Introduction
The immunoglobulin free light chain (FLC) is the protein precursor of the amyloid formed in primary systemic amyloidosis (AL),1,2 and recent literature suggests that the amyloidogenic FLC may be directly toxic in patients with AL.3 The present paradigm of therapy of AL is eradication of clonal bone marrow plasma cells in order to reduce or eliminate the circulating precursor protein.4-7 Until the advent of the FLC assay (Freelite; The Binding Site Limited, Birmingham, United Kingdom), serial quantitation of the amyloid protein precursor has been crude, relying on the quantity of the intact serum monoclonal immunoglobulin, the amount of urine light chain, and the degree of bone marrow plasmacytosis.5 The Freelite assay uses antibodies directed against FLC epitopes that are hidden in the process of light chains binding to immunoglobulin heavy chains, and has a sensitivity of less than 0.5 mg/L. This compares with typical detection limits of 150 mg/L to 500 mg/L by immunofixation and 500 mg/L to 2000 mg/L by electrophoresis.8,9
Not only is the Freelite assay more sensitive than conventional measures, but it also provides a tool to quantitate circulating FLC in patients with AL.9-11 Quantitation of monoclonal proteins has required protein electrophoresis, and only approximately 25% of patients with AL have “measurable” disease as defined by a serum protein electrophoresis M-spike of 10 g/L.11,12 In contrast, with the Freelite assay, more than half of patients with AL have “measurable disease” (ie, an FLC of more than 100 mg/L).9-12
Patients who achieve hematologic response by electrophoresis, immunofixation, and bone marrow biopsy have higher organ response rates and longer survival.4-7,13 The measurement of “hematologic response” in patients with AL, however, is difficult because of the inherent low tumor burden. Often the small changes that constitute a “hematologic response” in patients with AL are within the variability of the assays employed. Documenting a response in a cohort of patients who start with a median bone marrow plasmacytosis of 5% to 7% and median serum M-spike of 1 g/L is a challenge. More sensitive and reproducible methodology is required. There is one report from the United Kingdom in which the authors evaluated the role of changes of serum FLC, predominantly in a nonhematopoietic transplantation setting. These authors found that the 5-year overall survival was more than doubled in patients achieving a 50% decline in serum FLC regardless of the therapeutic strategy.11 Sanchorawala et al14 recently demonstrated that FLC improvement correlated with complete hematologic response and were more readily detected early after treatment than were changes in immunofixation and bone marrow studies. We hypothesized that FLC testing would make more patients evaluable for hematologic response and would also be more predictive for outcome than immunofixation and bone marrow studies due to the inherent subjectivity in interpreting the former and the sampling variability in the latter. In the current study, we determined the relevance of FLC in our cohort of patients with AL undergoing high-dose chemotherapy with peripheral blood stem cell transplantation (PBSCT).
Patients, materials, and methods
The study population consisted of patients with documented AL13 who underwent PBSCT and who had at least one immunoglobulin FLC measurement performed. No patients with coexistent multiple myeloma (ie, hypercalcemia, lytic bone disease, or hemoglobin level < 100 g/L) were included in the series. All patients had immunohistochemical documentation that their amyloid was composed of immunoglobulin light chain. Patients who underwent transplantation after August 1, 2004, were excluded to permit a 6-month follow-up in order to draw meaningful conclusions about hematologic and organ response as well as survival. There were 119 patients treated between March 1, 1996, and July 31, 2004, who satisfied these criteria. Of these, 10 died before their 3-month follow-up. Another patient was inevaluable for organ response due to renal allografting. This same patient was excluded from the overall survival analyses. Fifteen additional patients either had no repeat study performed (n = 11) or had it less than 1 month (n = 1) or more than 24 months (n = 3) after their transplantation, leaving 93 patients in whom changes in free light chains could be assessed. Ninety-three patients (excluding the 1 patient who had the renal allograft) had day-100 FLC measurements (median, 3.4 months; range, 1.0-11.9 months). Seventy-eight patients had measurements performed at 12 months (median, 12.2 months; range, 6-17 months); 3 of these had only the involved immunoglobulin measured with no ratio performed. Thirty-two patients had the FLC measurements performed on stored serum samples as previously reported.10 Organ response was defined as previously described.13,15 The study was approved by the Mayo Clinic Institutional Review Board. Informed consent was provided according to the Declaration of Helsinki.
Immunoglobulin FLC quantitation was carried out using a serum FLC assay (Freelite; The Binding Site Limited) performed on a Dade-Behring Nephelometer (Deerfield, IL). The FLC estimation consists of 2 separate assays: one to detect free-kappa and the other to detect free-lambda light chains.9 The reference range for kappa FLC is 3.3 mg/L to 19.4 mg/L; for lambda FLC is 5.7 mg/L to 26.3 mg/L, and for the ratio the range is 0.26 to 1.65. In order to pool the data of the monoclonal kappa and lambda patients, the clonal free light chain was considered the “involved” immunoglobulin free light chain for the purpose of the analyses and is henceforth referred to as FLC. The calculation of the reduction of FLC from baseline was calculated as the post-PBSCT value as a percentage of the pre-PBSCT value. Hematologic partial and complete response was defined according to the multiple myeloma European Blood and Marrow Transplant Criteria.12 Patients were considered evaluable for hematologic complete response if they did not meet the criteria before transplantation; that is, patients who were immunofixation negative and had fewer than 5% bone marrow plasma cells were considered inevaluable.
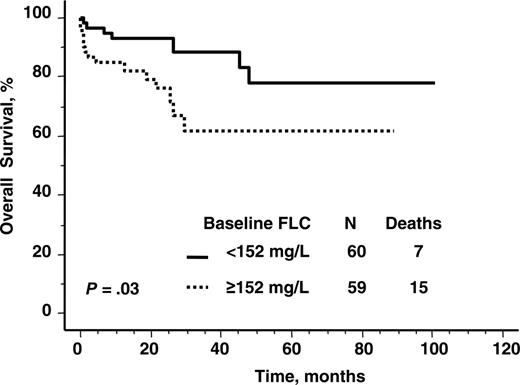
Overall survival according to baseline serum immunoglobulin free light chain level. Median value, 152 mg/L.
Overall survival according to baseline serum immunoglobulin free light chain level. Median value, 152 mg/L.
The prognostic effects of the size and the reduction of monoclonal FLC on survival were studied. These endpoints were computed using a Kaplan-Meier estimate16 ; curves were compared using the log-rank test. Survival was calculated from the date of the transplantation. The Spearman rank test was used to assess correlation between continuous variables. Differences between nominal variables and continuous variables were compared using Fisher exact and Mann-Whitney U tests, respectively. The pretransplantation factors evaluated included age, number of organs involved, sex, time from diagnosis to transplantation, serum creatinine, creatinine clearance, serum albumin, alkaline phosphatase, pretransplantation FLC, serum or urine M-spike, urine total protein, cardiac troponin T, bone marrow plasmacytosis or plasma cell labeling index, interventricular septal thickness, left ventricular ejection fraction, and intensity of melphalan conditioning. For these multiple comparisons, only P values less than or equal to .05 were considered significant. The effects of potential risk factors on survival were examined using a Cox proportional hazards model.17
Results
Patient characteristics
Tables 1 and 2 demonstrate characteristics of the 119 patients with a baseline FLC measurement performed. Fifty-nine percent were male, and the median age was 54 years. Median time from histologic diagnosis of AL to transplantation was 4.0 months. Thirty-six percent of patients had received prior therapy, most often a cycle or 2 of high-dose dexamethasone. Only 14% of patients had a “measurable” serum M-spike (ie, ≥ 10 g/L), whereas 73% had either no monoclonal protein on immunofixation or an M spike of less than 4 g/L. In contrast, 108 had an abnormal immunoglobulin free light chain, 104 abnormal FLC ratio, and 110 had both or either elevated.
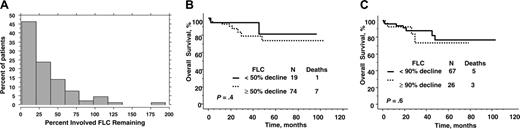
Reduction of serum immunoglobulin free light chain after peripheral blood stem cell transplantation. (A) Distribution of percent change after transplantation. (B) Overall survival by 50% reduction of immunoglobulin free light chain level from baseline. (C) Overall survival by 90% reduction of immunoglobulin free light chain level from baseline.
Reduction of serum immunoglobulin free light chain after peripheral blood stem cell transplantation. (A) Distribution of percent change after transplantation. (B) Overall survival by 50% reduction of immunoglobulin free light chain level from baseline. (C) Overall survival by 90% reduction of immunoglobulin free light chain level from baseline.
Twenty-two patients have died of their disease during a median follow-up of 19.0 months (range, 0-101 months). Median survival of patients has not yet been reached. The 1-year and 2-year estimated survivals are 90% and 80%, respectively. These numbers are comparable to the respective survival rates (82% and 79%) of the entire population of amyloid patients (n = 195) treated during the same time period, regardless of whether they had free light chain measurements available or not (data not shown).
Does the baseline value of immunoglobulin FLC predict for overall survival?
The baseline value of involved immunoglobulin FLC was prognostic for overall survival as a continuous variable, independent of the number of organs involved (HR 1.003; P = .013). The prognostic impact persisted when the 10 patients who died related to treatment (< 3 months after transplantation) were excluded (HR 1.004; P = .007). Using the median pretransplantation value of FLC (152 mg/L) for the cohort as a cut-off for Kaplan-Meier analysis, there was shorter survival in patients with higher baseline FLC (Figure 1). Those patients in this higher FLC burden category had a proportional hazard ratio of death of 2.6 (P < .04).
Comparisons between the baseline characteristics of those patients with a pre-PBSCT FLC of less than or greater than the median value (152 mg/L) were made. The only pretransplantation characteristics that differed between these 2 groups (Tables 1 and 2) were bone marrow plasmacytosis, number of organs involved, beta-2 microglobulin, serum cardiac troponin T, left ventricular interventricular septal thickness, and administration of reduced intensity conditioning. There was a weak correlation between cardiac troponin T levels and baseline immunoglobulin FLC (rho 0.46; P < .001). The correlation between bone marrow plasmacytosis and baseline free light chain (rho 0.34; P < .001) was even weaker.
Does posttransplantation immunoglobulin FLC predict for response?
The changes of involved FLC and FLC ratio at day 100 and 1 year are shown in Figure 2A and Table 3. The median percent of involved FLC remaining was 25% and 22% at 100 days and 1 year, respectively, whereas more than a quarter of patients had more than a 90% reduction in their FLC after transplantation. Pretransplantation factors that predicted for normal FLC 3 months after transplantation were lower pretransplantation FLC (P < .001) and fewer number of organs involved (P = .007). No other factors predicted for achievement of a normal FLC after transplantation.
Patients with normalized absolute FLC levels were most likely to have both hematologic and organ responses (Table 4). The same was true for the ratio, with the exception that the ratio at 100 days did not predict for organ response. Of the 93 patients with day-100 free light chain values available, 87 were assessed for complete hematologic response; 2 were excluded because they did not have all appropriate studies done to document a complete hematologic response after transplantation, and 4 were excluded because before transplantation, neither immunofixation of the serum or urine was positive nor was there more than 5% bone marrow plasmacytosis; that is, they were “inevaluable” by standard means. Forty-two (48%) had a complete hematologic response. Of the 74 patients with FLC measurements at 1 year, 35 (47%) achieved a complete hematologic response.
As shown in Table 4, normalization of immunoglobulin FLC predicted for organ response. Of the 93 patients with FLC measurements at day 100, 45 patients (48%) have had organ response documented. Of the 78 patients with FLC measurements at 1 year, 39 (50%) had organ response documented. The FLC ratio did not predict for organ response.
Does posttransplantation immunoglobulin FLC predict for overall survival?
The percent reduction of involved immunoglobulin FLC from baseline did not predict for overall survival, although achieving absolute low levels did. Overall survival was no different for those who had a 50% or 90% reduction at 3 months (Figure 2B-C) or at 1 year (data not shown). Even when the 5 patients with a normal baseline-involved FLC were excluded from the analyses, the percent reduction relative to baseline was not predictive for overall survival (data not shown).
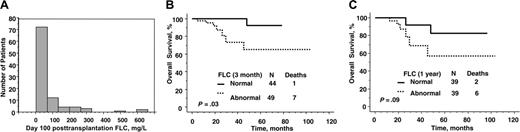
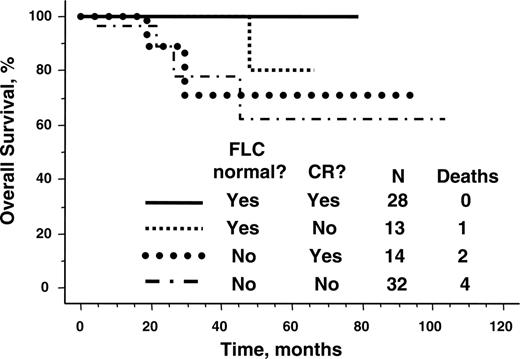
In contrast, survival was significantly better for those patients with the lowest immunoglobulin FLC after transplantation (Table 4). The median posttransplantation immunoglobulin free light chain values were 29 mg/L and 25 mg/L at day 100 and at 1 year, respectively, and fewer than one quarter of patients had involved free light chain more than 75 mg/L (Figure 3A). As shown in Figure 3B-C, normalization of the FLC was associated with a significantly improved survival. Normalization of FLC by day 100 was associated with a 0.13 hazard ratio of death. The ratio of involved to uninvolved FLC at 1 year approached significance as a predictor of survival; perhaps with longer follow-up and more events, it will be statistically significant. Even dichotomizing patients into normal and abnormal ratios was not predictive. Free light chain response was a more powerful predictor of survival than complete hematologic response (Figure 4).
Discussion
We have made 3 novel observations regarding the utility of immunoglobulin FLC in patients with primary systemic amyloidosis undergoing PBSCT in our retrospective analysis. The first is that absolute levels of immunoglobulin FLC appear to be more relevant than the FLC ratio in this clinical setting. The second is that attainment of a low absolute level of involved immunoglobulin free light chain—rather than a percentage reduction—is the best immunoglobulin FLC predictor for hematologic response, organ response, and overall survival after PBSCT. The third is that the pretransplantation immunoglobulin FLC has prognostic value for overall long-term survival and appears to be associated with more advanced disease.
At 100 days after transplantation, the absolute values of FLC were more predictive of outcome than were the ratio, the percent reduction of FLC, or complete hematologic response. One might expect that the ratio would be more informative than the value of involved FLC because the measurement of the FLC does not only include monoclonal FLC, but also polyclonal FLC. In instances of renal failure, circulating levels of polyclonal FLC increase, making the ratio an important number to consider—especially in individuals without clonal excess of plasma cells; however, in the context of amyloid patients—more than 90% of whom have measurable excess of the clonal light chain—reliance on the ratio appears to be less important, especially in the context of immunosuppressive therapies that also lower the uninvolved immunoglobulin FLC. Suppression of the uninvolved FLC results in extreme ratios, which are largely uninterpretable.
Relevance of absolute value of serum immunoglobulin free light chain after peripheral blood stem cell transplantation. (A) Distribution of levels 100 days after transplantation. (B) Overall survival by immunoglobulin free light chain at day 100. (C) Overall survival by immunoglobulin free light chain at 1 year.
Relevance of absolute value of serum immunoglobulin free light chain after peripheral blood stem cell transplantation. (A) Distribution of levels 100 days after transplantation. (B) Overall survival by immunoglobulin free light chain at day 100. (C) Overall survival by immunoglobulin free light chain at 1 year.
Unlike Lachmann et al,11 who have previously reported that a 50% reduction in FLC predicted for overall survival, we did not find this relative measure to be prognostic in our cohort. Their cohort included 137 patients with AL amyloid who received 1 of 3 types of therapy: a vincristine, doxorubicin, corticosteroid-based regimen; intermediate-dose melphalan (nonmyeloablative); or high-dose melphalan. The differential finding between the 2 studies may be related to the relative dose intensities administered in the 2 study populations. In their 3 treatment groups the median percent of FLC remaining after therapy relative to baseline was 35%, 43%, and 38%, respectively, as compared with 22% in our transplantation cohort. While 47% of their patients did not achieve an FLC reduction of 50% or more, only about 20% of our patients did not. Dose intensity can affect not only depth of response, but also durability of response.6,18 In addition, Lachmann et al11 reported that although a 50% reduction predicted for improved survival, a greater relative reduction did not further improve prognosis. This is in stark contrast to our findings. Hematologic response, using conventional means, has predicted for overall survival in patients with AL.5-7,13 Using the more sensitive and more quantitative FLC assay, we illustrate this same point. Whether one subscribes to the longstanding theory that the amyloid fibrils are the toxic species in patients with AL or the newer notion that the amyloidogenic light chains are actually the toxic species,3 maximal eradication of monoclonal FLC would appear to be desirable.
Although decreases in FLC predicted for both organ response and complete hematologic response, immunoglobulin FLC was a better predictor of survival than complete hematologic response, as defined by the European Blood and Marrow Transplant (EBMTR) myeloma response criteria. This finding is not surprising given the difficulties in reliably assigning complete hematologic response in amyloid patients. Interpretation of immunofixation of serum and urine and bone marrow plasmacytosis is confounded by the subjectivity encountered when assessing the former and the sampling variability of the latter. Moreover, reliance on a definition of bone marrow complete hematologic response as the absence of clonal plasma cells is subject to level of sensitivity of the assay used to make that assessment (immunohistochemistry, immunofluorescence, flow cytometry, or molecular methods). The variability in assignment of complete hematologic response should be abrogated by reliance on the immunoglobulin FLC assay. Our observation that attainment of low (or normal) FLC after transplantation better predicts survival than did complete hematologic response supports this notion.
Our observation that the baseline immunoglobulin FLC has independent prognostic value is also novel. The absolute levels of serum or urine monoclonal proteins have not been predictive for survival in patients with AL using electrophoretic measurements, whereas we have demonstrated that the values of FLC are. Even though the FLC assay does not restrict its measurement to the clonal FLC, this observation is not surprising given the fact that the FLCs are the precursors of amyloid fibrils. High levels of FLC also appear to be associated with more advanced disease. In our study, patients with higher baseline FLC had a greater number of organs involved, and baseline FLC levels correlated with levels of serum cardiac troponin T. One could question whether the association between cardiac troponin T and FLC is due to impaired renal clearance since both cardiac troponin T and FLC are affected by renal clearance,19,20 but renal function was not prognostic in this cohort of patients, nor was it significantly different between groups. In addition, only 6% of our patients had serum creatinine levels more than 176 μM (2 mg/dL).
In conclusion, our findings may have therapeutic implications in patients with AL undergoing PBSCT. Our data suggest that those patients who do not normalize their absolute FLC value after transplantation could be considered for further therapy. Based on the results of our study, however, we do not yet recommend this as a routine practice since the risk to a patient is likely a function not only of absolute quantity of FLC, but also its quality; that is, its intrinsic amyloidogeneicity. Ideally, our observations should be validated prospectively and by multiple centers.
Prepublished online as Blood First Edition Paper, January 5, 2006; DOI 10.1182/blood-2005-07-2922.
Supported in part by CA 62 242 (R.A.K.) from the National Cancer Institute, and the Robert A. Kyle Hematology Malignancies Fund, Mayo Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The Binding Site provided reagents for some of the testing.